Facile Synthesis of Copper Oxide-Cobalt Oxide/Nitrogen-Doped Carbon (Cu2O-Co3O4/CN) Composite for Efficient Water Splitting
Abstract
1. Introduction
2. Experimental
2.1. Chemical and Reagent
2.2. Synthesis of Cu2O-Co3O4/CN Composite
2.3. Characterization of Composite
2.4. Electrochemical Water Splitting
3. Results and Discussion
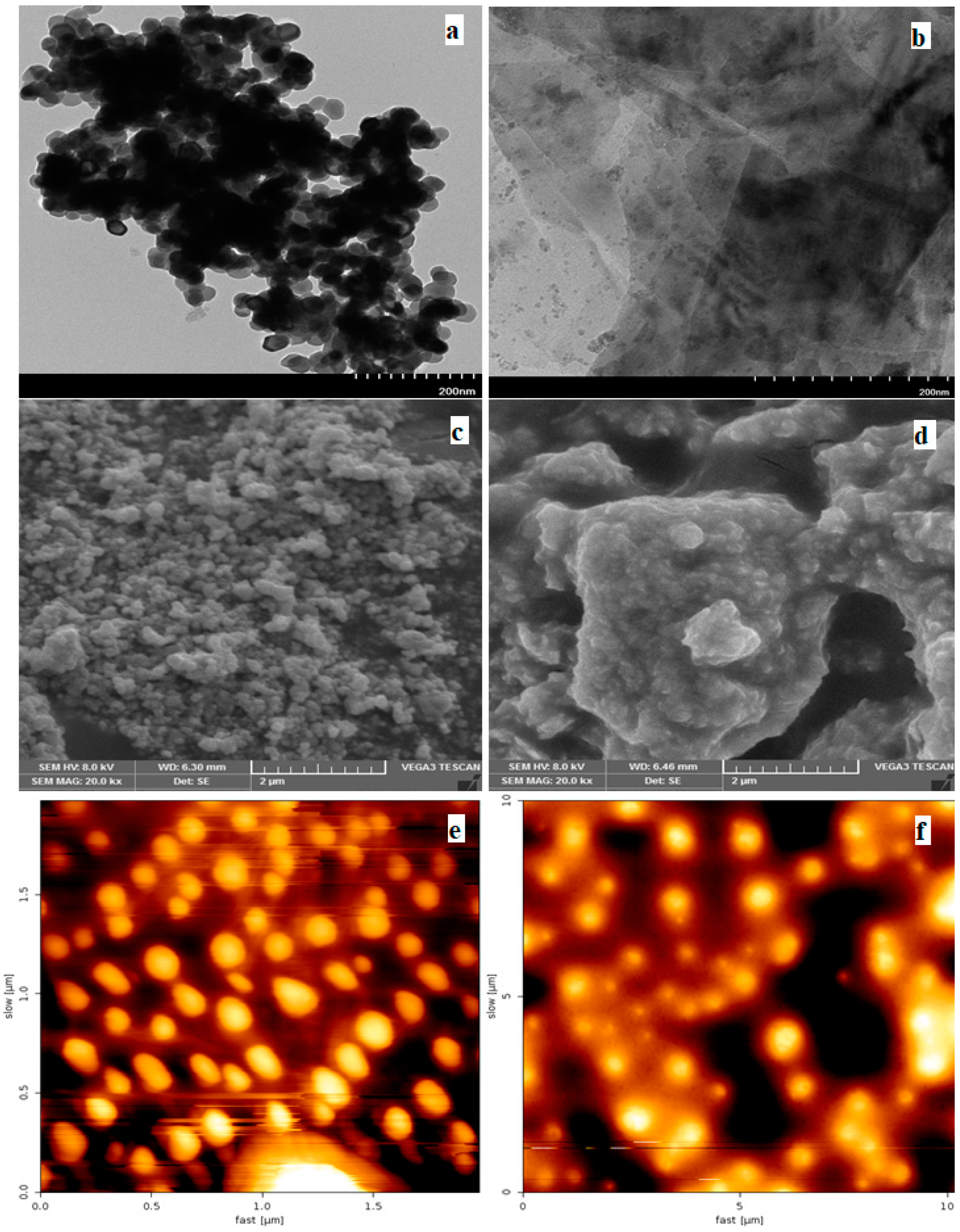
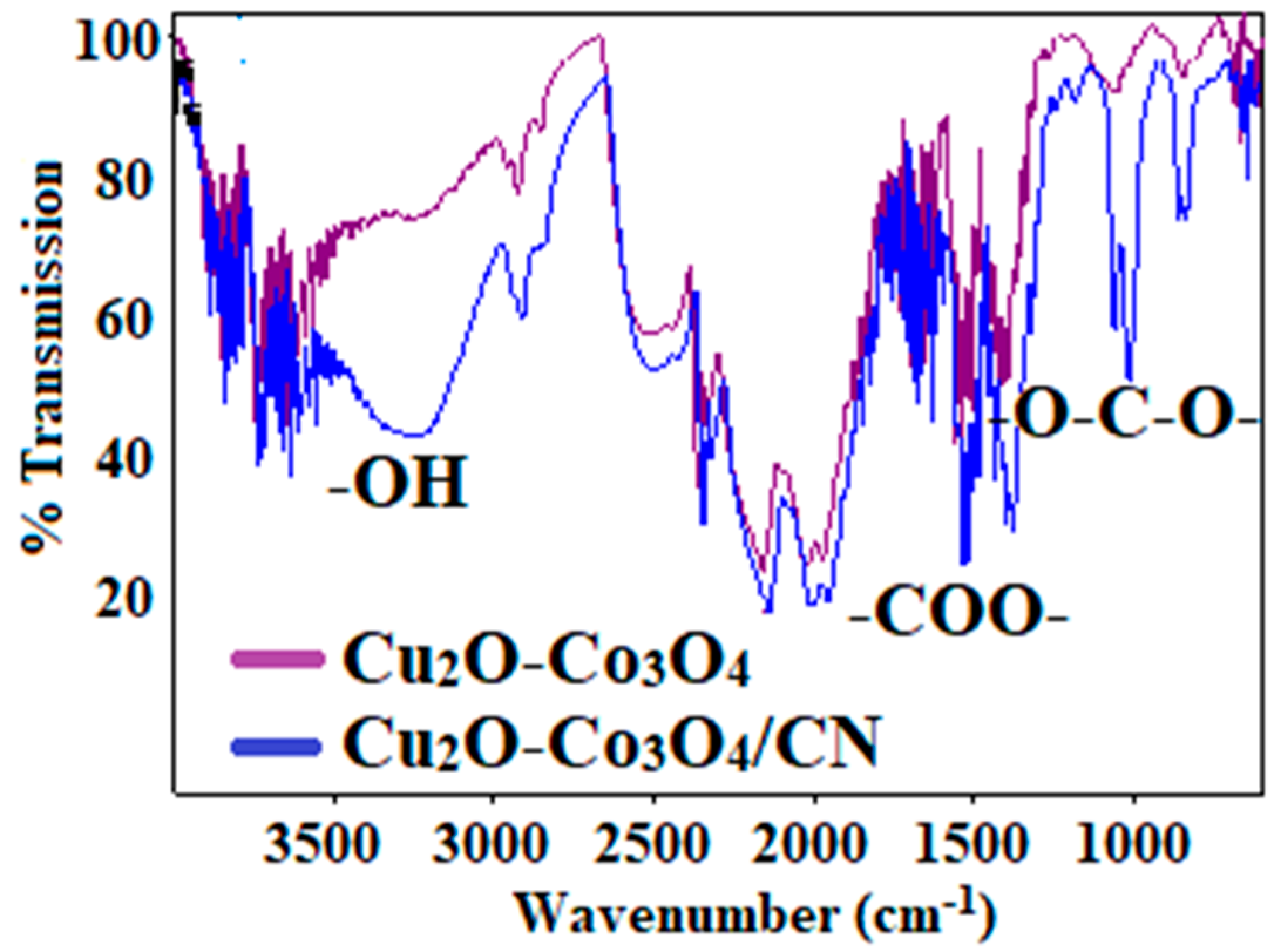
3.1. Characterization of Cu2O-Co3O4/CN Composite
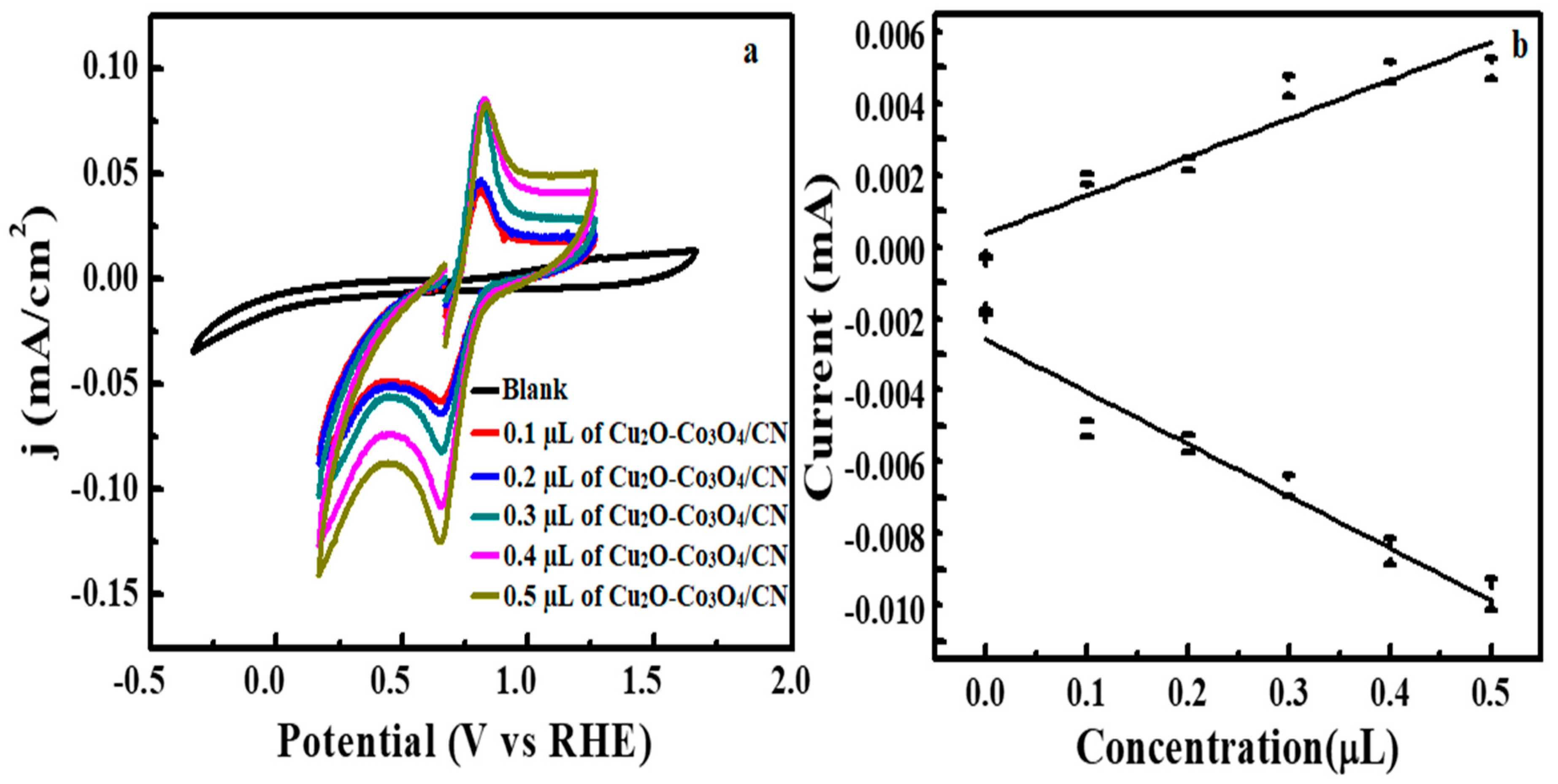
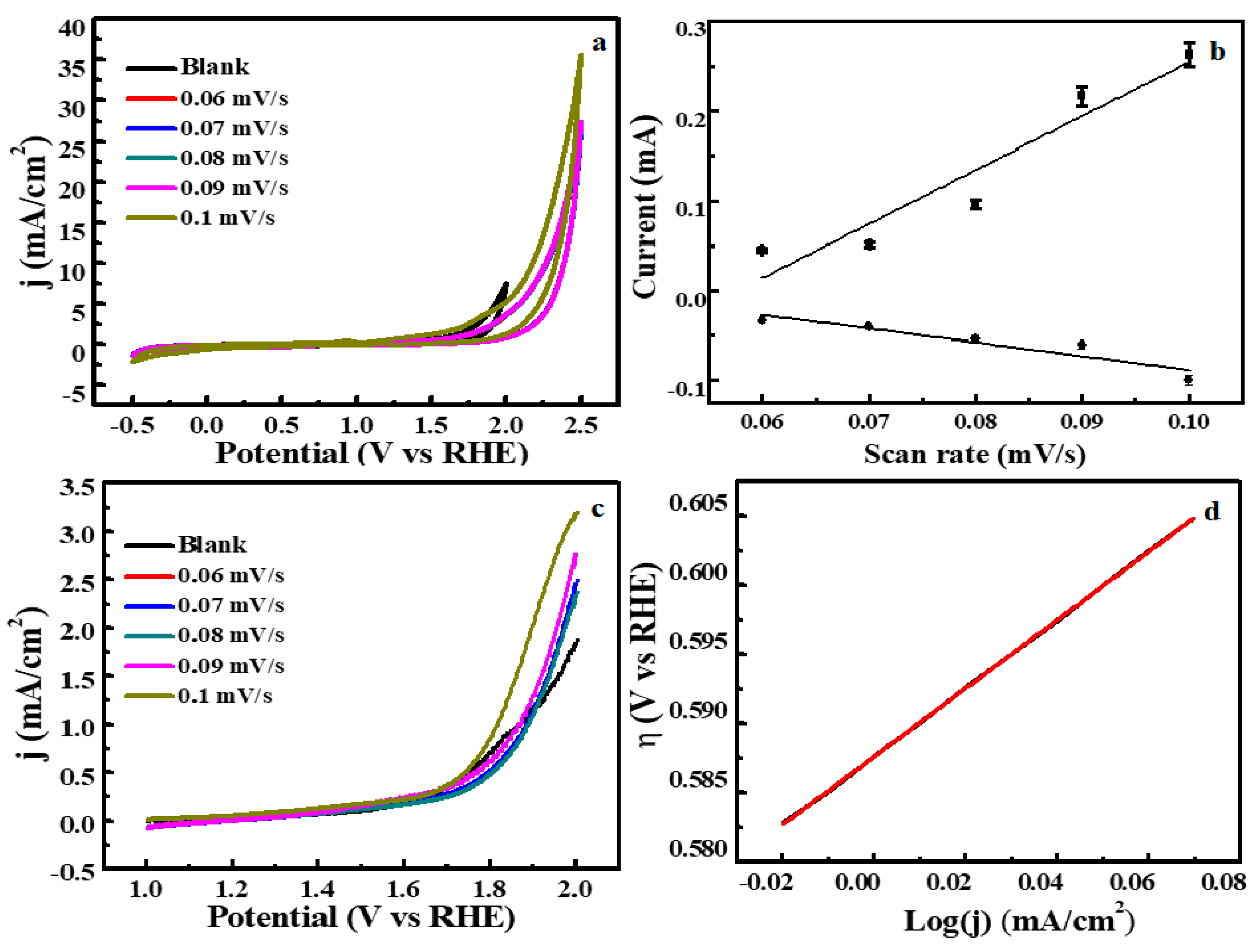
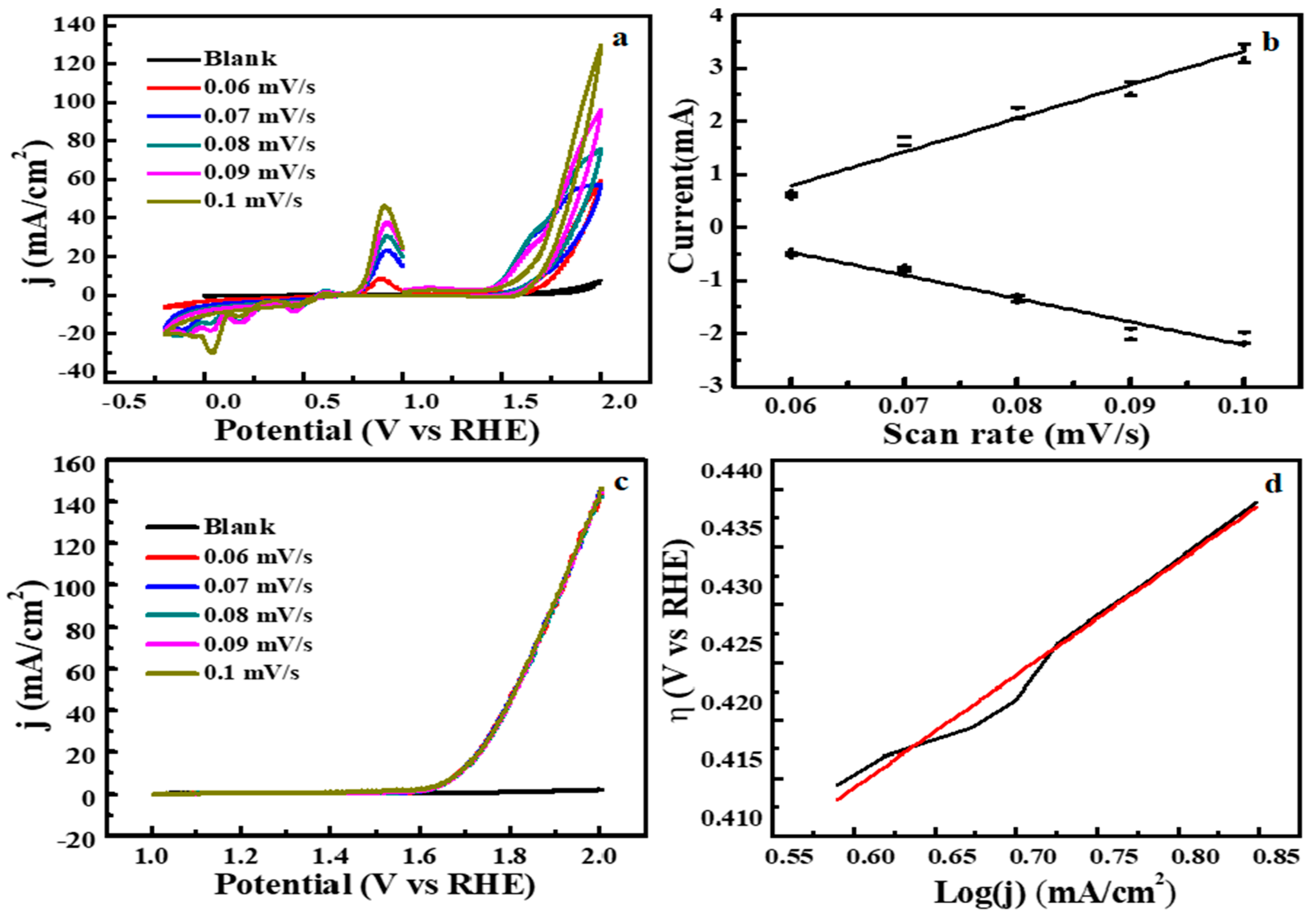
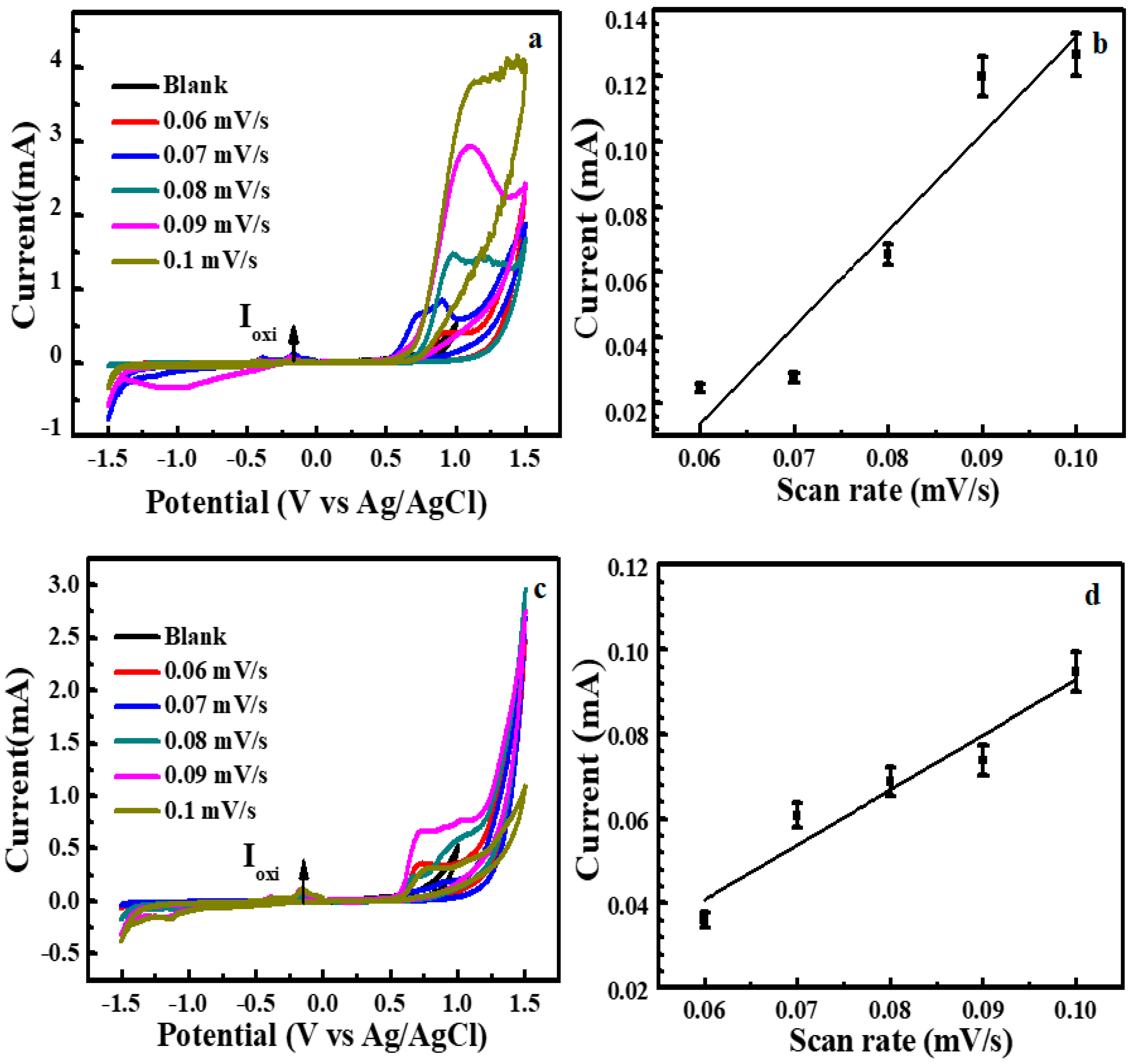
3.2. Electrochemical Water Splitting by Cu2O-Co3O4/CN Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bekun, F.V.; Alola, A.A.; Sarkodie, S.A. Toward a sustainable environment: Nexus between CO2 emissions, resource rent, renewable and nonrenewable energy in 16-EU countries. Sci. Total Environ. 2019, 657, 1023–1029. [Google Scholar] [CrossRef]
- Zafar, M.W.; Shahbaz, M.; Hou, F.; Sinha, A. From nonrenewable to renewable energy and its impact on economic growth: The role of research & development expenditures in Asia-Pacific Economic Cooperation countries. J. Clean. Prod. 2019, 212, 1166–1178. [Google Scholar]
- Hansen, K.; Mathiesen, B.V.; Skov, I.R. Full energy system transition towards 100% renewable energy in Germany in 2050. Renew. Sustain. Energy Rev. 2019, 102, 1–13. [Google Scholar] [CrossRef]
- Gu, G.H.; Noh, J.; Kim, I.; Jung, Y. Machine learning for renewable energy materials. J. Mater. Chem. A 2019, 7, 17096–17117. [Google Scholar] [CrossRef]
