Improvement of Working Conditions of Mining Workers by Reducing Nitrogen Oxide Emissions during Blasting Operations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alghofaili, Y.; Albattah, A.; Alrajeh, N.; Rassam, M.A.; Al-rimy, B.A.S. Secure Cloud Infrastructure: A Survey on Issues, Current Solutions, and Open Challenges. Appl. Sci. 2021, 11, 9005. [Google Scholar] [CrossRef]
- Cobos, J.E.; Knudby, C.; Søgaard, E.G. A Geothermal Plant from a Time-Scale Perspective. Energies 2021, 14, 6096. [Google Scholar] [CrossRef]
- Woodroffe, J.-D.; Lupton, D.V.; Garrison, M.D.; Nagel, E.M.; Siirila, M.J.; Harvey, B.G. Synthesis and fuel properties of high-energy density cyclopropanated monoterpenes. Fuel Process. Technol. 2021, 222, 106952. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Fan, J.; Li, L.; Liu, G. Life cycle assessment of CO2 emission reduction potential of carbon capture and utilization for liquid fuel and power cogeneration. Fuel Process. Technol. 2021, 221, 106924. [Google Scholar] [CrossRef]
- Imasiku, K.; Thomas, V.M. The Mining and Technology Industries as Catalysts for Sustainable Energy Development. Sustainability 2020, 12, 10410. [Google Scholar] [CrossRef]
- Khan, N.U.; Wei, H.; Yue, G.; Nazir, N.; Zainol, N.R. Exploring Themes of Sustainable Practices in Manufacturing Industry: Using Thematic Networks Approach. Sustainability 2021, 13, 10288. [Google Scholar] [CrossRef]
- Tian, P.; Wu, H.; Yang, T.; Zhang, W.; Jiang, F.; Zhang, Z.; Wu, T. Environmental Risk Assessment of Accidental Pollution Incidents in Drinking Water Source Areas: A Case Study of the Hongfeng Lake Watershed, China. Sustainability 2019, 11, 5403. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.N.; Redhwi, H.H.; Al-Arfaj, A.A.; Achilias, D.S. Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review. Sustainability 2021, 13, 10528. [Google Scholar] [CrossRef]
- Obiri, K.A.; Omotayo, T.S.; Bjeirmi, B.; Boateng, P. Long-Term Dynamic Behaviour of Human Resource Needs in Ghana’s Oil Sector: System Dynamics Approach. Sustainability 2021, 13, 3546. [Google Scholar] [CrossRef]
- González-Ruiz, J.D.; Mejia-Escobar, J.C.; Franco-Sepúlveda, G. Towards an Understanding of Project Finance in the Mining Sector in the Sustainability Context: A Scientometric Analysis. Sustainability 2021, 13, 10317. [Google Scholar] [CrossRef]
- Ponomarenko, T.; Marinina, O.; Nevskaya, M.; Kuryakova, K. Developing Corporate Sustainability Assessment Methods for Oil and Gas Companies. Economies 2021, 9, 58. [Google Scholar] [CrossRef]
- Meramo, S.; Puello, P.; Rodríguez, J. Sustainability Outlook of Thermochemical-Based Second-Generation Biofuel Production: Exergy Assessment. Appl. Sci. 2021, 11, 8851. [Google Scholar] [CrossRef]
- Fredj, M.; Hafsaoui, A.; Riheb, H.; Boukarm, R.; Saadoun, A. Back-analysis study on slope instability in an open pit mine (Algeria). Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2020, 2, 24–29. [Google Scholar] [CrossRef]
- Gendler, S.; Gridina, E.; Egorova, N. Calculation of the volume of air for ventilation of mining workings when operating self-propelled diesel equipment. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2019, 6, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Gendler, S.G. Ecological aspects of vehicle tunnels ventilation in the conditions of megalopolises. J. Min. Inst. 2016, 218, 313. Available online: https://pmi.spmi.ru/index.php/pmi/article/view/5112 (accessed on 12 August 2021).
