Antiproliferative Activity of Stokesia laevis Ethanolic Extract in Combination with Several Food-Related Bioactive Compounds; In Vitro (Caco-2) and In Silico Docking (TNKS1 and TNKS2) Studies
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Analytical Aspects of Ethanolic Extracts from Stokesia laevis
Polyphenol’s Appraisal in Extracts
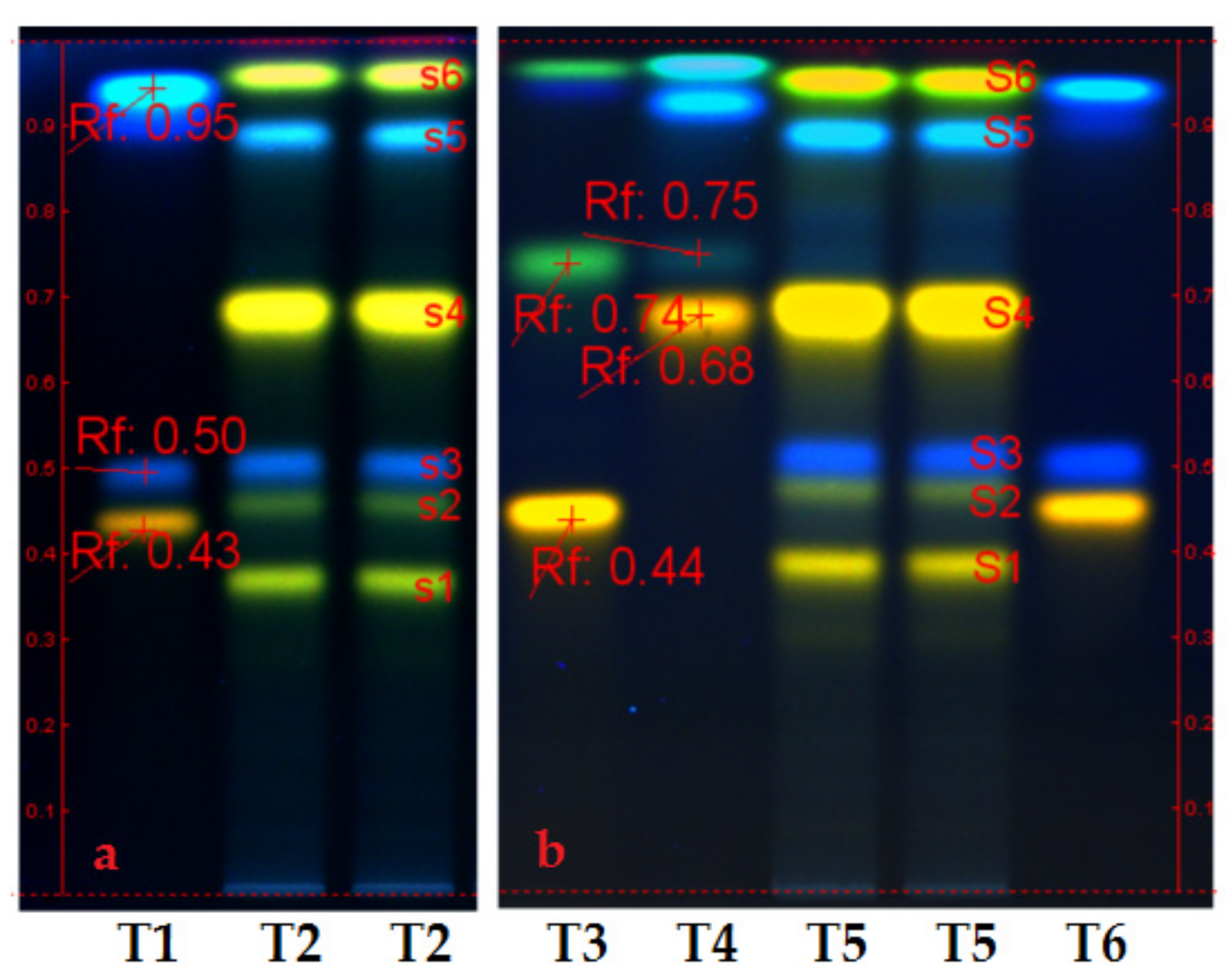
- Figure 1 (chromatograms a and b) presents the polyphenols profile of Slae and Slae26 ethanolic extracts. Therefore, HPTLC analyses of the two ethanolic extracts from Stokesia laevis plant material (Slae/a and Slae26/b) indicated identical qualitative aspects, and the prevalence of two main polyphenols subclasses: caffeic acid derivates (blue fluorescent/fl. spots s3 and s5), namely chlorogenic acid (s3) and isochlorogenic acid (s5), and several luteolin derivates (yellow fl. spots s1, s2, s4, and s6); the major compound in stokes aster ethanolic extracts is in the category of luteolin monoglycosides (punctually, luteolin-7-O-glucoside/s4). Additionally, HPTLC study suggests the presence of small quantities of ellagic acid in stokes aster, punctually the blue, fl. spot at the start point of chromatograms a and b, more evident in (70%) unrefined ethanolic extract Slae than in standardized (40%) ethanolic extract Slae26.
