Microencapsulation of an Extract of Sechium edule (Jacq.) Sw., with Antineoplastic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining the Extract
2.2. Manufacturing the Microspheres
2.3. Washing and Drying the Microspheres
2.4. Characterization and Release Tests of the Microspheres
2.5. Assessment of the Biological Activity
3. Results
3.1. Characterization of the Microencapsulated Product
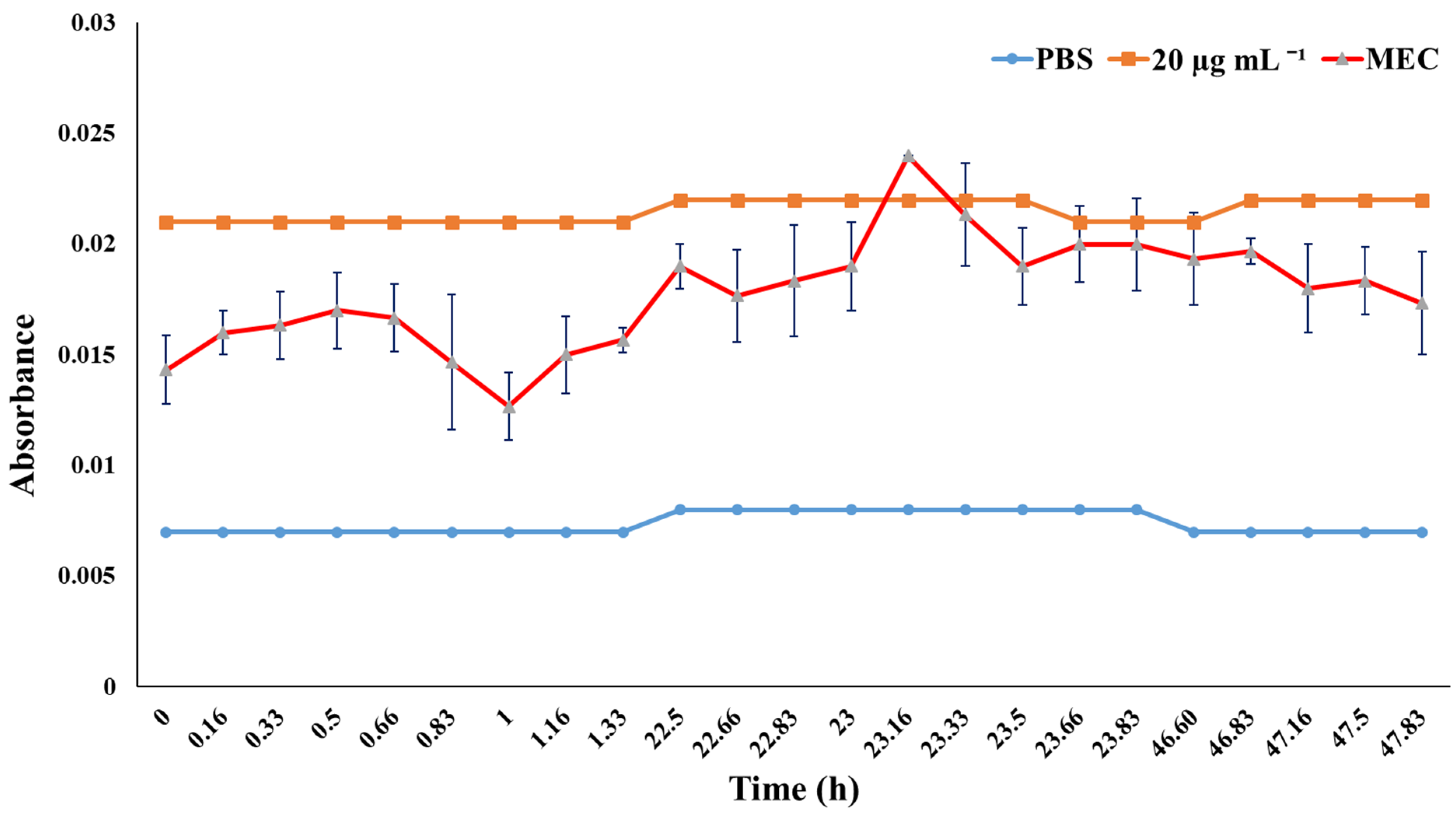
3.2. Release Tests
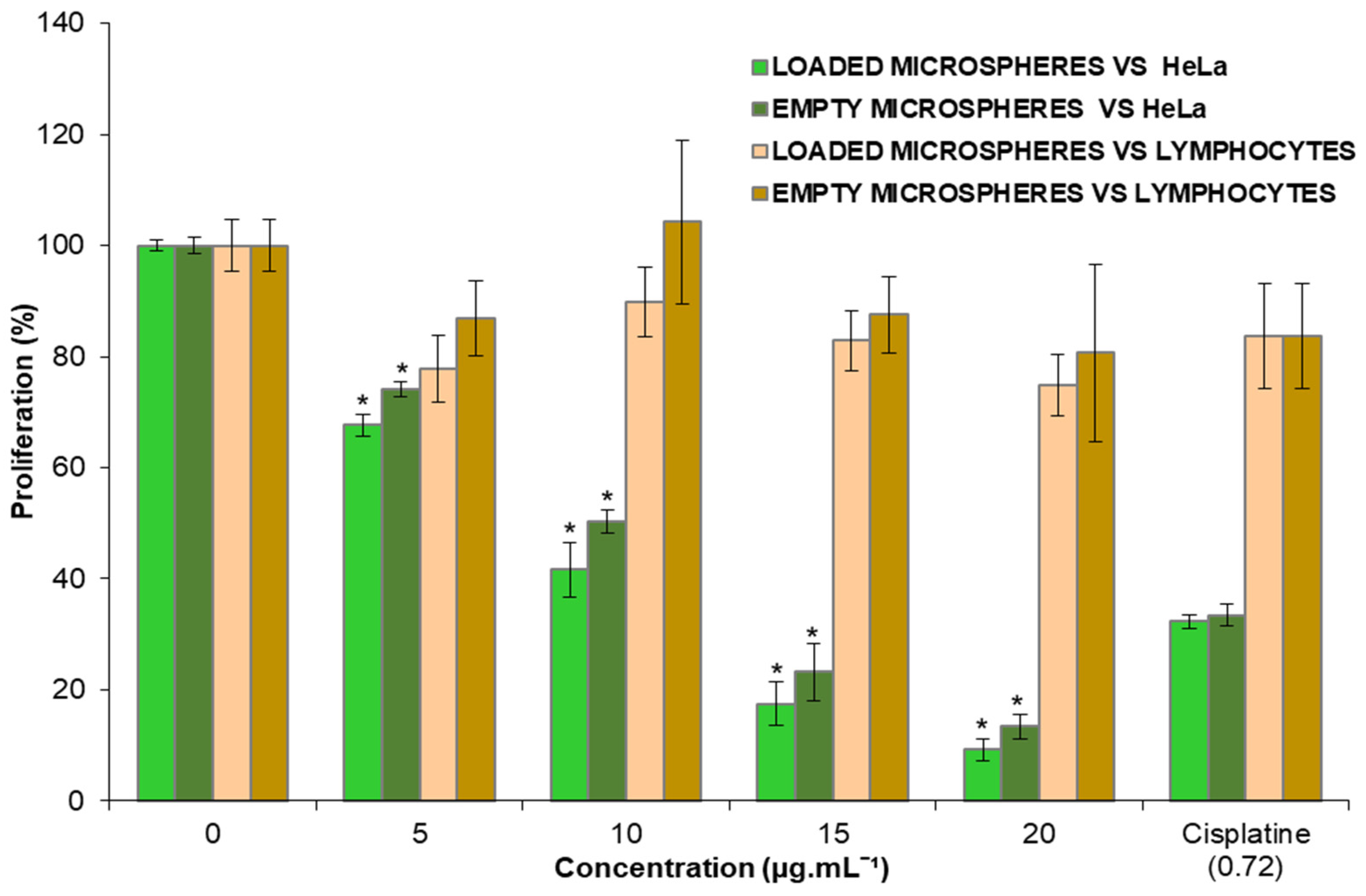
3.3. Effect of the Microspheres on HeLa and Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedraz, J.L.; Orive, G. La microencapsulación de células. Una nueva alternativa terapéutica? An. Real Acad. Nac. Farm 2004, 70, 777–787. [Google Scholar]
- López, H.O.; Márquez, T.; Mayo, C.O.; Toledo, A.C.S.; Pérez, Y.E.S. Características del aceite de semillas de Cucurbita pepo L. microencapsulado mediante secado por aspersión con maltodextrina y goma arábiga. Lat. Am. J. Pharm. 2009, 28, 628–632. [Google Scholar]
