Compatibility of Different Automotive Elastomers in Paraffinic Diesel Fuel
Abstract
1. Introduction
2. Materials and Methods
- Conventional diesel fuel, produced from crude oil without FAME—DF,
- Conventional diesel fuel, produced from crude oil with 7%(v/v) FAME—B7,
- Paraffinic diesel fuel, produced through synthesis from natural gas, by means of the gas to liquid process—GTL.
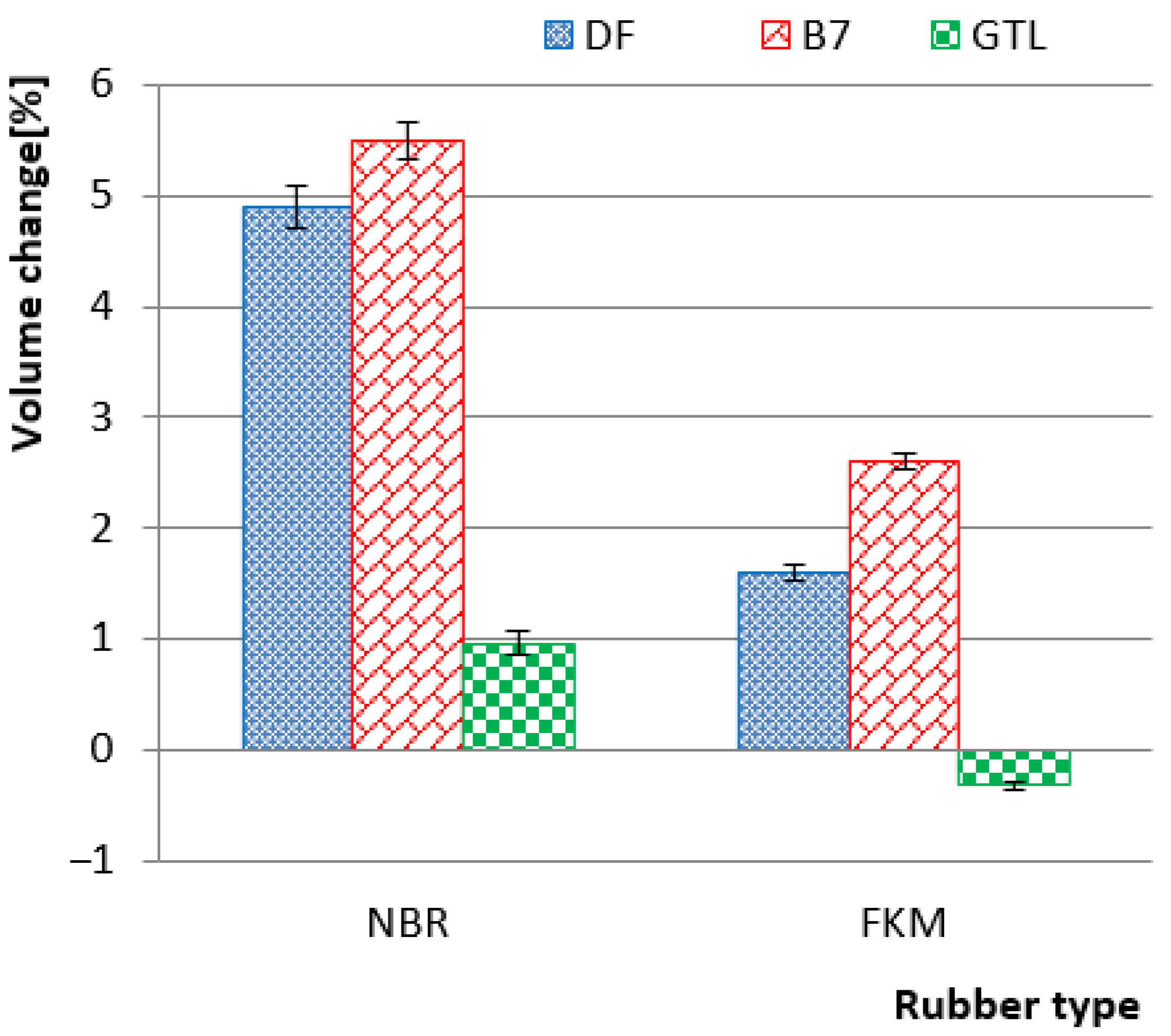
