Abstract
In this work, the optimization of two extraction methods, conventional CE and ultrasound-assisted UAE, to obtain extracts from cornelian cherry fruit with high antioxidant activity, which can be used to produce healthier jelly candies, is presented. In the CE process, the effects of temperature (30–50 °C), time (15–45 min), and hydroalcoholic mixtures (60–100% ethanol, v/v) were studied. The highest antioxidant activity (29.83 ± 0.85 mg TE/g dw) was found in the extracts obtained using 60% ethanol at 40 °C for 15 min. The UAE process led to comparable values of 26.60 ± 0.53 mg TE/g dw at 40% amplitude and pulsed sonication (5 s on and 5 s off) for 12.5 min. Under these experimental conditions, the specific energy consumed was 1.91 kJ/g. The vitamin C content and its inhibitory activity against metabolic enzymes were evaluated in extracts with different antioxidant activity. A significant inhibitory effect against carbohydrate-metabolism-associated enzymes was identified for all the tested extracts, with an inhibitory effect against α-glucosidase higher than 75%, but with a lower effect against α-amylase. The extract obtained by CE (60% ethanol, 40 °C, 15 min) provided the highest vitamin C content of 39.9 ± 1.2 mg ascorbic acid/100 g dw. Four variants of the healthier jelly candies were formulated, with a vitamin C content of 0.34 mg ascorbic acid/100 g dw in samples with agar-agar and 0.70 mg ascorbic acid/100 g dw in samples with gelatin.
1. Introduction
Recently, studies aiming at the valorization of plants as sources of innovative natural products and/or molecules for developing innovative targeted delivery systems and ingredients and/or nutraceuticals are re-emerging []. Therefore, many of the reports on the positive effects of bioactive compounds lead to the desirability of utilizing plant sources in different forms for human health.
The cornelian cherry (Cornus mas L.) is found mainly in Southeast Asia and Eastern Europe; it is a rich source of bioactives and is known for its strong antioxidant effects due to the content of more than one hundred compounds grouped into the following categories: polyphenols (phenolic acids and flavonoids such as anthocyanins []), monoterpenoids, triterpenoids, carotenoids, vitamins, carbohydrates, fatty acids, hydrocarbons, minerals, and pectin [,]. Additionally, different parts of the plant are rich in dietary fibers, sugars, proteins, carbohydrates, fats, and at least 15 amino acids [,]. The benefits of the cornelian cherry for human health are related to the cardiovascular, endocrine, immunity, gastrointestinal systems [], anti-inflammatory, hepatoprotective, anticancer, radioprotective, neuroprotective, the protection of the reproductive organs [,] and against nocturnal urinary incontinence for the children []. Also, the hypertriglyceridemia and atherosclerosis effects were tested on rats and rabbits [,].
Because of the potentially high bioactive compound concentration in the cornelian cherry, and its association with various health benefits, several studies have attempted to extract and quantify its bioactives with antioxidant activity [,,,]. Different combinations of solvents have been used to extract bioactives from the cornelian cherry, such as water, acetone, methanol, ethanol, or acidified water [,]. Hong et al. [] concluded that although acidified water promotes the ionization of the extraction solution, which assists with the breaking of the association between anthocyanins and pectin in plant matrices, anthocyanin stability can still be compromised as coumaroyl-, malonyl- and succinyl-based anthocyanins are unstable in highly acidified solution. Therefore, it is necessary to carefully select the suitable solvent that leads to the highest antioxidant activity of the extracts by preserving the bioactive compounds from degradation. In this sense, ethanol and aqueous mixtures of ethanol are preferred.
Besides the solvent, it is also known that the extraction method affects the stability of the bioactive compounds, especially the extraction temperature and the time they are in contact with the solvent. Therefore, it is necessary to have efficient extraction methods that reduce both the time and the energy consumed. Cornelian cherry extractions have been carried out using different methods, such as microwave-assisted extraction [], maceration, and ultrasound-assisted extraction (UAE) []. In UAE, ultrasound is applied to a suspension of the raw material in an appropriate solvent []. There are different ultrasonic devices, such as ultrasonic baths or ultrasonic probes. The latter has been proven to be more efficient for the recovery of bioactive compounds as the ultrasound is delivered directly to the sample []. As a consequence of the sonication process, small bubbles are formed; once they collapse, they generate strong shear forces in the so-called cavitation process, producing increases in temperature and changes of pressure, which affect the mass transfer properties of the solvent []. In this sense, UAE, using ultrasonic probes, is a versatile technique that has been used to intensify the extraction of bioactive molecules from a number of natural matrices [], but it has not been applied to the cornelian cherry to the best of the authors’ knowledge; therefore the UAE of the cornelian cherry is one of the aims of this work.
The excellent properties of the cornelian cherry extracts, due to their high content in bioactive compounds, can be applied to improve the nutritional profile of food, such as jellies and gummies. In the jelly-manufacturing process, consumers demand healthier alternatives with less added sugars or artificial flavorings. In this sense, the use of natural extracts instead of artificial additives can be used to produce a new generation of jelly candies with enhanced nutritional properties.
All in all, this research work consisted of two parts. The first part was to establish the optimal conditions for conventional and ultrasound-assisted extraction using a three-factor, three-level Box–Behnken design (BBD) in order to maximize the total polyphenol and total flavonoid content in the extracts as well as their antioxidant activity. In the second part of the study, the obtained extracts with the highest antioxidant activity were used in order to evaluate the vitamin C content and the efficiency against the enzymes relevant in hyperglycemia and inflammatory processes related with metabolic syndrome. Finally, the best cornelian cherry extract was used in the formulation of jelly candies, and the textural properties of the resulting candy were evaluated.
2. Materials and Methods
2.1. Materials
2.1.1. Raw Material
Ripe cornelian cherry fruits were collected in September 2017 from the spontaneous culture in the village of Slivna (46°5′5″N, 27°56′31″E), located in the northeastern part of Galați city (Romania). The fruits at full maturity (dark and cherry-red color) were washed and dried with paper to eliminate the excess of water. The pulp was removed from the seeds and stored at −18 °C. The pulp was freeze-dried at −42 °C under a pressure of 0.10 mbar for 72 h (Christ Alpha 1–4 LD plus, Osterode am Harz, Germany). The material balance indicated that from 1000 g of fresh fruits, 363 g of freeze-dried fruits were obtained. After freeze-drying, the fruits were ground for the extraction experiments.