- Khomenko, O.; Kononenko, M.; Myronova, I.; Sudakov, A.; Myronova, L. Increasing ecological safety during underground mining of iron-ore deposits. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2018, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Olufemi, A.C.; Mji, A.; Mukhola, M.S. Health risks of exposure to air pollutants among students in schools in the vicinities of coal mines. Energy Explor. Exploit. 2019, 37, 1638–1656. [Google Scholar] [CrossRef] [Green Version]
- Pavel, A.; Sergey, K.; Valentin, I. The Equation of State for Explosive Detonation Products. Int. J. Mech. Eng. Technol. 2018, 9, 865–868. Available online: http://www.iaeme.com/ijmet/issues.asp?JType=IJMET&VType=9&IType=13 (accessed on 12 August 2021).
- Nalisko, M.M. Modification of the method of large particles in the problem of calculation of an accidental explosion in mine atmosphere. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2017, 5, 108–116. [Google Scholar]
- Salonen, H.; Salthammer, T.; Morawska, L. Human exposure to NO2 in school and office indoor environments. Environ. Int. 2019, 130, 104887. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Wang, M.; Qian, Y.; Steenland, K.; Caudle, W.M.; Liu, Y.; Sarnat, J.; Papatheodorou, S.; Shi, L. Long-term exposure to nitrogen dioxide and mortality: A systematic review and meta-analysis. Sci. Total Environ. 2021, 776, 145968. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, T.; Nevskaya, M.; Jonek-Kowalska, I. Mineral Resource Depletion Assessment: Alternatives, Problems, Results. Sustainability 2021, 13, 862. [Google Scholar] [CrossRef]
- Mueller, J.T. Natural Resource Dependence and Rural American Economic Prosperity From 2000 to 2015. Econ. Dev. Q. 2020. [Google Scholar] [CrossRef]
- Shakhrai, S.G.; Kurchin, G.S.; Sorokin, A.G. New technical solutions for ventilation in deep quarries. J. Min. Inst. 2019, 240, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Korshunov, G.I.; Kabanov, E.I.; Cehlár, M. Occupational Risk Management in a Mining Enterprise with the Aid of an Improved Matrix Method for Risk Assessment. Acta Montan. Slovaca 2020, 25, 289–301. [Google Scholar]