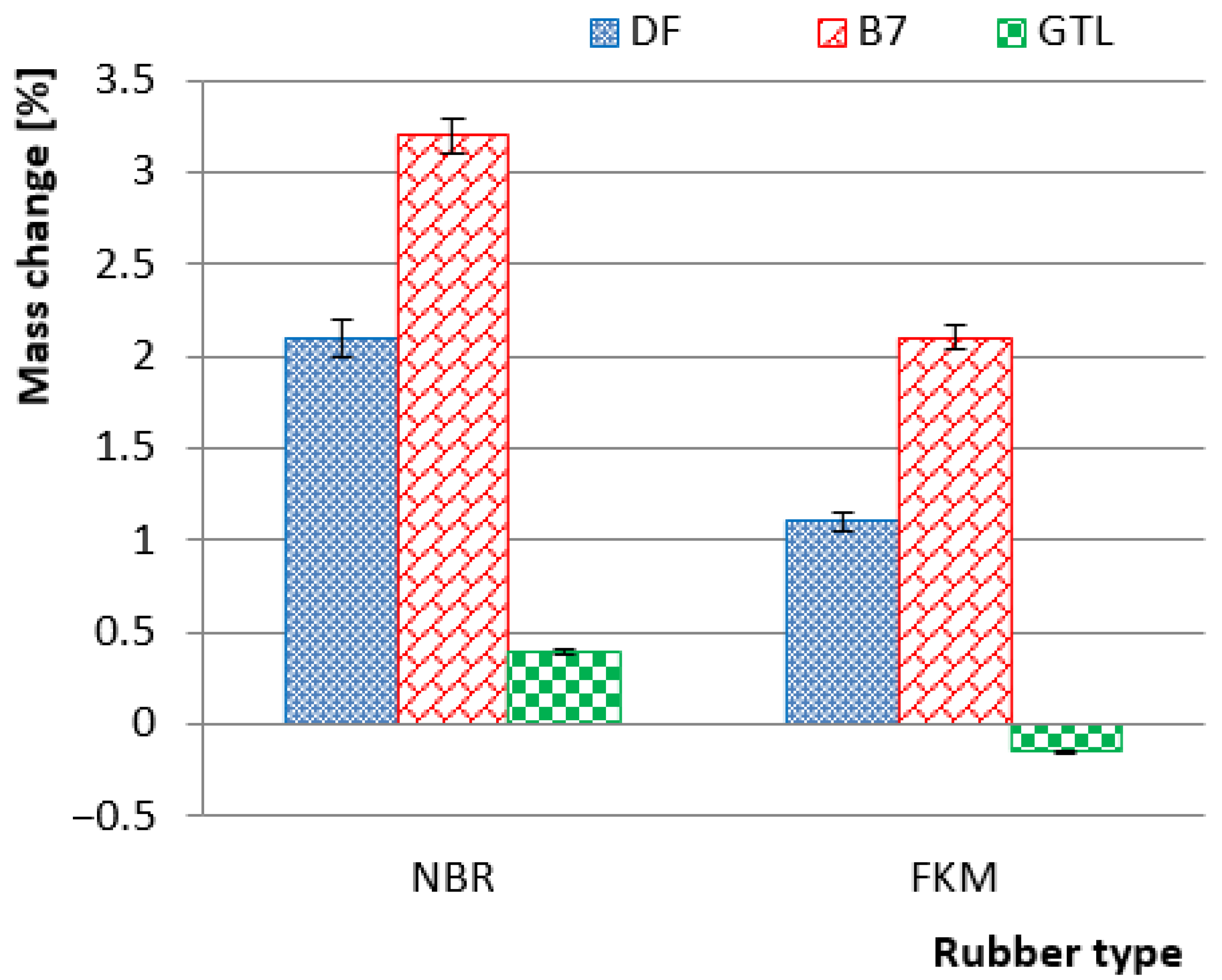
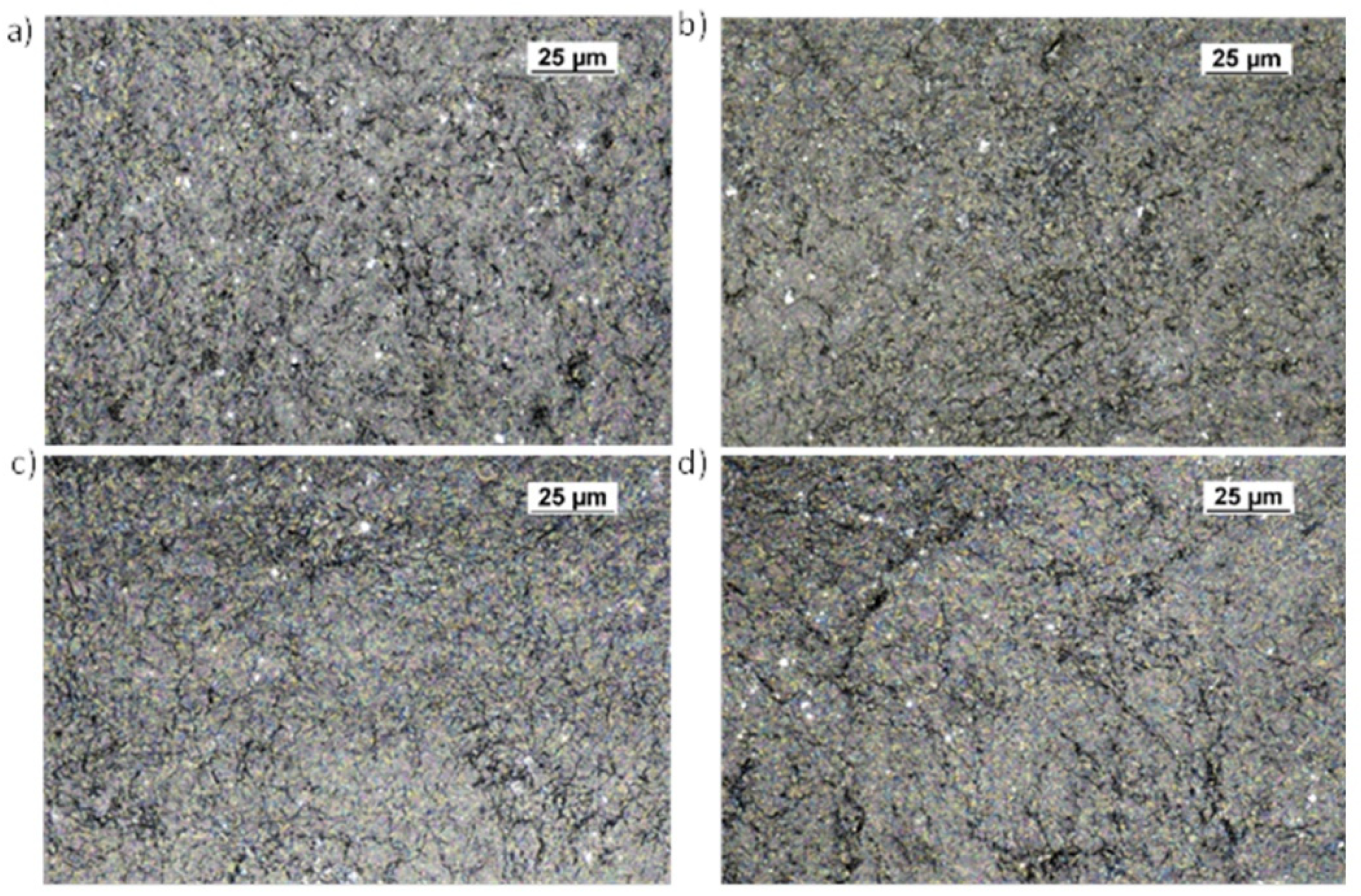
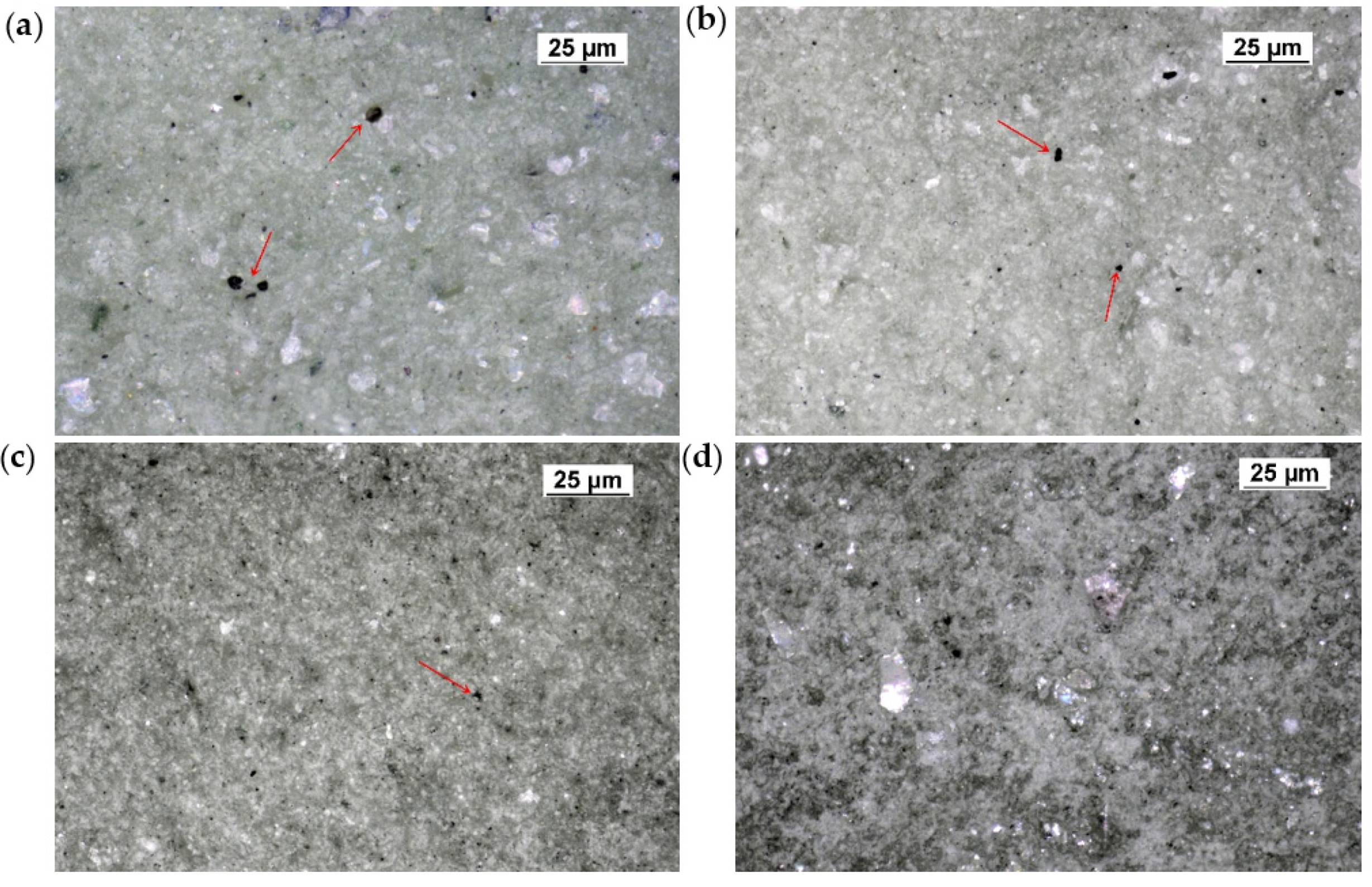
3. Results and Discussion
3.1. Properities of Rubbers
3.2. Surface Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diesel Fuels Technical Review (2007) Chevron Corporation. Available online: https://www.chevron.com/-/media/chevron/operations/documents/diesel-fuel-tech-review.pdf (accessed on 21 July 2021).