2.1.2. Chemicals
The aluminum chloride, potassium acetate, ethanol, Folin–Ciocalteu reagent, sodium carbonate, gallic acid, methanol, 2-Diphenyl-1-picrylhydrazyl (DDPH), 6-Hydroxy-2.5.7.8- tetramethylchromane-2-carboxylic acid (Trolox), hydrochloric acid (HCl), potassium iodate (KIO3), potassium iodide (KI), and starch were purchased from Sigma-Aldrich Steinheim, Germany. All the reagents used for the inhibitory activity, p-Nitrophenyl-α-ᴅ-glucopyranoside, α–glucosidase, and α-amylase were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
2.2. Cornelian Cherry Extraction Processes
2.2.1. Conventional Extraction (CE) Experiments
The conventional extractions were carried out in an orbital shaking water bath (Grant, OLS 200, Cambridge Ltd., Cambridge, UK). In each experiment, an amount of 1 g of cornelian cherry freeze-dried powder was transferred to a 15 mL falcon test tube, followed by the addition of 10 mL of solvent. The tube was placed in the water bath, where it was kept under mild shaking conditions (150 rpm) for the desired extraction time. After the extraction time was completed, the tubes were centrifuged at 5000 rpm and 9 °C for 20 min (Thermo Scientific Sorvall ST16R, Hudson, New York, USA), and the supernatant was kept at 4 °C until analysis.
A Box-Behnken experimental design was used to study the effect of three experimental variables on the bioactive compound’s recovery: X1, the extraction temperature (30, 40, and 50 °C); X2, the extraction time (15, 30 and 45 min); and X3, the solvent used (hydroalcoholic mixtures, 60, 80, and 100% ethanol, v/v). Three responses were studied: the TPC, the TFC, and the TAA content of the extracts. The experimental plan consisted of 15 runs, including three repetitions at the central point of the experimental design (hydroalcoholic solution with 80% ethanol (v/v), 40 °C, and 30 min) in order to evaluate the reproducibility of the experimental results and to estimate the curvature of the model and the nonlinear responses. All the experiments were randomized. In the optimization process, the target was to find the combination of the experimental variables that simultaneously maximized the TPC, the TFC, and the TAA of the extracts.
2.2.2. Ultrasound-Assisted Extraction (UAE)
The UAE experiments were performed in an ultrasonic system (Vibra-Cell 75043, 750 W, Bioblock Scientific, Newtown, CT, USA) at constant ultrasound frequency (20 kHz). The identical amount of cornelian cherry freeze-dried powder and volume of the solvent were used for all the experiments. An amount of 5 g of the powder of cornelian cherry was mixed with 50 mL of a hydroalcoholic solution with a 60% ethanol concentration (v/v) and located in a 250 mL (internal diameter 4.8 cm) jacketed vessel. The temperature was maintained at a constant 40 °C by circulating cold water through the jacket. The ultrasonic processor was equipped with a 13 mm titanium probe that was submerged into the sample at a depth of 2 cm from the bottom of the vessel. The total delivered energy was registered, then the solid–liquid mixture was centrifuged at 5000 rpm at 9 °C for 20 min (Thermo Scientific Sorvall™ ST16R, Hudson, New York, NY, USA), and the supernatant was collected and kept at 4 °C prior to analysis.
A Box-Behnken experimental design was used, considering three experimental variables at three of levels each one: X1, the amplitude (40, 60, and 80%), X2, the sonication mode: pulsed/continuous (0.5, 0.75, and 1), and X3, the extraction time (5, 10, and 15 min). At continuous sonication the pulses = 1, whereas the other settings indicate pulsed sonication (on/off switching times of the ultrasonic processor); as an example: a value of 0.5 means that the power discharges for 5 s and pauses for 5 s, or at the value of 0.75, which means that the power discharges for 7.5 s and pauses for 2.5 s. The main benefit of pulsed sonication over continuous sonication is a better control of the energy released to the sample, which involves a slower temperature increase []. The maximum amplitude of oscillation of the probe used in the experiments was 79 μm, corresponding to the display setting value of 100%.
Initially, the experimental plan consisted of 15 runs (including three repetitions at the central point of the experimental design (60% amplitude, 0.75 pulses, and 10 min) plus two additional experiments at 100% amplitude (in order to evaluate the effect of harsher working conditions). Three responses were studied, the TPC, the TFC, and the TAA content of the extracts, in order to find the combination of the experimental variables that maximizes the bioactive compound concentration recovery.
2.3. Jelly Candy Formulation
In order to demonstrate the potential benefits of adding the cornelian cherry extracts to the jelly candy formulation, the extract obtained by CE at 40 °C for 15 min with 60% hydroalcoholic solution was concentrated at 40 °C under vacuum conditions (Martin Christ, Osterode am Harz, Germany). The concentrated extract, rich in the antioxidants, vitamin C, and natural pigments was used for the following variants of jelly candies, coded as follows: AM—2% agar-agar control sample without extract; AEC—2% agar-agar sample with extract; GM—10% gelatin control sample without extract; GEC—10% gelatin sample with extract. The gelling agents were prepared as following: the gelatin (10% w/w) was hydrated in 100 mL of ultrapure water for 10 min, and the agar-agar (2% w/w) aqueous solution was boiled for 5 min, then cooled at 40 °C, followed by the addition of the concentrated extract (3% w/w). Then, the obtained solutions follow the conventional jelly candy manufacturing steps of deposition in silicone molds, cooling, drying, and demolding []. The vitamin C content of the jelly candies was evaluated according to the method described in Section 3.4. Furthermore, the textural parameters were evaluated for all the obtained jelly candy samples.
2.4. Analytical Methods
2.4.1. Total Polyphenol Content (TPC)
Total polyphenol content (TPC) was evaluated using the Folin–Ciocâlteu method adapted from Turturicӑ et al. []. Briefly, 0.1 mL of diluted extract was mixed with 7.9 mL of distilled water and 0.5 mL of Folin–Ciocalteu solution and kept for 10 min to allow interaction. Then, 1.5 mL of sodium bicarbonate (20% w/v) was added, and the samples were kept in the dark for 60 min at room temperature. The absorbance was measured using a UV-VIS spectrophotometer (Jasco V-750, Tokyo, Japan) connected with an immersion thermostat with a digital control Digiterm S150, Jasco PAC-743R and with a color LCD touch screen and Spectra Manager™ II software against the blank at 765 nm. A calibration curve with standard solutions of gallic acid was prepared and the results were expressed as mg Gallic Acid Equivalents/g dry weight raw material (mg GAE/g dw).
2.4.2. Total Flavonoid Content (TFC)
TFC content was measured according to the colorimetric method with aluminum chloride adapted after Kaur and Mondal []: 0.5 mL of extract was mixed with 1.5 mL of 96% ethanol, 0.1 mL of potassium acetate (1 M), 0.1 mL of aluminum chloride (10%, w/v), and 2.8 mL of distilled water. The samples were kept in the dark for 30 min at room temperature. The absorbance was measured with a UV-VIS spectrophotometer (Jasco V-750, Tokyo, Japan) against the blank at 415 nm. A calibration curve with standard solutions of quercetin was prepared and the results were expressed as mg Quercetin Equivalent/g dry weight raw material (mg QE/g dw).