3.2. Pharmacological In Vitro Cytotoxicity and Antiproliferative Results
3.2.1. In Vitro Cytotoxicity MTS Results at 24 h and 48 h
- Cytotoxicity tests on Caco-2 cells have at the purpose to evaluate the effects of the five reference compounds (luteolin-7-O-glucoside, luteolin-8-C-glucoside, caffeic acid, gentisic acid and PABA) at punctual dilution series (10, 25, 50 and 100 μg/mL sample) comparatively to the solvent sample control series (70% ethanol series), and the negative control sample (blank), respectively. Thereby, the cytotoxicity tests also considered the evaluation of the effects of ethanol solvent in culture medium.
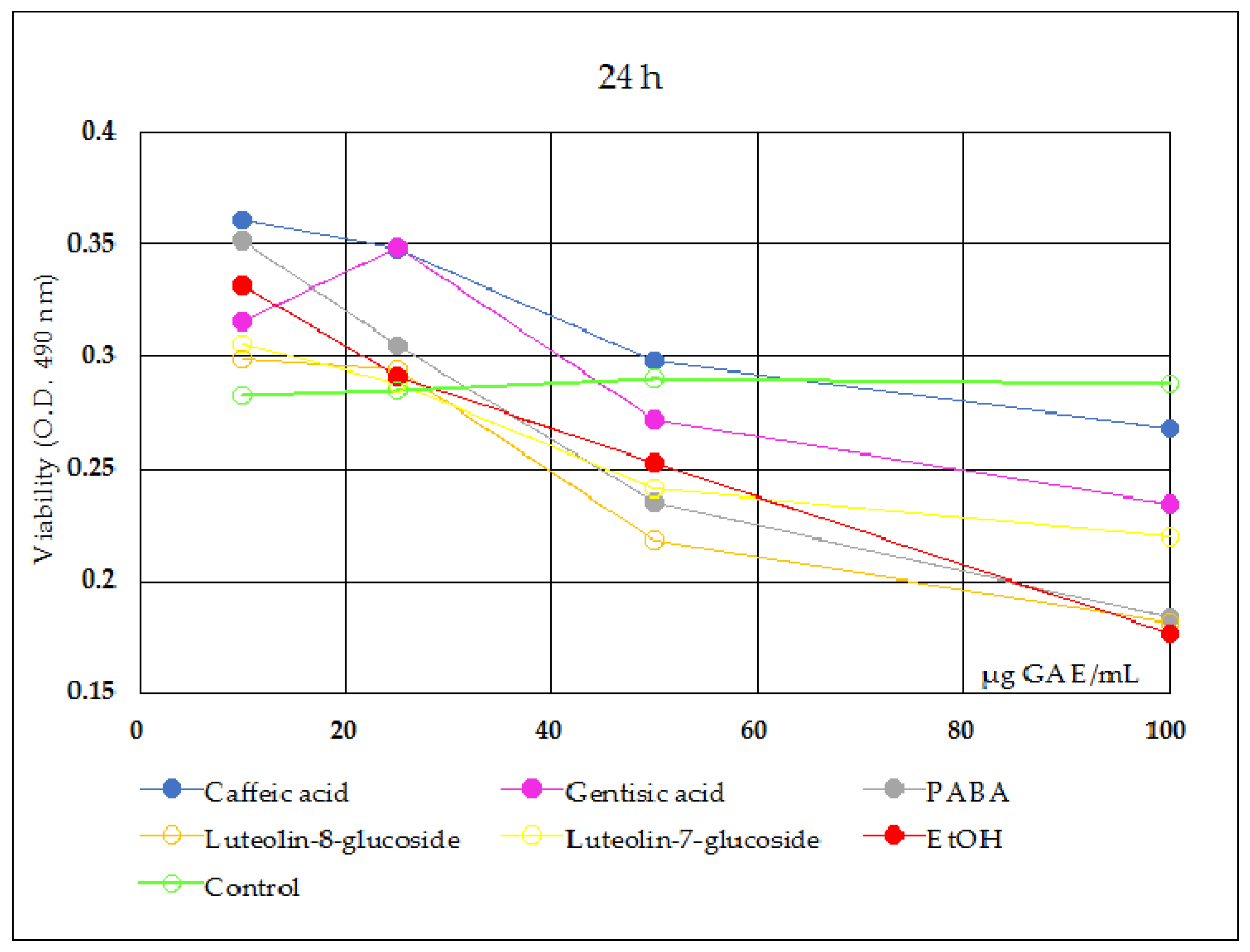
- The cytotoxicity results at 24 h (Figure 2) indicated that, in comparison with the negative control sample (blank), all reference compounds at concentrations smaller than 25 μg/mL induced a stimulatory effect upon the viability of Caco-2 cells, after which they induced an inhibitory effect. The cytotoxicity results on Caco-2 cell viability at 48 h (Figure 3) showed certain inhibitory effects for all reference compounds tested and at all point series, but considering the augmented inhibitory effects of the solvent sample control series (70% ethanol), the only conclusion to be drawn is the protective effect of luteolin-7-O-glucoside, caffeic acid and gentisic acid against the cytotoxic effects of ethanol in culture medium; in the concentration interval less than 50 μg/mL sample, caffeic acid and gentisic acid only were effective against the alcohol damages in the intestinal cells.