- Speight, J.G. Chapter 3—Hydrocarbons from crude oil. In Handbook of Industrial Hydrocarbon Processes, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA, 2020; pp. 95–142. [Google Scholar]
- Martyr, A.J.; Rogers, D.R. Engine Testing (Fifth Edition), Chapter 7—Energy Storage; Butterworth-Heinemann: Oxford, UK, 2021; pp. 191–215. [Google Scholar]
- Boichenko, S.V.; Zakharchuk, M.M. Aviation Fuels and Lubricants. Manual, Ministry of Education and Science; Youth and Sport of Ukraine, National Aviation University: Kiev, Ukraine, 2012. [Google Scholar]
- Schobert, H. Chemistry of Fossil Fuels and Biofuels (Cambridge Series in Chemical Engineering); Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Zhu, L.; Cheung, C.S.; Zhang, W.G.; Huang, Z. Compatibility of different biodiesel composition with acrylonitrile butadiene rubber (NBR). Fuel 2015, 158, 288–292. [Google Scholar] [CrossRef]
- Białecki, T. Reducing carbon footprint direction of the development of aviation fuels. J. KONBiN 2015, 29, 59–67. [Google Scholar] [CrossRef][Green Version]
- Sulek, M.W.; Kulczycki, A.; Malysa, A. Assessment of lubricity of compositions of fuel oil with biocomponents derived from rape-seed. Wear 2010, 268, 104–108. [Google Scholar] [CrossRef]
- Şanli, H. Influences of biodiesel fuels produced from highly degraded waste animal fats on the injection and emission characteristics of a CRDI diesel engine. Int. J. Automot. Eng. Technol. 2019, 8, 11–21. [Google Scholar] [CrossRef]
- Puricelli, S.; Cardellini, G.; Casadei, S.; Faedo, D.; van den Oever, A.E.M.; Grosso, M. A review on biofuels for light-duty vehicles in Europe. Renew. Sust. Energ. Rev. 2021, 137, 110398. [Google Scholar] [CrossRef]
- Dzięgielewski, W.; Gawron, B.; Kulczycki, A. Low temperature properties of fuel mixtures of kerosene and fame type used to supply turbine engines in marine and other non-aeronautical applications. Pol. Marit. Res. 2015, 22, 101–105. [Google Scholar] [CrossRef][Green Version]
- Worldwide Fuel Charter Gasoline and Diesel Fuel, 6th ed.; World-Wide Fuel Charter Committee: Paris, France, 2019.
- Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 116817. [Google Scholar] [CrossRef]
- Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018L2001 (accessed on 28 June 2021).
- Poliscanova, J.; Earl, T.; Ambel, C.C.; Archer, G. Roadmap on How to Decarbonise European Cars by 2050; Transport & Environment: Brussels, Belgium, 2018. [Google Scholar]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Makky, A.A.; Baroutaji, A.; Carton, J.G.; Olabi, A.G. Developments of electric cars and fuel cell hydrogen electric cars. Int. J. Hydrog. Energy 2017, 42, 25695–25734. [Google Scholar] [CrossRef]
- Suthisripok, T.; Semsamran, P. The impact of biodiesel B100 on a small agricultural diesel engine. Tribol. Int. 2018, 128, 397–409. [Google Scholar] [CrossRef]
- Dabi, M.; Saha, U.K. Application potential of vegetable oils as alternative to diesel fuels in compression ignition engines: A review. J. Energy Inst. 2019, 92, 1710–1726. [Google Scholar] [CrossRef]
- Stepanenko, D.; Kneba, Z. DME as alternative fuel for compression ignition engines—A review. Combust. Engines 2019, 177, 172–179. [Google Scholar] [CrossRef]
- Tipanluisa, L.; Fonseca, N.; Casanova, J.; López, J.-M. Effect of n-butanol/diesel blends on performance and emissions of a heavy-duty diesel engine tested under the World Harmonised Steady-State cycle. Fuel 2021, 302, 121204. [Google Scholar] [CrossRef]
- Suchocki, T.; Lampart, P.; Kazimierski, P.; Januszewicz, K.; Gawron, B.; Witanowski, Ł. Experimental investigation of performance and emission characteristics of a miniature gas turbine supplied by blends of kerosene and waste tyre pyrolysis oil. Energy 2021, 215, 119125. [Google Scholar] [CrossRef]
- Parravicini, M.; Barro, C.; Boulouchos, K. Experimental characterization of GTL, HVO, and OME based alternative fuels for diesel engines. Fuel 2021, 292, 120177. [Google Scholar] [CrossRef]
- EN 15940 Automotive Fuels—Paraffinic Diesel Fuel from Synthesis or Hydrotreatment—Requirements and Test Methods; European Committee for Standardization: Brussels, Belgium, 2019.
- Białecki, T.; Gawron, B.; Giemza, B.; Głąb, J. Influence of synthetic fuel on nitrile rubbers used in aviation. Transp. Probl. 2020, 15, 29–41. [Google Scholar] [CrossRef]
- EN 590 Automotive Fuels—Diesel—Requirements and Test Methods; European Committee for Standardization: Brussels, Belgium, 2021.