- Lopretti, M.; Barreiro, F.; Fernandes, I.; Damboriarena, A.; Ottati, C.; Olivera, A. Microencapsulación de compuestos de actividad biológica. INNOTEC 2011, 2, 19–23. [Google Scholar] [CrossRef]
- Sáez, V.; Hernández, E.; Sanz, A.L.; Katime, I. Liberación controlada fármacos. Micropartículas. Rev. Iberoam. Polim. 2004, 5, 87–101. [Google Scholar]
- Martín, V.M.J.; Morales, H.M.E.; Gallardo, L.V.; Ruiz, M.M.A. Técnicas de microencapsulación: Una propuesta para microencapsular probióticos. Ars Pharm. 2009, 50, 43–50. [Google Scholar]
- Cabanè, P.; Alvo, A.; Neira-Carrillo, A.; Caviedes, P.; Gac, P. Microencapsulación de células y tejido para terapia celular. Rev. Cir. 2011, 63, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Marte, Y.A.; Marte, Y.A.; Tejada, A.E.; Berruezo, G.R. Effect of different concentrations of pulverized mesocarp of Citrus paradisi Macf. on the bromatological characteristics of spray-dried lemon juice powder. Food Sci. Nutr. 2018, 6, 1261–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, H.O.D. Microencapsulación de sustancias oleosas mediante secado por aspersión. Rev. Cuba. Farm 2010, 44, 381–389. [Google Scholar]
- López-Hernández, O.D.; Gómez-Carril, M.; Padrón-Yaquis, A.S.; Toledo-Rivero, A.; Toledo-Sanchez, C.A.; Reyes-Sanchez, E.; Reguera-Ruiz, E.; Duque-Rodríguez, J.; López-Pelaez, B. Microencapsulación de tramadol en ácido poliláctico mediante secado por aspersión. Rev. Cuba. Farm 2010, 44, 432–442. [Google Scholar]
- Lárez, V.C. Algunos usos del quitosano en sistemas acuosos. Rev. Iberoam. Polim. 2003, 4, 91–109. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.; Amninabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Acosta, N.; Heras, A. New Drug Delivery Systems Based on Chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341. [Google Scholar] [CrossRef]
- Expósito, R. Quitosano, un Biopolímero con Aplicaciones en Sistemas de Liberación Controlada de Fármacos. Ph.D. Thesis, Universidad Complutense de Madrid, Facultad de Ciencias Biológica, Departamento de Bioquímica y Molecular I, Madrid, Spain, 2010; p. 171. Available online: https://eprints.ucm.es/id/eprint/11160/1/T32051.pdf (accessed on 30 November 2021).