2.4.3. Total Antioxidant Activity (TAA)
The total antioxidant activity was determined using the DPPH method suggested by Oancea et al. []. Briefly, 0.06 mL of extract was mixed with 2.94 mL of DPPH. The samples were kept at room temperature for 60 min. The absorbance was measured with a UV-VIS spectrophotometer (Jasco V-750, Tokyo, Japan) against the blank at 517 nm. The calibration curve was obtained using seven different dilutions of Trolox reagent, respectively: 0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 mM. The color obtained for the samples after 60 min at room temperature in dark conditions indicated the degree of binding of the free radical DPPH with the antioxidants from the cornelian cherry fruits extracts. Therefore, the less colored the sample, the higher was the antioxidant activity. The total antioxidant activity was expressed in mg Trolox Equivalent/g dry weight raw material (mg TE/g dw).
2.4.4. Vitamin C Content
The iodometric titration method described by Spínola et al. [] was used as a general norm for vitamin C content determination. Briefly, 3 g of six selected extracts (coded as: C1, C5, and C8, according to Table 1 and U1, U5, and U10, according to Table 3, Section 3.2) were mixed with 20 mL of HCl 2% solution (v/v) to avoid the oxidation, and the mixture was completed with distillate water to the final volume of 100 mL. After 10 min of reaction, the mixture was filtered using a narrow-pore, dense paper filter with 0.16 mm thickness for slow filtering. Then, the filtrate (n = 3) was mixed with 30 mL of distillate water, 5 mL of KI 1% solution (v/v), and 1% starch solution (v/v), homogenized, and then titrated with 0.002 mol/L KIO3, previously standardized, until the mixture became dark blue and the color persisted for more than 60 s. All of the solutions were prepared and standardized daily. Each mL of 0.002 mol/L KIO3 is equivalent to 0.8806 mg of L-ascorbic acid.

Table 1.
Box-Behnken experimental design (BBD) with independent variables and experimental values for the responses of TPC, TFC, and TAA from cornelian cherry extracts obtained by conventional extraction.
The vitamin C content was calculated with the Equation (1):
where t is the titre of 0.002 mol/L KIO3 solution reported for the ascorbic acid (0.8806 mg); V is the volume of 0.002 mol/L KIO3 solution used for the titration, mL; fd is the dilution factor; and m is the mass of the sample, g.
2.4.5. Inhibitory Activity against Metabolically Important Enzymes
The methods described by Costamagna et al. [] for the inhibitory effect against α-amylase and α-glucosidase were applied to evaluate the antidiabetic potential of the six selected extracts (coded as: C1, C5, and C8, according to Table 1 and U1, U5, and U10, according to Table 3, Section 3.2). In brief, for the α-amylase inhibitory effect, equal volumes (200 μL) of extracts in 0.1 M phosphate buffer (pH 6.9) and 1% (w/v) of starch solution were incubated in Eppendorf tubes at 25 °C for 10 min. A volume of 200 μL of α-amylase (1 mg/mL in 0.1 M phosphate buffer, pH 6.9) was added to each tube and the reaction mixtures were incubated at 25 °C for a further 10 min. The reaction was stopped with the addition of 1 mL of 3,5-dinitrosalicyclic acid reagent solution, followed by incubation at 100 °C for 5 min. The mixtures were diluted with 5 mL of MilliQ water, and the absorbance was measured at 540 nm.
For α-glucosidase, the procedure involved the addition of equal volumes (50 μL) of extracts in 0.1 M phosphate buffer (pH 6.9) and an enzyme solution (1 mg/mL in 0.1 M phosphate buffer, pH 6.9), followed by incubation at 37 °C for 20 min. Further to this, 20 μL of 25 mM p-nitrophenyl-α-D-glucopyranoside in phosphate buffer 0.1 M, pH 6.9, was added and incubation at 37 °C for 40 min in the darkness followed. Acarbose was used as a positive control. The amount of p-nitrophenol released was quantified at 405 nm.
Enzyme inhibition was calculated using the Equation (2):
where A0 is the absorbance of the control (blank, without extracts addition), and As is the absorbance in the presence of the extracts.
2.4.6. Textural Analysis of Jelly Candies
The Texture Profile Analysis (TPA) was used to determine the textural properties of the jellies. This method was achieved with a Brookfield CT3-1000 Texture Analyzer. The samples were cut into cylindrical pieces, with a 10 mm diameter and a 10 mm height. Each piece was subjected to a double compression with 1 mm/s speed until the deformation of 5 mm was reached. The textural parameters (firmness, adhesiveness, cohesiveness, springiness, gumminess, and chewiness) were collected using TexturePro CT V1.5 software. The results are expressed as the mean of five determinations.
2.4.7. Color Measurement
The colorimetric parameters were determined by using Chromameter CR-400 (Konica-Minolta Sensing Inc., Osaka, Japan), programmed in the CieLab system. The color measurements were performed for the jelly candies after the samples were put in Petri dishes. The equipment was calibrated with the white calibration plate before any reading. Chroma (C*), the hue values (H*), and the total color difference (ΔE*) values were calculated by Equations (3)–(5).
where L* (a lower value indicates a darker color, black: L* = 0 and white: L* = 100), a* (indicates the balance between red (>0) and green (<0) color), and b* (the balance between yellow (>0) and blue (<0) color). All measurements were performed in triplicate.
2.5. Statistical Analysis Optimization Procedure
All the experiments carried out in the present study were performed in duplicate. The results were expressed in terms of an average followed by standard deviation. For both experimental plans (CE and UAE), the calculations were conducted by means of Statgraphics Centurion XVII Statistical Software. A generalized second-order polynomial model, as shown in Equation (3), was used to fit the experimental results.
In that polynomial, Y is the response variable to be optimized, β0, βj, βjj, and βij are the regression coefficients for the intercept, linearity, quadratic, and interaction, respectively; Xj is the uncoded independent factor and the terms XiXj and Xj2 represent the interaction and quadratic terms, respectively. An analysis of variance (ANOVA) with a 95% confidence level was performed for each response variable to test the model significance and suitability. The Durbin–Watson statistic test was performed, and the p-value was less than 0.05. The correlation between the different responses used in this work was carried out using the Pearson product-moment correlation at a 95% confidence level.
From the polynomials obtained by means of Statgraphics, it was possible to calculate the experimental conditions that maximized all the responses (TPC, TFC, and TAA) simultaneously, obtaining one desirability function coefficient. In order to validate the prediction made by the statistical software, a new experiment (in duplicate) was run in the optimal conditions and the three responses measured. The experimental results and theorical results for the three responses were then compared and the prediction validated.