- Shell GTL Fuel. Knowledge Guide; Version 2.0, Shell. Available online: https://www.bremer-mineraloel.de/wp-content/uploads/2018/06/Shell-GTL-Fuel-techn.-Details.pdf (accessed on 20 July 2021).
- Neste Renewable Diesel Handbook, Neste Corporation; 2016; Available online: https://www.neste.com/sites/default/files/attachments/neste_renewable_diesel_handbook.pdf (accessed on 10 August 2021).
- Hunicz, J.; Matijošius, J.; Rimkus, A.; Kilikevičius, A.; Kordos, P.; Mikulski, M. Efficient hydrotreated vegetable oil combustion under partially premixed conditions with heavy exhaust gas recirculation. Fuel 2020, 268, 117350. [Google Scholar] [CrossRef]
- Choi, K.; Park, S.; Roh, H.G.; Lee, C.S. Combustion and Emission Reduction Characteristics of GTL-Biodiesel Fuel in a Single-Cylinder Diesel Engine. Energies 2019, 12, 2201. [Google Scholar] [CrossRef]
- ASTM D7566 Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons; ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D4054 Standard Practice for Evaluation of New Aviation Turbine Fuels and Fuel Additives; ASTM International: West Conshohocken, PA, USA, 2021.
- Farfan-Cabrera, L.I.; Pérez-González, J.; Gallardo-Hernández, E.A. Deterioration of seals of automotive fuel systems upon exposure to straight Jatropha oil and diesel. Renew. Energy 2018, 127, 125–133. [Google Scholar] [CrossRef]
- Kass, M.; Janke, C.; Connatser, R.; West, B.; Szybist, J.; Sluder, S. Influence of biodiesel decomposition chemistry on elastomer compatibility. Fuel 2018, 233, 714–723. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Pourrahimi, A.M.; Hedenqvist, M.S.; Sjöstedt, C.; Bellander, M.; Gedde, U.W. Degradation of carbon-black-filled acrylonitrile butadiene rubber in alternative fuels: Transesterified and hydrotreated vegetable oils. Polym. Degrad. Stab. 2016, 123, 69–79. [Google Scholar] [CrossRef]
- Fazala, M.A.; Rubaieeb, S.; Al-Zahrani, A. Overview of the interactions between automotive materials and biodiesel obtained from different feedstocks. Fuel Process. Technol. 2019, 15, 106178. [Google Scholar] [CrossRef]
- Chandran, D. Compatibility of diesel engine materials with biodiesel fuel. Renew. Energy 2020, 147, 89–99. [Google Scholar] [CrossRef]
- Komariah, L.N.; Arita, S.; Aprianjaya, F.; Novaldi, M.G.; Fathullah, M.F. O-Rings Material Deterioration due to Contact with Biodiesel Blends in a Dynamic Fuel Flow. J. Phys. Conf. Ser. 2019, 1167, 012050. [Google Scholar] [CrossRef]
- Linhares, F.N.; Gabriel, C.F.S.; De Sousa, A.M.F.; Leite, M.C.A.M.; Furtado, C.R.G. Nitrile rubber and carboxylated nitrile rubber resistance to soybean biodiesel. Polimeros 2018, 28, 23–29. [Google Scholar] [CrossRef]
- Sa’at, N.; Samsuri, A.; Latif, N.A.; Nasir, N.F.; Madon, R.H.; Osman, S.A. Compatibility of Palm Biodiesel Blends on the Existing Elastomer Fuel Hose in Diesel Engine with Approach of Dynamic Test Rig: A Concept Study. Mater. Sci. Forum 2020, 1010, 172–177. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Gedde, U.W.; Hedenqvist, M.S.; Conde Braña, M.T.; Bellander, M. Deterioration of automotive rubbers in liquid biofuels: A review. Renew. Sust. Energ. Rev. 2015, 43, 1238–1248. [Google Scholar] [CrossRef]
- Westbrook, S.R. Guidance Document for Alternative Diesel Fuels Proposed as “Drop-in” Fuels to Displace Diesel Fuels as Specified by ASTM Specification D975; Interim Report TFLRF No. 451, 2014. Available online: https://apps.dtic.mil/sti/pdfs/ADA626569.pdf (accessed on 28 June 2021).