- Ivakhnenko, A.; Bakytzhan, B. Characterization of Economic and Ecological Advantages and Challenges in Development of Conventional and Unconventional Hydrocarbon, Non-Hydrocarbon and Renewable Energy Sources for Resource-Based Economy in Kazakhstan. In Proceedings of the EGU General Assembly Conference, online, 4–8 May 2020. [Google Scholar]
- Winebrake, J.J.; Wang, M.Q.; He, D. Toxic emissions from mobile sources: A total fuel-cycle analysis for conventional and alternative fuel vehicles. J. Air Waste Manag. Assoc. 2001, 51, 1073–1086. [Google Scholar] [CrossRef][Green Version]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110–4131. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and nonrenewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Maggio, G.; Nicita, A.; Squadrito, G. How the hydrogen production from RES could change energy and fuel markets: A review of recent literature. Int. J. Hydrogen Energy 2019, 44, 11371–11384. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Salomão, P.E.; Gomes, D.S.; Ferreira, E.J.C.; Moura, F.; Nascimento, L.L.; Patrocínio, A.O.T.; Pereira, M.C. Photoelectrochemical hydrogen production from water splitting using heterostructured nanowire arrays of Bi2O3/BiAl oxides as a photocathode. Sol. Energy Mater. Sol. Cells 2019, 194, 276–284. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Zhou, J.; Wang, K.; Zheng, Y.-Z.; Tao, X. Uniformly assembling n-type metal oxide nanostructures (TiO2 nanoparticles and SnO2 nanowires) onto P doped g-C3N4 nanosheets for efficient photocatalytic water splitting. Appl. Catalys. B Environ. 