The textural and color parameters were performed in triplicate and the mean values ± standard deviations were calculated. In order to identify significant differences, the experimental data were subjected to a one-way analysis of variance (ANOVA) after running the normality and homoscedasticity tests. Tukey’s test at the 95% confidence interval was employed for post-hoc analysis; p < 0.05 was considered to be statistically significant. The statistical analysis was carried out using Minitab 18 software.
3. Results
3.1. Influence of the Conventional Extraction Parameters on the TPC, TFC, and TAA of the Extracts
The knowledge of the extraction conditions and their influences is essential in order to obtain the highest concentration of biologically active compounds from the raw material. In our study, the three-factor, three-level Box–Behnken design (BBD) with three responses in the central point was applied in order to study the effects of different extraction parameters on the TPC, the TFC, and the TAA of the cornelian cherry extracts. The BBD experimental design for the conventional extraction and the experimental results are presented in Table 1.
The antioxidant capacity of the cornelian cherry depends on the cultivar and cultivation conditions (geographical region, climatic parameters, maturity, and collection period) []. In our study, the highest TAA value (29.83 ± 0.85 mg TE/g dw) was obtained in conventional extraction with hydroalcoholic solution at an ethanol concentration of 60% (v/v) after 15 min of extraction at 40 °C. In the same extraction conditions, the highest concentration of TPC (29.27 ± 1.09 mg GAE/g dw) was found. Increasing the extraction time up to 30 min, at the temperature of 30 °C, allowed us to obtain the highest concentration of TFC (1.61 ± 0.01 mg QE/g dw), but a lower value of TPC (27.62 ± 0.36 mg GAE/g dw), approximatively 6% lower than the value obtained after 15 min of extraction at 40 °C. When the temperature was increased up to 50 °C in the same extraction time (30 min) and at the same solvent concentration (60% EtOH), 26.68 ± 0.52 mg GAE/g dw was found, a value 10% lower than the result obtained at 40 °C and 15 min.
Our results indicate that the concentration of the hydroalcoholic solution was the main independent variable influencing the extraction, and the higher concentrations of bioactive compound recovery from the cornelian cherry extracts were obtained at 60% (v/v). These results are in good agreement with Dumitrașcu et al. [] who reported the highest values for TPC at temperatures up to 35 °C and an ethanol concentration between 50–70%. The analysis of the influence of the independent variables on the TAA (mg TE/g dw) and on the TPC concentration (mg GAE/g dw) allowed us to obtain a good correlation between both response parameters (Figure 1).
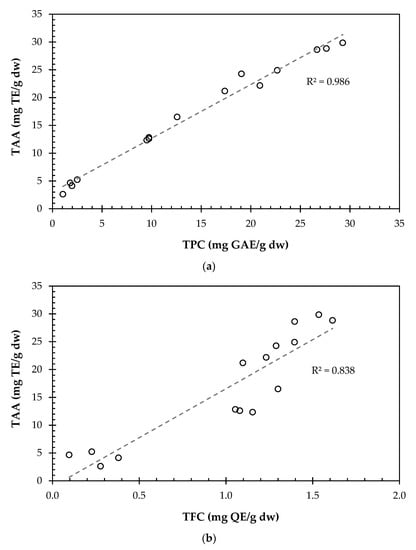
Figure 1.
Correlation of TPC (a) and TFC (b) values with the TAA for the conventional extraction.
As expected, Figure 1a shows an increase in the antioxidant activity with the TPC content of the extracts, as well as with the TFC content (Figure 1b). The Pearson product-moment correlation between the TAA and the TPC revealed a statistical correlation between those two variables (p-value was 0.000, at a 95% confidence level; with a correlation coefficient of 0.99), as well as between the TAA and the TFC (p-value was 0.000, at a 95% confidence level; with a correlation coefficient of 0.93).
The statistical software allowed us to obtain the F-test ratio, the coefficient of regression R2, the R2 adjusted for D.F., the Durbin–Watson statistic, and the p-values of the predicted model for the TPC, the TFC, and the TAA from the cornelian cherry conventional extracts (Table 2).

Table 2.
F-test ratio, coefficient of regression R2, Durbin–Watson statistic and p-values of the predicted model for TPC, TFC and TAA from cornelian cherry conventional extracts.
The ANOVA table partitions were applied in order to observe the variability of the response variables in separate pieces for each of the effects. The test of the statistical significance for each effect was applied by comparing the mean square against an estimate of the experimental error. In Table 2, it can be observed that in the case of all three response variables (TPC, TFC and TAA) the amount of ethanol in the hydroalcoholic solution has a statistically significant effect (p < 0.05), as also described by the Pareto diagram (Figure 2). The model fits the experimental values for the TFC, the TPC, and the TAA to a great extent as R2 is close to 1 for all the responses, according to Table 2. The Durbin–Watson statistic tests indicate the residuals to determine if there is any significant correlation based on the order in which they occur in the data file. As for all the response variables, the p > 0.05; there is no indication of a serial autocorrelation in the residuals at the 5.0% significance level.
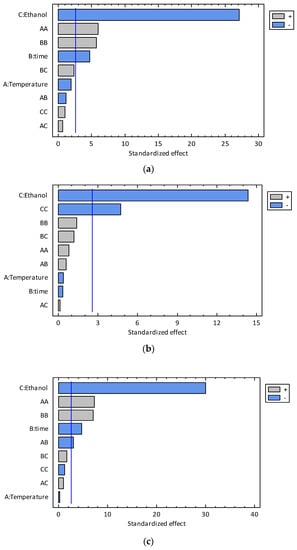
Figure 2.
Pareto charts for TPC (a), TFC (b), and TAA (c) for the conventional extraction experiments.
As can be observed from the Pareto charts (Figure 2), the concentration of the hydroalcoholic solution (X3) is the independent variable with the biggest statistical effect on the TPC, TFC, and TAA responses. The other independent variables showed no statistical influence on the response variables, as can be observed from the Pareto diagrams (Figure 2). In the case of the extraction time, it would probably be necessary to perform a kinetic study of the phenolics and flavonoid extraction in order to study the effect of the time at shorter times than those used in the experimental design. It seems that the extraction process is very fast, and the time range used in the experimental design only covers the diffusion-controlled period.