- Sorate, K.A.; Bhale, P.V.; Dhaolakiya, B.Z. A Material Compatibility Study of Automotive Elastomers with high FFA based Biodiesel. Energy Procedia 2015, 75, 105–110. [Google Scholar] [CrossRef][Green Version]
- Damanabi, A.T.; Bahadori, F. Improving GTL process by CO2 utilization in tri-reforming reactor and application of membranes in Fisher Tropsch reactor. J. CO2 Util. 2017, 21, 227–237. [Google Scholar] [CrossRef]
- ASTM D471 Standard Test Method for Rubber Property—Effect of Liquids; ASTM International: West Conshohocken, PA, USA, 2021.
- Leniart, A.A.; Pula, P.; Sitkiewicz, A.; Majewski, P.W. Macroscopic Alignment of Block Copolymers on Silicon Substrates by Laser Annealing. ACS Nano 2020, 14, 4805–4815. [Google Scholar] [CrossRef]
- Wei, X.-F.; Meng, Q.; Kallio, K.J.; Olsson, R.T.; Hedenqvist, M.S. Ageing properties of a polyoxymethylene copolymer exposed to (bio) diesel and hydrogenated vegetable oil (HVO) in demanding high temperature conditions. Polym. Degrad. Stab. 2021, 185, 109491. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Rasheed, T.; Sher, F. An Experimental Investigation on Tribological Behaviour of Tire-Derived Pyrolysis Oil Blended with Biodiesel Fuel. Sustainability 2020, 12, 9975. [Google Scholar] [CrossRef]
- ASTM D7566 Standard Specification for Aviation Turbine Fuels; ASTM International: West Conshohocken, PA, USA, 2021.

| Property | Unit | Test Method | EN 590 Requirement | Results | ||
|---|---|---|---|---|---|---|
| DF | B7 | GTL | ||||
| Cetane index | – | EN ISO 4264 | min 46.0 | 56.2 | 53.1 | 84.2 |
| Density at 15 °C | kg/m3 | EN ISO 12185 | 820.0–845.0 | 822.9 | 833.9 | 777.9 |
| Viscosity at 40 °C | mm2/s | EN ISO 3104 | 2.000–4.500 | 2.685 | 2.772 | 2.660 |
| Flash point | °C | EN ISO 2719 | min 55.0 | 58.5 | 62.5 | 70.0 |
| Sulphur content | mg/kg | EN ISO 20846 | max 10.0 | 6.8 | 6.9 | <3.0 |
| Cold filter plugging point | °C | EN 116 | −10 for D grade −15 for E grade −20 for F grade | −16 | −15 | −21 |
| Lubricity HFRR at 60 °C | μm | EN ISO 12156-1 | max 460 | 413 | 202 | 476 |
| Aromatics | % (v/v) | D1319 | - | 20.3 | 19.7 | <1.0 |
| FAME content | % (v/v) | EN 14078 | max 7.0 | <0.1 | 6.6 | <0.1 |
| Copper strip corrosion (3 h, 50 °C) | classification | EN ISO 2160 | 1 | 1a | 1a | 1a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białecki, T.; Sitkiewicz, A.; Giemza, B.; Sarnecki, J.; Skolniak, M.; Gawron, B. Compatibility of Different Automotive Elastomers in Paraffinic Diesel Fuel. Appl. Sci. 2021, 11, 11312. https://doi.org/10.3390/app112311312
Białecki T, Sitkiewicz A, Giemza B, Sarnecki J, Skolniak M, Gawron B. Compatibility of Different Automotive Elastomers in Paraffinic Diesel Fuel. Applied Sciences. 2021; 11(23):11312. https://doi.org/10.3390/app112311312
Chicago/Turabian StyleBiałecki, Tomasz, Andrzej Sitkiewicz, Bolesław Giemza, Jarosław Sarnecki, Marta Skolniak, and Bartosz Gawron. 2021. "Compatibility of Different Automotive Elastomers in Paraffinic Diesel Fuel" Applied Sciences 11, no. 23: 11312. https://doi.org/10.3390/app112311312
APA StyleBiałecki, T., Sitkiewicz, A., Giemza, B., Sarnecki, J., Skolniak, M., & Gawron, B. (2021). Compatibility of Different Automotive Elastomers in Paraffinic Diesel Fuel. Applied Sciences, 11(23), 11312. https://doi.org/10.3390/app112311312