- Mármol, Z.; Páez, G.; Rincón, M.; Araujo, K.; Aiello, C.; Chandler, C.; Gutiérrez, E. Quitina y Quitosano polímeros amigable. Una revisión de sus aplicaciones. Rev. Tecnocient. 2011, 1, 53–58. [Google Scholar]
- Patel, V.; Patel, M.; Patel, R. Chitosan: A Unique Pharmaceutical Excipient. Drug Deliv. Technol. 2005, 6, 30–40. [Google Scholar]
- Veiga, O.M.D.; Ruiz, C.R. El Quitosano: Usos farmacéuticos y biológicos. Rev. OFIL 2004, 14, 33–42. Available online: https://ilaphar.org/wp-content/uploads/2014/01/OFILn142.pdf (accessed on 17 August 2021).
- Rodríguez-Pedroso, A.T.; Ramírez-Arrebato, M.A.; Rivero-González, D.; Bosquez-Molina, E.; Barrera-Necha, L.L.; Bautista-Baños, S. Propiedades químico-estructurales y actividad biológica de la quitosana en microorganismos fitopatógenos. Rev. Chapingo Ser. Hortic. 2009, 15, 307–317. [Google Scholar]
- Khanvilkar, K.; Donovan, M.D.; Flanagan, D.R. Drug transfer through mucus. Adv. Drug Deliv. Rev. 2001, 48, 173–193. [Google Scholar] [CrossRef]
- Tiyaboonchai, W. Chitosan Nanoparticles. A Promising System for Drug Delivery. Naresuan Univ. J. 2003, 11, 51–66. [Google Scholar]
- De Enríquez, S.A.; Diebold, Y.; Calonge, M.; García-Vazquez, C.; Callejo, S.; Vila, A.; Alonso, M.J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: Toxicity, uptake mechanism and in vivo tolerance. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1416–1425. [Google Scholar] [CrossRef]
- Genta, I.; Costantini, M.; Asti, A.; Conti, B.; Montanari, L. Influence of glutaraldehyde on drug release and mucoadhesive properties of chitosan microspheres. Carbohydr. Polym. 1998, 36, 81–88. [Google Scholar] [CrossRef]
- Baki, D.E.; Ottenbrite, R.M. Perspectives on: Chitosan drug delivery systems based on their geometries. J. Bioact. Compat. Polym. 2006, 21, 351–368. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Henninknnin, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Shaji, J.; Jain, V.; Lodha, S. Chitosan: A novel pharmaceutical excipient. International. J. Pharm. Sci. 2010, 1, 11–28. [Google Scholar]
- Morris, G.A.; Samil, K.M.; Harding, S.E.; Adams, G.G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 257–284. Available online: https://www.tandfonline.com/doi/pdf/10.1080/02648725.2010.10648153 (accessed on 20 August 2021). [CrossRef]
- Chen, C.-S.; Liau, W.-Y.; Tsai, G.-J. Antibacterial Effects of N-Sulfonated and N-Sulfobenzoyl Chitosan and Application to Oyster Preservation. J. Food Prot. 1998, 61, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Aguilar, S.; Ruiz-Posadas, L.D.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Rivera-Martínez, A.R.; Aguirre-Medina, J.F. Sechium edule (Jacq.) Swartz, a New Cultivar with Antiproliferative Potential in a Human Cervical Cancer HeLa Cell Line. Nutricients 2017, 9, 798. [Google Scholar] [CrossRef] [Green Version]
- Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Avendaño-Arrazate, C.H.; Soto-Hernández, R.M.; Ruiz-Posadas, L.M.; Santiago-Osorio, E.; Acosta-Ramos, M.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Ochoa-Martínez, D. Production, genetics, postharvest management, and pharmacological characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. [Google Scholar]
- Cadena-Iñiguez, J.; Soto, H.M.; Arevalo, G.M.L.; Avendaño, A.C.H.; Aguirre, M.J.F.; Ruiz, P.L.M. Caracterización bioquímica de variedades domesticadas de Sechium edule (Jacq.) Sw. comparadas con parientes silvestres. Rev. Chapingo Ser. Hortic. 2011, 17, 45–55. [Google Scholar]
- Che, C.; Fang, X.; Phoebe, C.H.; Kiunghorn, A.D.; Farnswworth, N.R. High-field 1H-NRM spectral analysis of some cucurbitacins. J. Nat. Prod. 1985, 48, 429–434. [Google Scholar] [CrossRef]
- Afifi, M.S.; Ross, S.A.; ElSohly, M.A.; Naeem, Z.E.; Halaweish, F.T. Cucurbitacins of Cucumis prophetarum and Cucumis prophetarum. J. Chem. Ecol. 1999, 25, 847–859. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J. Caracterización Morfoestructural, Fisiológica, Química y Genética de Diferentes Tipos de Chayote (Sechium edule (Jacq.) Sw). Ph.D. Thesis, Colegio de Postgraduados, Texcoco, Mexico, 2005; p. 156. Available online: www.colpos.mx (accessed on 12 July 2021).