Different variables were used in the literature to obtain bioactive-rich extracts from the cornelian cherry. Kadam et al. [] used the conventional solvent extraction with 70% ethanol and obtained extracts with antioxidant activity ranging from 4.70–9.32 mg TE/g. Apel at al. [] obtained extracts with a TPC ranging from 4.94–7.04 mg GAE/g by using ethanol in a concentration of 80%, whereas Espada-Bellido et al. [] obtained extracts with a TFC of 3.21–6.69 mg catechin equivalent/g F.W. by using methanol and hydrochloric acid. These differences could be explained by the genetic factors, the environmental conditions, and the ability to synthesize secondary metabolites, but also could be due to the different methods, solvents, and conditions used for the extraction. It is known that phenolic extracts of plant materials are always a mixture of different classes of phenolics, which are soluble in the solvent used. The solubility of phenolic compounds is controlled by the solvent polarity (which is a function of the solvent and the temperature), as recently demonstrated by Benito-Román et al. [,]. The degree of polymerization of phenolics, the interaction of phenolics with other structural components constituents, and the ability of the solvent to deal with these facts affects the extraction performance. Related to that the extraction time as the extraction curve of phenolics from natural matrices has different periods []: (1) the cell structure swells (extract not readily accessible for the solvent), (2) the maximum extraction rate, and (3) the solid depletion, no more extraction of bioactive compounds is achieved, despite the increase in the extraction time. The combination of solvent, temperature, and extraction time will affect the amount and nature of the phenolics compounds extracted, indicating that there is no uniform procedure that is suitable for the extraction of all the phenolics in plant materials, and each raw material has to be treated individually, being different in the comparison of results among the authors.
3.2. UAE Parameters Influence on the TPC, TFC and TAA
The ultrasound-assisted extraction experiments were performed at a constant temperature (40 °C) with a 60% hydroalcoholic solution, (v/v) considering three independent variables (Table 3) in order to find the optimal extraction conditions to maximize the TPC, TFC and TAA concentrations.

Table 3.
Box-Behnken experimental design (BBD) with independent variables and experimental values for the responses of total polyphenols, total flavonoids, and total antioxidant activity from cornelian cherry extracts obtained by UAE.
The three-level, three-factor Box–Behnken design was used to study the effect of the independent variables (X1—amplitude, X2—sonication mode, X3—time). The amplitude term refers to the amplitude of oscillation generated by the probe. In our experiments, the maximum amplitude of oscillation of the titanium probe was 79 μm (100%, Table 3). The central point of the experimental design was performed in triplicate at the conditions 60% amplitude, 10 min, and 0.75 sonication mode (U13–U15, Table 3). The results obtained at the three replicates of this central point presented some differences among them due to the possibility of the temperature increasing during sonication. Furthermore, at higher amplitudes higher accelerations were generated, which increased the probability of cavitation phenomena due to the higher-pressure differences. Increased cavitation leads to higher extraction rates. At the same amplitude (60%), if the extraction time decreased down to 5 min, but with continuous sonication action, the TPC value was 32.42 ± 1.13 mg GAE/g dw (U10). At the maximum ultrasound amplitude and continuous sonication (run U17), the TPC concentration (31.44 ± 0.49 mg GAE/g dw) was close to the result obtained at 60% amplitude (U10). This means that the amplitude during ultrasound extraction had no effect on the recovery of the TPC concentration. Our results are in accordance with Kadam et al. [] who obtained no amplitude influence on the recovery of total phenols from brown seaweed Ascophyllum nodosum. Moreover, Apel et al. [] concluded that the amplitude in the range of 50–100% had no significant effect on the extraction yields of the individual and total glycoalkaloids of potato peel.
In Table 4, it can be observed that the fitting of the UAE experiments is poor; the independent variables do not affect the responses studied.

Table 4.
F-test ratio, coefficient of regression R2, Durbin-Watson statistic and p-values of the predicted model for TPC, TFC, and TAA from cornelian cherry ultrasound-assisted extraction.
According to the literature, the UAE performance may be quantified by some variables, such as the solid/liquid (S/L) ratio, temperature, and ultrasound amplitude [,,,,,,]. Other authors report that suboptimal UAE conditions may lead to reduced cavitation phenomena, resulting in reduced recovery bioactives []. Dumitrașcu et al. [] reported the highest TPC concentration (>25 mg GAE/g dw) after 15 min of UAE with a hydroalcoholic solution and an ethanol concentration between 50–70%. Cosmulescu and Trandafir [] obtained cornelian cherry extracts with 10% methanol (v/v) and 1% hydrochloric acid (v/v) at 30 °C during 60 min in a water bath with ultrasound. They used the cornelian cherry fruits from the spontaneous flora of the western part of Romania, collected in 2017, and indicated a content from 163.69 mg GAE/100g fresh weight (Strimba-Jiu village, Gorj county) to 359.28 mg GAE 100/g fresh weight (Hartagani village in Hunedoara) of total phenolic compounds.
In order to analyze the ultrasound effect on the internal mass transfer, the TPC, TFC and TAA values were plotted versus the specific energy consumption (Figure 3). It can be observed that the use of ultrasound does not increase the highest TPC (Figure 3a) value obtained by conventional extraction (C8, Table 1), which indicates that there is no internal resistance to the mass transfer when using the cornelian cherry to recover bioactive compounds. The same conclusion could be formulated in the case of the TAA (Figure 3c) and the TFC (Figure 3b).
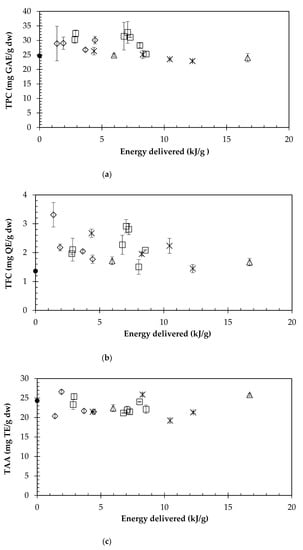
Figure 3.
Effect of the specific energy delivered in the UAE extraction on TPC (a), TFC (b), and TAA (c), as a function of the sonication amplitude. Key: ◊, 40%; ☐, 60%; ™ ⃝, 80%; △, 100%; ●, conventional extraction.
In the UAE experiments, we did not obtain a good trend for the response variables; so, from our results, the conventional extraction allowed us the higher recovery of the bioactives from Cornus mas fruits. In addition, the antioxidant power of cornelian cherry fruits depends on the genotype (cultivar) as well the geographical region of its cultivation, the climatic condition, the maturity, and the collection or storage and preparation conditions []. Dumitrașcu et al. [] reported that for the cornelian cherry from a controlled culture, the TPC ranged from 2.04 g GAE/ 100 g dw to 4.42 g GAE/100 g dw for the extracts obtained by ultrasound-assisted extraction. For the methanol extracts obtained at 30 °C under ultrasound action for 60 min, Cosmulescu and Trandafir [] found an average content of flavonoids of 59.37 mg QE /100 g for the cornelian cherry collected from the village of Baita, Hunedoara county. The cornelian cherry fruits possess high antioxidant activity with a huge variation among Cornus mas genotypes. The six wild cornelian cherry fruits from the western part of Romania have a variation of the total antioxidant activity between 1.24 and 2.71 mMol TE/ 100 g FW. The higher antioxidant activity was found for the genotype collected from the village of Hartagani, Hunedoara county [].