2020, 278, 119301. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Wang, H.; Gao, C.; Li, R.; Peng, Z.; Yang, J.; Gao, J.; Yang, Y.; Li, S.; Li, B.; Liu, Z. Ruthenium-cobalt nanoalloy embedded within hollow carbon spheres as a bifunctionally robust catalyst for hydrogen generation from water splitting and ammonia borane hydrolysis. ACS Sustain. Chem. Eng. 2019, 7, 18744–18752. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-supported transition-metal-based electro-catalysts for hydrogen and oxygen evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Joe, J.; Yang, H.; Bae, C.; Shin, H. Metal chalcogenides on silicon photocathodes for efficient water splitting: A mini overview. Catalysts 2019, 9, 149. [Google Scholar] [CrossRef]
- Kötz, R.; Stucki, S. Oxygen Evolution and Corrosion on Ruthenium-Iridium Alloys. J. Electrochem. Soc. 1985, 132, 103–107. [Google Scholar] [CrossRef]
- Devarayapalli, K.C.; Lee, K.; Nam, N.D.; Vattikuti, S.V.P.; Shim, J. Microwave synthesized nano-photosensitizer of CdS QD/MoO3–OV/g–C3N4 heterojunction catalyst for hydrogen evolution under full-spectrum light. Ceram. Int. 2020, 46, 28467–28480. [Google Scholar] [CrossRef]
- Devarayapalli, K.C.; Vattikuti, S.V.P.; Sreekanth, T.V.M.; Nagajyothi, P.C.; Shim, J. Pyrolysis-Synthesized g-C3N4/Nb2O5 Nanocomposite for Enhanced Photocatalytic Activity under White LED Light Irradiation. ChemistrySelect 2019, 4, 13250–13258. [Google Scholar] [CrossRef]