- Akbuğa, J.; Bergişadi, N. 5-Fluorouracil-loaded chitosan microspheres: Preparation and release characteristics. J. Microencapsul. 1996, 13, 161–168. [Google Scholar] [CrossRef]
- Olivera, A.D.; Barreiro, M.F.; Lopretti, M. Microesferas de quitosano como potenciales transportadores de ácidos nucleicos y otros bioactivos. Rev. Iberoam. Polim. 2012, 13, 238–244. [Google Scholar]
- NOM-253-SSA1-2012. Para la Disposición de Sangre Humana y sus Componentes con Fines Terapéuticos. Diario Oficial. Tercera Sección Poder Ejecutivo. Secretaria de Salud. 2012. Available online: http://www.cnts.salud.gob.mx/descargas/NOM-253-SSA1-2012.pdf (accessed on 30 November 2021).
- Kueng, W.; Silver, E.; Eppnberg, V. Quantification of cell cultured on 96-wels plates. Anal. Biochem. 1989, 186, 16–19. [Google Scholar] [CrossRef]
- Gillies, R.; Didier, N.; Denton, M. Determination of cell number in monolayer cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar] [CrossRef]
- Olivera, A.D.; Barreiro, M.F.; Lopretti, M. Proteínas de actividad bilógica microencapsuladas, ultraestructura y distribución de carga. Rev. Iberoam. Polim. 2014, 15, 219–228. [Google Scholar]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Agrawal, A.D. Pharmacological Activities of Flavonoids: A Review. Int. J. Pharm. Sci. Nanotechnol. 2011, 4, 1394–1398. [Google Scholar] [CrossRef]
- Kaur, S.H.; Kumar, B.; Prasher, S.; Tiwari, P.; Salhan, M.; Sharma, P. A Review of phytochemistry and pharmacology of flavonoids. Sci. Pharm. 2011, 1, 25–41. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Hindawi Sci. World J. 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Qin, C.; Du, Y.; Xiao, L.; Li, Z.; Gao, X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol. 2002, 31, 111–117. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, B.; Zeng, L.; Zhang, Z.; Liu, Y.; Du, Y.; Xiao, L. The physicochemical properties and antitumor activity of cellulase-treated chitosan. J. Agric. Food Chem. 2004, 84, 107–115. [Google Scholar] [CrossRef]
- Nam, K.S.; Shon, Y.H. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J. Microbiol. Biotechnol. 2009, 19, 629–633. [Google Scholar] [CrossRef]
- Park, J.K.; Chung, M.J.; Na Choi, H.; Park, Y.I. Effects of the Molecular Weight and the Degree of Deacetylation of Chitosan Oligosaccharides on Antitumor Activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.; Xu, Z. In vivo antitumor activity of chitosan nanoparticles. Bioorg. Med. Chem. Lett. 2006, 16, 4243–4245. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Colina, M.; Molina, J.; Vargas, J.; Rincon, D.; Medina, J.; Rosales, L.; Cardenas, H. Evaluación de la actividad antifúngica del quitosano contra el hongo Mycosphaerella fijiensis Morelet que produce la sigatoka negra que ataca el plátano. Rev. Iberoam. Polim. 2014, 15, 312–338. [Google Scholar]
- González-Fernández, E.; Fernández, M.S.; Lorenzo, A. Linfocitos T y B. Clasificación. Receptores. Generación de diversidad: Mecanismos moleculares. Capacidades funcionales. Rev. Med. 2005, 9, 1–24. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=1252689 (accessed on 27 August 2021).