- Belin, V.A.; Paramonov, G.P.; Jamiyan, J. Peculiarities of Manufacturing and Application of Mixed Explosives of ANFO Type at Mining Enterprises of Mongolia. J. Min. Inst. 2018, 232, 364–367. [Google Scholar] [CrossRef]
- Hollodenko, T.F.; Ustimenko, E.B.; Kirichenko, A.L.; Pavlichenko, A.V. Environmental substantiation of the possibility of using emulsion explosives of the ERA brand at mining enterprises. Forum of miners: Materials of an international scientific and practical conference. Dnipro 2016, 2, S203–S208. [Google Scholar]
- Afanasev, P.; Makhmudov, K. Assessment of the Parameters of a Shock Wave on the Wall of an Explosion Cavity with the Refraction of a Detonation Wave of Emulsion Explosives. Appl. Sci. 2021, 11, 3976. [Google Scholar] [CrossRef]
- Castedo, R.; Natale, M.; López, L.; Sanchidrian, J.; Santos, A.; Navarro, J.; Segarra, P. Estimation of Jones-Wilkins-Lee parameters of emulsion explosives using cylinder tests and their numerical validation. Int. J. Rock Mech. Min. Sci. 2018, 112, 290–301. [Google Scholar] [CrossRef]
- Isheyskiy, V.; Sanchidrián, J.A. Prospects of Applying MWD Technology for Quality Management of Drilling and Blasting Operations at Mining Enterprises. Minerals 2020, 10, 925. [Google Scholar] [CrossRef]
- Abdollahisharif, J.; Bakhtavar, E.; Nourizadeh, H. Green biocompatible approach to reduce the toxic gases and dust caused by the blasting in surface mining. Environ. Earth Sci. 2016, 75, 1–12. [Google Scholar] [CrossRef]
- Choi, S.-G.; Chang, I.; Lee, M.; Lee, J.-H.; Han, J.-T.; Kwon, T.-H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Zara, M.; Boersma, K.F.; Eskes, H.; van der Gon, H.D.; de Arellano, J.V.-G.; Krol, M.; van der Swaluw, E.; Schuch, W.; Velders, G.J. Reductions in nitrogen oxides over the Netherlands between 2005 and 2018 observed from space and on the ground: Decreasing emissions and increasing O3 indicate changing NOx chemistry. Atmos. Environ. X 2021, 9, 100104. [Google Scholar] [CrossRef]
- Kabanov, E.; Korshunov, G.; Gridina, E. Algorithmic provisions for data processing under spatial analysis of risk of accidents at hazardous production facilities. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2019, 6, 117–121. [Google Scholar] [CrossRef]