- Devarayapalli, K.; Vattikuti, S.V.P.; Sreekanth, T.V.M.; Yoo, K.S.; Nagajyothi, P.C.; Shim, J. Hydrogen production and photocatalytic activity of g-C3N4/Co-MOF (ZIF-67) nanocomposite under visible light irradiation. Appl. Organomet. Chem. 2020, 34, e5376. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Devarayapalli, K.C.; Nagajyothi, P.C.; Shim, J. Microwave synthesized dry leaf-like mesoporous MoSe2 nanostructure as an efficient catalyst for enhanced hydrogen evolution and supercapacitor applications. Microchem. J. 2020, 153, 104446. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Manabe, H.; Sato, E. Oxygen Evolution on La1-xSrx Fe1-y Co y O3 Series Oxides. J. Electrochem. Soc. 1980, 127, 2360–2364. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, M.; Chai, J.; Tang, Z.; Shao, M.; Kwok, C.T.; Yang, M.; Wang, Z.; Chua, D.; Wang, S.; et al. Facile synthesis of vanadium-doped Ni3S2 nanowire arrays as active electro-catalyst for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 5959–5967. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Sun, Z. Novel 2D Transition-Metal Carbides: Ultrahigh Performance Electrocatalysts for Overall Water Splitting and Oxygen Reduction. Adv. Funct. Mater. 2020, 30, 2000570. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Cheng, H.; Li, N.; Ma, T.Y.; Su, Y.-Z. ZnCo2O4 quantum dots anchored on nitrogen-doped carbon nanotubes as reversible oxygen reduction/evolution electro-catalysts. Adv. Mater. 2016, 28, 3777–3784. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ji, S.; Wang, H.; Gai, H.; Liu, F.; Linkov, V.; Wang, R. Mesoporous nickel-sulfide/nickel/N-doped carbon as HER and OER bifunctional electro-catalyst for water electrolysis. Int. J. Hydrogen Energy 2019, 44, 2832–2840. [Google Scholar] [CrossRef]