3.3. Optimization and Validation of Extraction Conditions
The experimental results presented in Section 3.1 for the conventional extraction of bioactive compounds from the cornelian cherry revealed that the extraction of the TPC, the TFC, and the TAA could be fitted to a second-order polynomial, according to the R2 values presented in Table 2.
The optimization section is obtained from the statistical program that provides the optimal conditions and the predicted values for the variables. As the fitting for the UAE experiments is poor, no optimal conditions will be calculated as there is no statistically significant effect of any of the experimental variables on the responses studied.
The final model for conventional extraction of cornelian cherry fruits in terms of three factors, coded with X1—temperature (°C); X2—time (min); and X3—ethanol concentration (%) was as follows:
TPC = 169.67 − 1.374X2 − 1.1097X3 + 0.04019X12 + 0.017X22
TFC = 1.02 + 0.08X3 − 0.00074X32
TAA = 142 − 1.03X2 − 0.522X3 + 0.043X12 − 0.01125X1X2 + 0.018X22
Finally, using the optimization tool provided by the Statgraphics 18.0 statistical software it was possible to determine the experimental conditions that maximized the three responses simultaneously. This procedure tries to find the levels of the experimental factors that maximize a combined desirability function, which is a function that expresses the desirability of a solution involving the three responses studied (TPC, TFC and TAA). The results are presented in Table 5. In order to verify the optimal parameters proposed by the polynomial equation and the statistical software, the experiments were conducted with the following parameters: 40 °C, 15 min, 60% ethanol (v/v), which are the optimal extraction conditions predicted by the statistical software. For validation, the values of the experimental answers were compared with the values predicted by the quadratic (polynomial) model expressed by the Equations (4)–(6).

Table 5.
Validation of the polynomial (quadratic) Box-Behnken model for conventional extraction by cornbelian cherry fruits.
The percentage error between the experimental values and the predicted values was determined by applying the Equation (7):
where Xexp—experimental (actual) value and Xpred—predicted value.
Err (%) = ǀXexp − Xpred/ Xpredǀ × 100
In Table 5, the experimental results are compared with the results predicted by the polynomial models. The proximity of the experimental and predicted values demonstrated the high validity and adequacy of the fitted model.
3.4. Inhibitory Activity against Metabolically Important Enzymes
Diabetes mellitus is a disorder of the metabolism that is characterized by hyperglycemia []. The control of diabetes mellitus, especially the non-insulin-dependent Type II, involves preventing the excessive rise of the blood glucose level through the inhibition of starch digestive enzymes, i.e., pancreatic α-amylase and α-glucosidase in the digestive system. Various investigations stated that oral anti-hyperglycemic agents derived from medicinal plants can be used in traditional medicine and many of the medicinal plants were found to have potential antidiabetic properties []. In our study, six extracts (coded as: C1, C5, and C8, according to Table 1 and U1, U5, and U10, according to Table 3), selected on the basis of the antioxidant higher values, were selected to be tested as potential inhibitory agents of the two enzymes known as being responsible for hydrocarbon degradation and intestinal absorption, α-amylase and α-glucosidase. Their inhibition was assessed as an effective means of the potential use of the extracts in the prevention of type 2 diabetes. All the extracts showed an effective inhibitory effect on α-glucosidase with values higher than 75%; more specifically, they were in the range from 76.9 ± 2.7% (experiment C8) and 87.7 ± 2.0% (experiment C1). Except for U1, the ultrasound-assisted extracts showed a higher inhibitory effect against α-glucosidase (p < 0.05). A significant lower inhibitory activity was found for α-amylase, a maximum of 16% for C5, and a minimum of 7% for U5. However, some extracts, such as C1, C8, and U10, showed no activity against α-amylase. Our results are in accordance with the results reported for other plants used traditionally to control diabetes or hyperglycemia which present a strong inhibition on α-glucosidase and a moderate or negligible effect on α-amylase activity [].
3.5. Vitamin C Content of the Cornelian Cherry Extracts and Jelly Candies
Studies have shown that the cornelian cherry fruits contain vitamin C (ascorbic acid), which varies with many factors, such as genotype, cultivar, geographic area, and the solvents used for the extractions []. The iodometric titration method was applied due to the following main advantages: simplicity, very elementary equipment, easily available reagents of low cost, and the speed of reaction of iodine with L-ascorbic acid [].
The ascorbic acid content was evaluated for the six selected extracts, coded as C1, C5, and C8 (according to Table 1) and U1, U5, and U10 (according to Table 3). The vitamin C content was significantly higher for the extract obtained by the CE method at optimal conditions (40 °C, 15 min and 60% ethanol, v/v), 39.90 ± 1.22 mg ascorbic acid/100 g dw. At the lower extraction temperature (30 °C), the vitamin C content was 36.15 ± 1.24 mg ascorbic acid/100 g dw. The vitamin C content for the ultrasound extracts ranged from 21.16 ± 1.03 to 26.39 ± 0.01 mg ascorbic acid/100 g dw. The same amount of concentrated extract obtained by the CE method (3% w/w) was used for the jelly candy formulations.
Other reports present the vitamin C content obtained by titration method from the cornelian cherry fruits as 196.31 ± 0.23 mg/100 g f.w. [] and 54.90–75.97 mg/100 g f.w. [].
The vitamin C content for the jelly candies ranges from 0.34 ± 0.01 in samples with agar-agar to 0.67 ± 0.06 mg ascorbic acid/100 g dw for the jelly candies obtained with gelatin.
3.6. Textural and Color Analysis of Jelly Food
For a comparative study, two gelling agents, the gelatin as the common gelling agent used by industry and the agar-agar characterized by stability, clarity, metabolic inertness, and nontoxicity were used. The texture profile analysis results for the formulated jelly candies are given in Table 6. Texture profile analysis was composed of firmness, adhesiveness, cohesiveness, springiness, gumminess, and chewiness properties.

Table 6.
Values of textural parameters.