- Smirniakova, V.; Smirniakov, V.; Almosova, Y.; Kargopolova, A. “Vision Zero” Concept as a Tool for the Effective Occupational Safety Management System Formation in JSC “SUEK-Kuzbass”. Sustainability 2021, 13, 6335. [Google Scholar] [CrossRef]
- Gendler, S.G.; Tumanov, M.V.; Levin, L.Y. Principles for selecting, training and maintaining skills for safe work of personnel for mining industry enterprises. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2021, 2, 156–162. [Google Scholar] [CrossRef]
- Gendler, S.G.; Grishina, A.M.; Kochetkova, E.A. Optimization of expenditures for labor protection at deep mining. Eurasian Min. 2017, 2, 35–39. [Google Scholar] [CrossRef]
- Russian Federation. On Approval of Hygienic Standards GN 2.2.5.3532-18 MPC. Resolution of the Chief State Sanitary Doctor of the Russian Federation of February 13, 2018 No. 25; Collected Legislation of the Russian Federation: Moscow, Russia, 2018. [Google Scholar]
- Safe Work Australia. Standards for Workplace Exposure to Airborne Contaminants, National Standard of Australia; Safe Work Australia: Canberra, Australia, 2020; ISBN 978-1-76051-898-1.
- Queensland Government. Mining and Quarrying Safety and Health Act, 1999; Resources Safety and Health Queensland: Brisbane, Australia, 1999. Available online: https://www.legislation.qld.gov.au/view/html/inforce/current/act-1999-040 (accessed on 12 August 2021).
- Victorian Legislation. The Occupational Health and Safety Act (OHS Act), 2004, National Standard of Australia; Victorian Legislation: Melbourne, Australia, 2004. Available online: https://www.legislation.vic.gov.au/in-force/acts/occupational-health-and-safety-act-2004/036 (accessed on 12 August 2021).
- Katanov, I.B.; Skachilov, P.G. Improvement of the design of the borehole charge with foam-gel stemming. Bull. Kuzbass State Tech. Univ. 2015, 5, S43–S46. [Google Scholar]
- Murin, K.M. Damming as a factor in increasing the efficiency and safety of blasting operations. Min. Inf. Anal. Bull. Sci. Tech. J. 2011, 4, 390–395. [Google Scholar]