- Wu, A.; Xie, Y.; Ma, H.; Tian, C.; Gu, Y.; Yan, H.; Zhang, X.; Yang, G.; Fu, H. Integrating the active OER and HER components as the heterostructures for the efficient overall water splitting. Nano Energy 2018, 44, 353–363. [Google Scholar] [CrossRef]
- Zhang, H.; Maijenburg, A.W.; Li, X.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Jiang, M.; Zhang, Q.; He, P.; Sun, X. 3D self-supported Fe-doped Ni2P nanosheet arrays as bifunctional catalysts for overall water splitting. Adv. Funct. Mater. 2017, 27, 1702513. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Huang, F.; Qu, L.; Li, J.; Owusu, K.A.; Liu, Z.; Lin, Z.; Xiang, B.; Liu, X.; et al. Copper-Nickel Nitride Nanosheets as Efficient Bifunctional Catalysts for Hydrazine-Assisted Electrolytic Hydrogen Production. Adv. Energy Mater. 2019, 9, 1900390. [Google Scholar] [CrossRef]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 0003. [Google Scholar] [CrossRef]
- Wang, J.; Choi, S.; Kim, J.; Cha, S.W.; Lim, J. Recent Advances of First d-Block Metal-Based Perovskite Oxide Electrocatalysts for Alkaline Water Splitting. Catalysts 2020, 10, 770. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Gao, Y.; Chen, D.; Chen, C.; Saccoccio, M.; Ciucci, F. Ba0. 5Sr0. 5Co0. 8Fe0. 2O3− δ on N-doped mesoporous carbon derived from organic waste as a bi-functional oxygen catalyst. Int. J. Hydrogen Energy 2016, 41, 10744–10754. [Google Scholar] [CrossRef]