The jellies obtained with gelatin registered lower firmness values (1.33 N and 2.39 N) compared to those obtained with agar-agar (5.01 N and 7.39 N). It can also be noticed that the addition of cornelian cherry extract softened the texture of both kind of jellies. Adhesiveness registered higher values in the case of agar jellies (0.07 mJ) and the addition of fruits did not significantly modify the values of adhesiveness. The cohesiveness, springiness, gumminess, and chewiness showed the highest values for the jellies obtained with gelatin. From these results, it can be concluded that the gelatin jellies are more elastic than the agar jellies, and the Cornus mas extract did not change this behavior. The fruit-concentrated extracts reduced the values of chewiness for the gelatin and agar jellies, showing that these samples require less energy to be disintegrated during mastication. The color attributes of the gelatin and agar-agar jelly containing cornelian cherry extracts are shown in Table 7.

Table 7.
Color parameters of the jelly candies.
The incorporation of the cornelian cherry affected the product color. Increases in the redness (a*) and blueness (b*) values were observed for the GEC and AEC samples. The a* value was slightly higher in the formulated agar jelly candies AEC than in the AM (p ≤ 0.05), indicating that the formation of the agar gels was able to provide red color intensity to the product. The control samples GM, as expected, had the highest color with the lowest a* parameter value and the highest lightness L* (79,76). It was also observed that the L* value was higher in the formulation containing gelatin GEC, while the lowest L* value was noted in the AEC sample. The gelatin jelly candies showed a significant increase in total color difference (ΔE*) value (p < 0.05); so, these products can provide good visual color variation. The GEC showed the higher color intensity due to the higher Chroma value C*, of 25.6 ± 0.6. Rawdkue et al. (2020) [] reported a modification of the gelatin films’ color when containing resazurin. S.C.S.R. de Moura et al. (2019) [] reported an increase of the a* value for the free hibiscus extract jelly, probably due to an increase in total phenolic compounds and the anthocyanin contents being reduced during the storage period.
4. Conclusions
The cornelian cherry (Cornus mas L.) is an edible fruit plant rich in bioactive compounds growing wild and recently cultivated in some controlled cultures in Romania. The Box–Behnken design was used in order to optimize the influence of different independent variables on the conventional (CE) and ultrasound-assisted extraction (UAE) processes. In the CE process, the responses were mainly affected by the ethanol content in the extraction aqueous mixtures used as solvent. The total polyphenol content was dependent on both the extraction time and the solvent used. The highest value for the TPC concentration (29.27 ± 1.09 mg GAE/g dw) was obtained at 40 °C and 15 min when using a 60% ethanol concentration (v/v) for the conventional extraction method. The TPC, TFC, and TAA measured for the extracts correlated to a great extent. Regarding the UAE process, according to our results, the use of ultrasound did not enhance the extraction process, and similar results in terms of the antioxidant activity of the extracts were obtained in the CE process. These results indicate that the extraction of bioactive compounds from the cornelian cherry is not controlled by the internal mass transfer. As an application for the cornelian cherry extracts obtained in this work, their use was proposed as natural additives to improve the formulation of jelly candies. The addition of cornelian cherry extracts affected the texture of the jellies, making them softer.
In conclusion, our results suggest that the cornelian cherry fruits from the spontaneous crops are a good source of the bioactive compounds that can be efficiently extracted by conventional extraction and can therefore be used as a raw material to obtain bioactive compounds that can enhance the properties of jelly candies.
Author Contributions
Conceptualization, I.M.E., G.C., Ó.B.-R., L.M. and M.T.S.: methodology, N.S., D.G.A., G.C. and I.M.E.; software, Ó.B.-R. and G.C.; validation, M.T.S. and Ó.B.-R.; formal analysis, I.M.E., N.S. and D.G.A.; investigation I.M.E., N.S. and D.G.A.; resources, M.T.S.; data curation, L.M., Ó.B.-R. and M.T.S.; writing—original draft preparation, I.M.E., L.M., G.C., N.S. and D.G.A.; writing—review and editing, L.M. and G.C.; visualization, L.M., Ó.B.-R., and M.T.S.; supervision, M.T.S. and C.V.; project administration, L.M.; funding acquisition, M.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by mobility grants of the Romanian Ministry of Research and Innovation, CNCS-UEFISCDI, project numbers PN-III-P1-1.1-MC-2019-1905 and PN-III-P1-1.1-MC-2019-1908, within PNCDI III. The APC was funded by M.T.S. The authors are also grateful to Junta de Castilla y León (Spain) and ERDF for the financial support of project BU050P20. The Ó. Benito–Román post-doctoral contract was funded by Junta de Castilla y León (Spain) and ERDF through project BU050P20 and by Agencia Estatal de Investigación through project PID2020-116716RJ-I00/AEI/10.13039/501100011033.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The first author is grateful to and thanks Eliza Țupu from the Museal Complex of Natural Sciences “Răsvan Angheluţă” Galaţi. The experiments carried out in this work, regarding the extractions, were made using the infrastructure of the BIOND research Group, at the University of Burgos, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodrigues, M.J.; Oliveira, M.; Neves, V.; Ovelheiro, A.; Pereira, C.G.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Coupling sea lavender (Limonium algarvense Erben) and green tea (Camellia sinensis (L.) Kuntze) to produce an innovative herbal beverage with enhanced enzymatic inhibitory properties. S. Afr. J. Bot. 2019, 120, 87–94. [Google Scholar] [CrossRef]
- Radbeh, Z.; Asefi, N.; Hamishehkar, H.; Roufegarinejad, L.; Pezeshki, A. Novel carriers ensuring enhanced anti-cancer activity of Cornus mas (cornelian cherry) bioactive compounds. Biomed. Pharmacother. 2020, 125, 109906. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef] [PubMed]
- Mattera, R.; Molnar, T.; Struwe, L. Cornus× elwinortonii and Cornus× rutgersensis (Cornaceae), new names for two artificially produced hybrids of big-bracted dogwoods. PhytoKeys 2015, 55, 93–111. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC-TOF-MS Characterization and Identification of Bioactive Iridoids in Cornus mas Fruit. J. Anal. Methods Chem. 2013, 2013, 710972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikaili, P.; Koohirostamkolaei, M.; Babaeimarzangou, S.S.; Aghajanshakeri, S.; Moloudizargari, M.; Gamchi, N.S.; Toloomoghaddam, S. Therapeutic uses and pharmacological effects of Cornus mas: A review. J. Pharm. Biomed. Sci. 2013, 35, 1732–1738. [Google Scholar]
- Ahmadipour, S.H.; Vakili, M.; Ahmadipour, S. Phytotherapy for children’s nocturnal enuresis. J. Med. Biomed. Sci. 2018, 6, 23–29. [Google Scholar] [CrossRef]