- Naboychenko, S.S. Experience in the use of salt stemming during blasting operations at the mine. Gottwald. Non-Ferr. Metall. 1964, 14, S58–S64. [Google Scholar]
- Mikhailov, V.A.; Beresnevich, P.V. Struggle with dust and poisonous gases during drilling and blasting operations in open pits. Nedra 1971, 262. [Google Scholar]
- Andreev, K.K.; Glazkov, A.P. A Comment to the Theory of Permissible Explosives. Dokl. Acad. Nauk. SSSR 1952, 86, 801. [Google Scholar]
- Andreev, K.K.; Purkaln, M.M. The explosion and the explosives. Title. Dokl. Acad. Nauk. SSSR 1946, 51, 444. [Google Scholar]
- Glazkova, A.P. Catalysis of Combustion of Explosives. Nauka 1976, 264. [Google Scholar]
- Kozlovskaya, T.F.; Chebenko, V.N.; Vitusko, O.N. Research of Catalysts Influence on Gas Burst Volume Decline during Detonation of Different Types of Explosives; Naukovyi Visnyk Natsionalnoho Hirnychoho Universytetu: Dnipro, Ukraine, 2010; pp. 11–12. [Google Scholar]
- Babkin, R.S.; Paramonov, G.P. Applicant and Patentee of the Federal State Budgetary Educational Institution of Higher Education “St. Petersburg Mining University”. Downhole Stemming. Russia: IPC51 F 42 D 1/08/. No. 2018120782. Patent 182481, 21 August 2018. [Google Scholar]
- Mainiero, R.J.; Rowland , J.H., III; Harris, M.L.; Sapko, M.J. Behavior of Nitrogen Oxides in the Product Gases from Explosive Detonations. In Proceedings of the Annual Conference on Explosives and Blasting Technique, Dallas, TX, USA, 29 January–1 February 2006; Volume 2, p. 10. [Google Scholar]

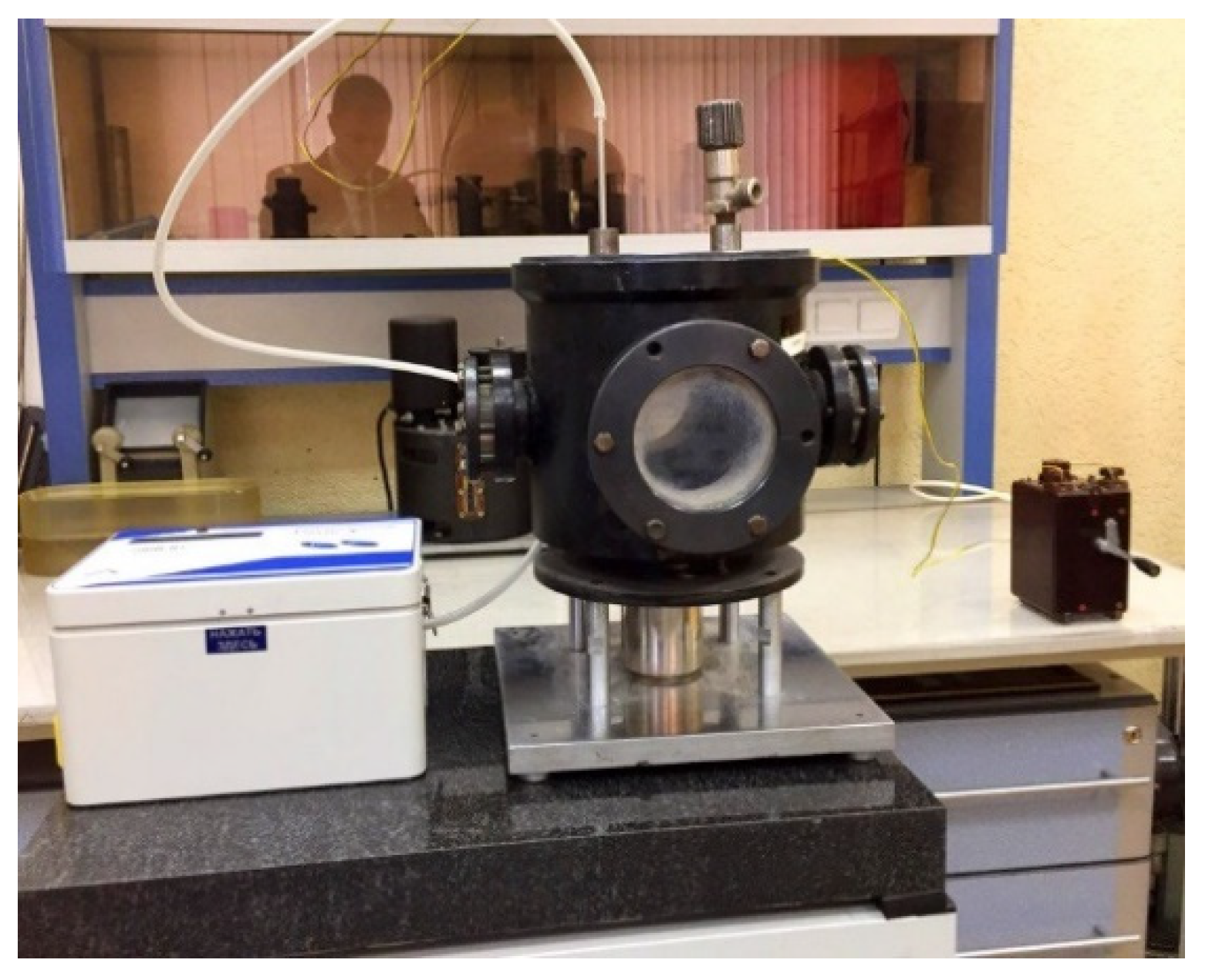


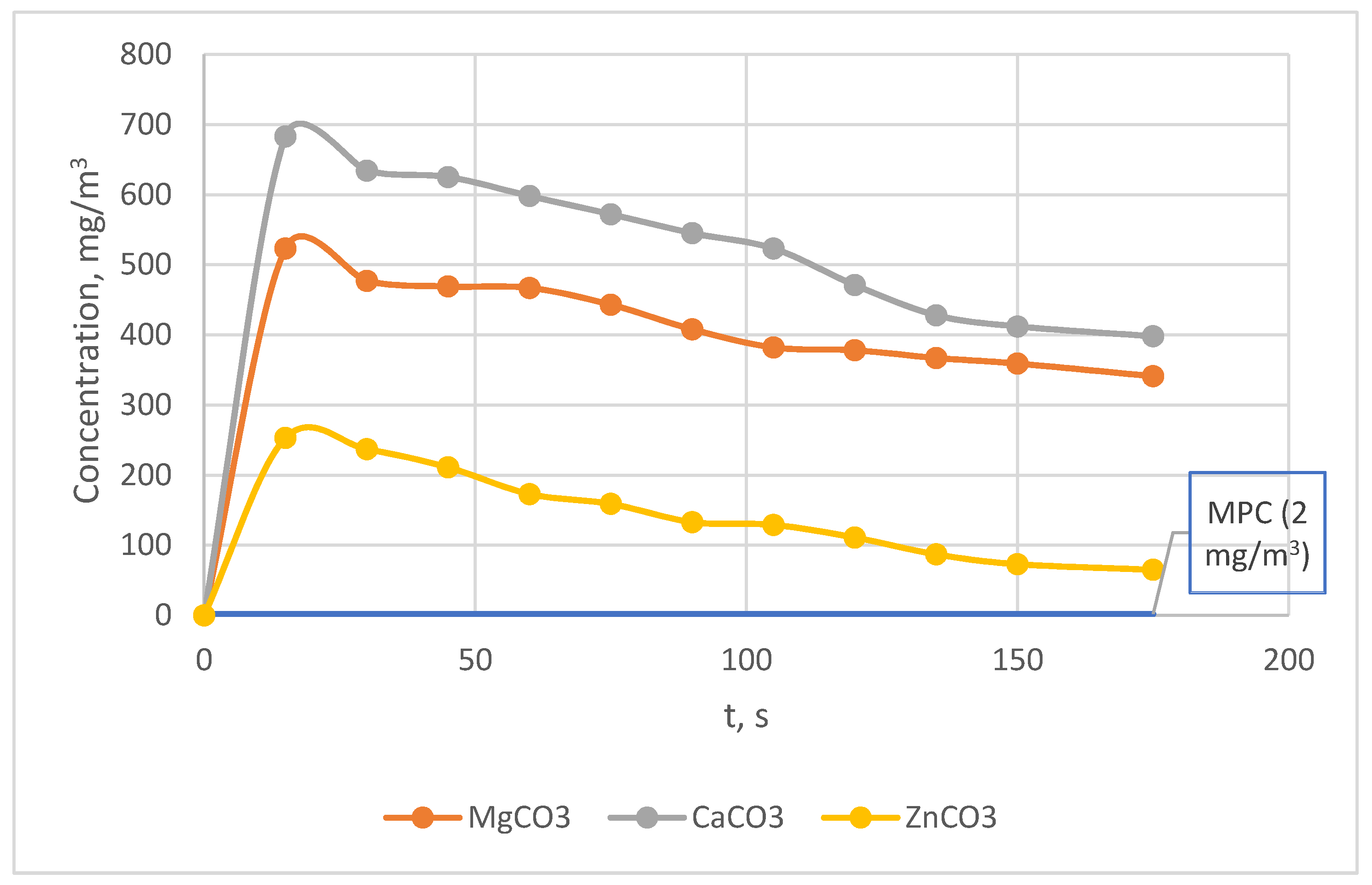

| t, s | Concentration NO2, mg/m3 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| 0 | 653 | 360 | 550 | 670 | 480 | |
| 15 | 579 | 560 | 470 | 580 | 440 | |
| 30 | 564 | 533 | 482 | 585 | 421 | |
| 45 | 548 | 521 | 451 | 530 | 389 | |
| 60 | 470 | 490 | 433 | 470 | 375 | |
| 75 | 454 | 430 | 412 | 468 | 343 | |
| 90 | 365 | 380 | 396 | 437 | 351 | |
| 105 | 345 | 355 | 365 | 402 | 328 | |
| 120 | 368 | 340 | 320 | 380 | 315 | |
| 135 | 283 | 290 | 301 | 391 | 289 | |