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, 91–101. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Soto-Hernandez, M.; Torres-Salas, A.; Aguiñiga-Sánchez, I.; Ruiz-Posadas, L.; Rivera-Martínez, A.R.; Avendaño-Arrazate, C.; Santiago-Osorio, E. The antiproliferative effect of chayote varieties (Sechium edule (Jacq.) Sw.) on tumour cell lines. J. Med. Plant. 2013, 7, 455–460. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Cadena-Íñiguez, J.; Santiago-Osorio, E.; Gómez-García, G.; Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Ruíz-Ramos, M.; Cisneros-Solano, V.M.; Ledesma-Martínez, E.; Delgado-Bordonave, A.D.J.; et al. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechium edule var. nigrum spinosum. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar] [CrossRef]

| Metabolite (Triterpenoid) | Concentration (µg mg−1) | Flavonoid | Concentration (µg mg−1) |
|---|---|---|---|
| Cucurbitacin B | 137.00 | Rutin | 0.34 |
| Cucurbitacin D | 935.94 | Myricetin | 1.85 |
| Cucurbitacin E | 98.21 | Quercetin | 0.25 |
| Cucurbitacin I | 40.37 | Naringenin | 2.26 |
| Phloretin | 4.72 | ||
| Galangin | 0.43 | ||
| Apigenin | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Aguilar, S.; Ruiz-Posadas, L.d.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Olivera, Á.D. Microencapsulation of an Extract of Sechium edule (Jacq.) Sw., with Antineoplastic Activity. Appl. Sci. 2022, 12, 24. https://doi.org/10.3390/app12010024
Salazar-Aguilar S, Ruiz-Posadas LdM, Cadena-Iñiguez J, Soto-Hernández M, Santiago-Osorio E, Aguiñiga-Sánchez I, Cisneros-Solano VM, Aguirre-Medina JF, Olivera ÁD. Microencapsulation of an Extract of Sechium edule (Jacq.) Sw., with Antineoplastic Activity. Applied Sciences. 2022; 12(1):24. https://doi.org/10.3390/app12010024
Chicago/Turabian StyleSalazar-Aguilar, Sandra, Lucero del Mar Ruiz-Posadas, Jorge Cadena-Iñiguez, Marcos Soto-Hernández, Edelmiro Santiago-Osorio, Itzen Aguiñiga-Sánchez, Víctor M. Cisneros-Solano, Juan F. Aguirre-Medina, and Álvaro D. Olivera. 2022. "Microencapsulation of an Extract of Sechium edule (Jacq.) Sw., with Antineoplastic Activity" Applied Sciences 12, no. 1: 24. https://doi.org/10.3390/app12010024
APA StyleSalazar-Aguilar, S., Ruiz-Posadas, L. d. M., Cadena-Iñiguez, J., Soto-Hernández, M., Santiago-Osorio, E., Aguiñiga-Sánchez, I., Cisneros-Solano, V. M., Aguirre-Medina, J. F., & Olivera, Á. D. (2022). Microencapsulation of an Extract of Sechium edule (Jacq.) Sw., with Antineoplastic Activity. Applied Sciences, 12(1), 24. https://doi.org/10.3390/app12010024