- Shamsi, F.; Asgari, S.; Rafieian-Kopaei, M.; Kazemi, S.; Adelnia, A. Effects of Cornus mas L. on blood glucose, insulin and histopathology of pancreas in alloxan-induced diabetic rats. J. Isfahan Med. Sch. 2011, 29, 929–938. [Google Scholar]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Kramarova, D.; Jurikova, T. Selected cultivars of cornelian cherry (Cornus mas L.) as a new food source for human nutrition. Afr. J. Biotechnol. 2010, 9, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef] [Green Version]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Ersoy, N.; Bagci, Y.; Gok, V. Antioxidant properties of 12 cornelian cherry fruit types (Cornus mas L.) selected from Turkey. Sci. Res. Essays 2011, 6, 98–102. [Google Scholar] [CrossRef]
- Juranović Cindrić, I.; Zeiner, M.; Krpetić, M.; Stingeder, G. ICP-AES determination of minor and major elements in Cornelian cherry (Cornus mas L.) after microwave assisted digestion. Microchem. J. 2012, 105, 72–76. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Enachi, E.; Stănciuc, N.; Aprodu, I. Optimization of ultrasound assisted extraction of phenolic compounds from cornelian cherry fruits using response surface methodology. CyTA-J. Food 2019, 17, 814–823. [Google Scholar] [CrossRef]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Optimisation of extraction procedure and development of LC–DAD–MS methodology for anthocyanin analysis in anthocyanin-pigmented corn kernels. Food Chem. 2020, 319, 126515. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Alonso, E.; Cocero, M.J. Ultrasound-assisted extraction of β-glucans from barley. LWT-Food Sci. Technol. 2013, 50, 57–63. [Google Scholar] [CrossRef]
- Ran, X.-L.; Zhang, M.; Wang, Y.; Adhikari, B. Novel technologies applied for recovery and value addition of high value compounds from plant byproducts: A review. Crit. Rev. Food Sci. Nutr. 2017, 59, 450–461. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- de Avelar, M.H.M.; Efraim, P. Alginate/pectin cold-set gelation as a potential sustainable method for jelly candy production. LWT 2020, 123, 109119. [Google Scholar] [CrossRef]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of thermal treatment on phenolic compounds from plum (Prunus domestica) extracts—A kinetic study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]
- Kaur, S.; Mondal, P. Study of Total Phenolic and Flavonoid Content, Antioxidant Activity and Antimicrobial Properties of Medicinal Plants. J. Microbiol. Exp. 2014, 1, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Oancea, A.-M.; Aprodu, I.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Bahrim, G.; Râpeanu, G.; Stănciuc, N. A bottom-up approach for encapsulation of sour cherries anthocyanins by using β-lactoglobulin as matrices. J. Food Eng. 2017, 210, 83–90. [Google Scholar] [CrossRef]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs iodometric titration as analytical methods. LWT-Food Sci. Technol. 2013, 50, 489–495. [Google Scholar] [CrossRef]
- Costamagna, M.; Zampini, I.; Alberto, M.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Golos, B.R.; Ninic, J.I.; Bijelic, S.M.; Popovic, B.M. Physicochemical Fruit Characteristics of Cornelian Cherry (Cornus mas L.) Genotypes from Serbia. HortScience 2011, 46, 849–853. [Google Scholar]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochem. 2015, 23, 308–316. [Google Scholar] [CrossRef]
- Apel, C.; Lyng, J.G.; Papoutsis, K.; Harrison, S.M.; Brunton, N.P. Screening the effect of different extraction methods (ultrasound-assisted extraction and solid–liquid extraction) on the recovery of glycoalkaloids from potato peels: Optimisation of the extraction conditions using chemometric tools. Food Bioprod. Process. 2020, 119, 277–286. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Freeze-dried extract from onion (Allium cepa cv. Horcal) skin wastes: Extraction intensification and flavonoids identification. Food Bioprod. Process. 2021, 130, 92–105. [Google Scholar] [CrossRef]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Maran, J.P.; Manikandan, S.; Nivetha, C.V.; Dinesh, R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017, 10, S1145–S1157. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, I.G.; Maran, J.P.; Ilakya, S.; Anitha, S.L.; Sabarima, S.P.; Priya, B. Ultrasound assisted extraction of pectin from waste Artocarpus heterophyllus fruit peel. Ultrason. Sonochem. 2017, 34, 525–530. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process. Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Brás, T.; Paulino, A.F.C.; Neves, L.A.; Crespo, J.G.; Duarte, M.F. Ultrasound assisted extraction of cynaropicrin from Cynara cardunculus leaves: Optimization using the response surface methodology and the effect of pulse mode. Ind. Crop. Prod. 2020, 150, 112395. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.N.; Trandafir, I.; Cornescu, F. Antioxidant Capacity, Total Phenols, Total Flavonoids and Colour Component of Cornelian Cherry (Cornus mas L.) Wild Genotypes. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, K.U.; Ercisli, S.; Zengin, Y.; Sengul, M.; Kafkas, E.Y. Preliminary characterisation of cornelian cherry (Cornus mas L.) genotypes for their physico-chemical properties. Food Chem. 2009, 114, 408–412. [Google Scholar] [CrossRef]
- Cornescu, F.-C.; Cosmulescu, S.N. Morphological and Biochemical Characteristics of Fruits of Different Cornelian Cherry (Cornus mas L.) Genotypes from Spontaneous Flora. Not. Sci. Biol. 2017, 9, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Singh, R.K.; Tewari, L. Antioxidative potential of herbal hypoglycemic agents in diabetes—An overview. SFRR Ind. Bull. 2004, 3, 24–26. [Google Scholar]
- Kesari, A.N.; Kesari, S.; Singh, S.K.; Gupta, R.K.; Watal, G. Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J. Ethnopharmacol. 2007, 112, 305–311. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory poten-tial against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Bayram, H.M.; Ozturkcan, S.A. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Kostecka, M.; Szot, I.; Czernecki, T.; Szot, P. Vitamin C content of new ecotypes of cornelian cherry (Cornus mas L.) determined by various analytical methods. Acta Sci. Pol. Hortorum Cultus 2017, 16, 53–61. [Google Scholar] [CrossRef]
- Szot, I.; Lipa, T.; Sosnowska, B. Evaluation of yield and fruit quality of several ecotypes of cornelian cherry (Cornus mas L.) in polish condiotions. Acta Sci. Pol. Hortorum Cultus 2019, 18, 141–150. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Faseha, A.; Benjakul, S.; Kaewprachu, P. Application of anthocyanin as a color indi-cator in gelatin films. Food Biosci. 2020, 36, 100603. [Google Scholar] [CrossRef]
- de Moura, S.C.; Berling, C.L.; Garcia, A.O.; Queiroz, M.B.; Alvim, I.D.; Hubinger, M.D. Release of anthocyanins from the hibiscus extract encapsulated by ionic gelation and application of microparticles in jelly candy. Food Res. Int. 2019, 121, 542–552. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).