| 150 | 285 | 276 | 290 | 359 | 280 | |
| 165 | 250 | 244 | 255 | 345 | 276 | |
| 180 | 235 | 226 | 243 | 321 | 257 | |
| 195 | 213 | 221 | 218 | 298 | 255 | |
| 210 | 220 | 225 | 210 | 278 | 243 | |
| 225 | 212 | 218 | 206 | 266 | 232 | |
| 240 | 208 | 216 | 204 | 244 | 211 | |
| 255 | 210 | 216 | 208 | 238 | 202 |
| t, s | Concentration NO2, mg/m3 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| 0 | 380 | 402 | 398 | 356 | 412 | |
| 0 | 380 | 402 | 398 | 356 | 412 | |
| 15 | 360 | 387 | 402 | 334 | 400 | |
| 30 | 352 | 366 | 387 | 320 | 380 | |
| 45 | 334 | 340 | 366 | 334 | 376 | |
| 60 | 318 | 332 | 354 | 340 | 366 | |
| 75 | 306 | 323 | 348 | 345 | 340 | |
| 90 | 292 | 305 | 332 | 323 | 321 | |
| 105 | 280 | 298 | 320 | 320 | 310 | |
| 120 | 276 | 280 | 312 | 318 | 298 | |
| 135 | 264 | 282 | 295 | 315 | 290 | |
| 150 | 258 | 278 | 280 | 310 | 288 | |
| 165 | 240 | 266 | 275 | 287 | 280 | |
| 180 | 227 | 243 | 267 | 280 | 275 | |
| 195 | 219 | 233 | 265 | 270 | 276 | |
| 210 | 205 | 220 | 244 | 266 | 266 | |
| 225 | 198 | 221 | 232 | 246 | 235 | |
| 240 | 194 | 214 | 220 | 240 | 220 | |
| 255 | 188 | 206 | 214 | 233 | 221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudakov, M.; Babkin, R.; Medova, E. Improvement of Working Conditions of Mining Workers by Reducing Nitrogen Oxide Emissions during Blasting Operations. Appl. Sci. 2021, 11, 9969. https://doi.org/10.3390/app11219969
Rudakov M, Babkin R, Medova E. Improvement of Working Conditions of Mining Workers by Reducing Nitrogen Oxide Emissions during Blasting Operations. Applied Sciences. 2021; 11(21):9969. https://doi.org/10.3390/app11219969
Chicago/Turabian StyleRudakov, Marat, Ruslan Babkin, and Ekaterina Medova. 2021. "Improvement of Working Conditions of Mining Workers by Reducing Nitrogen Oxide Emissions during Blasting Operations" Applied Sciences 11, no. 21: 9969. https://doi.org/10.3390/app11219969
APA StyleRudakov, M., Babkin, R., & Medova, E. (2021). Improvement of Working Conditions of Mining Workers by Reducing Nitrogen Oxide Emissions during Blasting Operations. Applied Sciences, 11(21), 9969. https://doi.org/10.3390/app11219969