- Janani, G.; Yuvaraj, S.; Surendran, S.; Chae, Y.; Sim, Y.; Song, S.-J.; Park, W.; Kim, M.-J.; Sim, U. Enhanced bifunctional electrocatalytic activity of Ni-Co bimetallic chalcogenides for efficient water-splitting application. J. Alloy. Compd. 2020, 846, 156389. [Google Scholar] [CrossRef]
- Wang, J.; Ciucci, F. Boosting Bifunctional Oxygen Electrolysis for N-Doped Carbon via Bimetal Addition. Small 2017, 13, 1604103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, J.; Choi, S.; Wang, H.; Lim, J. A Review of Carbon-Supported Nonprecious Metals as Energy-Related Electrocatalysts. Small Methods 2020, 4, 2000621. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Progr. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Surendran, S.; Sivanantham, A.; Shanmugam, S.; Sim, U.; Kalai Selvan, R. Ni2P2O7 microsheets as efficient Bi-functional electrocatalysts for water splitting application. Sustain. Energy Fuels 2019, 3, 2435–2446. [Google Scholar] [CrossRef]
- An, T.-Y.; Surendran, S.; Kim, H.; Choe, W.-S.; Kim, J.K.; Sim, U. A polydopamine-mediated biomimetic facile synthesis of molybdenum carbide phosphide nanodots encapsulated in carbon shell for electrochemical hydrogen evolution reaction with long-term durability. Compos. Part B Eng. 2019, 175, 107071. [Google Scholar] [CrossRef]
- Sim, Y.; Kim, S.J.; Janani, G.; Chae, Y.; Surendran, S.; Kim, H.; Yoo, S.; Seok, D.C.; Jung, Y.H.; Jeon, C.; et al. The synergistic effect of nitrogen and fluorine co-doping in graphene quantum dot catalysts for full water splitting and supercapacitor. Appl. Surf. Sci. 2020, 507, 145157. [Google Scholar] [CrossRef]
- Dayong, N.; Mikhaylov, A.; Bratanovsky, S.; Shaikh, Z.A.; Stepanova, D. Mathematical modeling of the technological processes of catering products production. J. Food Process Eng. 2020, 43, e13340. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Sun, Y.; Qi, K.; Jin, Z.; Wei, S.; Li, W.; Zhang, L.; Zheng, W. Nanoporous Sulfur-Doped Copper Oxide (Cu2OxS1−x) for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 745–752. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Yun, J.-H.; Hu, Y.; Ziao, M.; Du, A.; Wang, L. New Iron-Cobalt Oxide Catalysts Promoting BiVO4 Films for Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2018, 28, 1802685. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Ramasamy, H.; Janani, G.; Kalpana, D.; Lee, Y.S.; Sim, U.; Selvan, R.K. Hydrothermal deposition of CoS nanostructures and its multifunctional applications in supercapattery and water electrolyzer. Appl. Surf. Sci. 2019, 494, 916–928. [Google Scholar] [CrossRef]
- Yumashev, A.; Ślusarczyk, B.; Kondrashev, S.; Mikhaylov, A. Global Indicators of Sustainable Development: Evaluation of the Influence of the Human Development Index on Consumption and Quality of Energy. Energies 2020, 13, 2768. [Google Scholar] [CrossRef]
- Nie, D.; Panfilova, E.; Samusenkov, V.; Mikhaylov, A. E-Learning Financing Models in Russia for Sustainable Development. Sustainability 2020, 12, 4412. [Google Scholar] [CrossRef]
- An, J.; Mikhaylov, A.; Jung, S.-U. The Strategy of South Korea in the Global Oil Market. Energies 2020, 13, 2491. [Google Scholar] [CrossRef]
- An, J.; Mikhaylov, A.; Kim, K. Machine Learning Approach in Heterogeneous Group of Algorithms for Transport Safety-Critical System. Appl. Sci. 2020, 10, 2670. [Google Scholar] [CrossRef]
- Yumashev, A.; Mikhaylov, A. Development of Polymer Film Coatings with High Adhesion to Steel Alloys and High Wear Resistance. Polym. Compos. 2020, 41, 2875–2880. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Cho, M.H.; Kang, M.; Han, T.H. Carbothermal process-derived porous N-doped carbon for flexible energy storage: Influence of carbon surface area and conductivity. Chem. Eng. J. 2019, 378, 122158. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, Z.A.; Moiseev, N.; Mikhaylov, A.; Yüksel, S. Facile Synthesis of Copper Oxide-Cobalt Oxide/Nitrogen-Doped Carbon (Cu2O-Co3O4/CN) Composite for Efficient Water Splitting. Appl. Sci. 2021, 11, 9974. https://doi.org/10.3390/app11219974
Shaikh ZA, Moiseev N, Mikhaylov A, Yüksel S. Facile Synthesis of Copper Oxide-Cobalt Oxide/Nitrogen-Doped Carbon (Cu2O-Co3O4/CN) Composite for Efficient Water Splitting. Applied Sciences. 2021; 11(21):9974. https://doi.org/10.3390/app11219974
Chicago/Turabian StyleShaikh, Zaffar Ahmed, Nikita Moiseev, Alexey Mikhaylov, and Serhat Yüksel. 2021. "Facile Synthesis of Copper Oxide-Cobalt Oxide/Nitrogen-Doped Carbon (Cu2O-Co3O4/CN) Composite for Efficient Water Splitting" Applied Sciences 11, no. 21: 9974. https://doi.org/10.3390/app11219974
APA StyleShaikh, Z. A., Moiseev, N., Mikhaylov, A., & Yüksel, S. (2021). Facile Synthesis of Copper Oxide-Cobalt Oxide/Nitrogen-Doped Carbon (Cu2O-Co3O4/CN) Composite for Efficient Water Splitting. Applied Sciences, 11(21), 9974. https://doi.org/10.3390/app11219974