3.2.2. In Vitro Antiproliferative MTS Test Results at 48 h
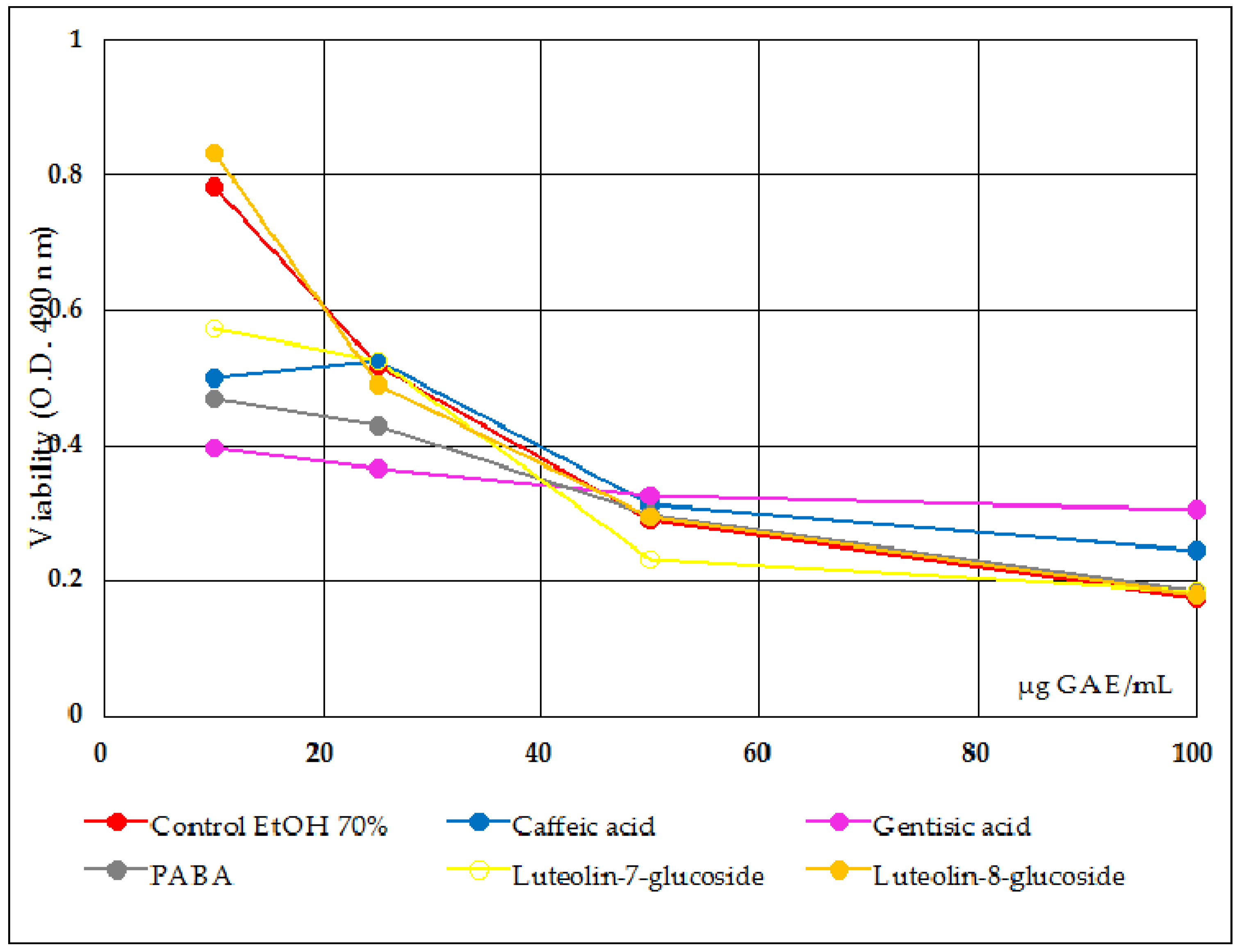
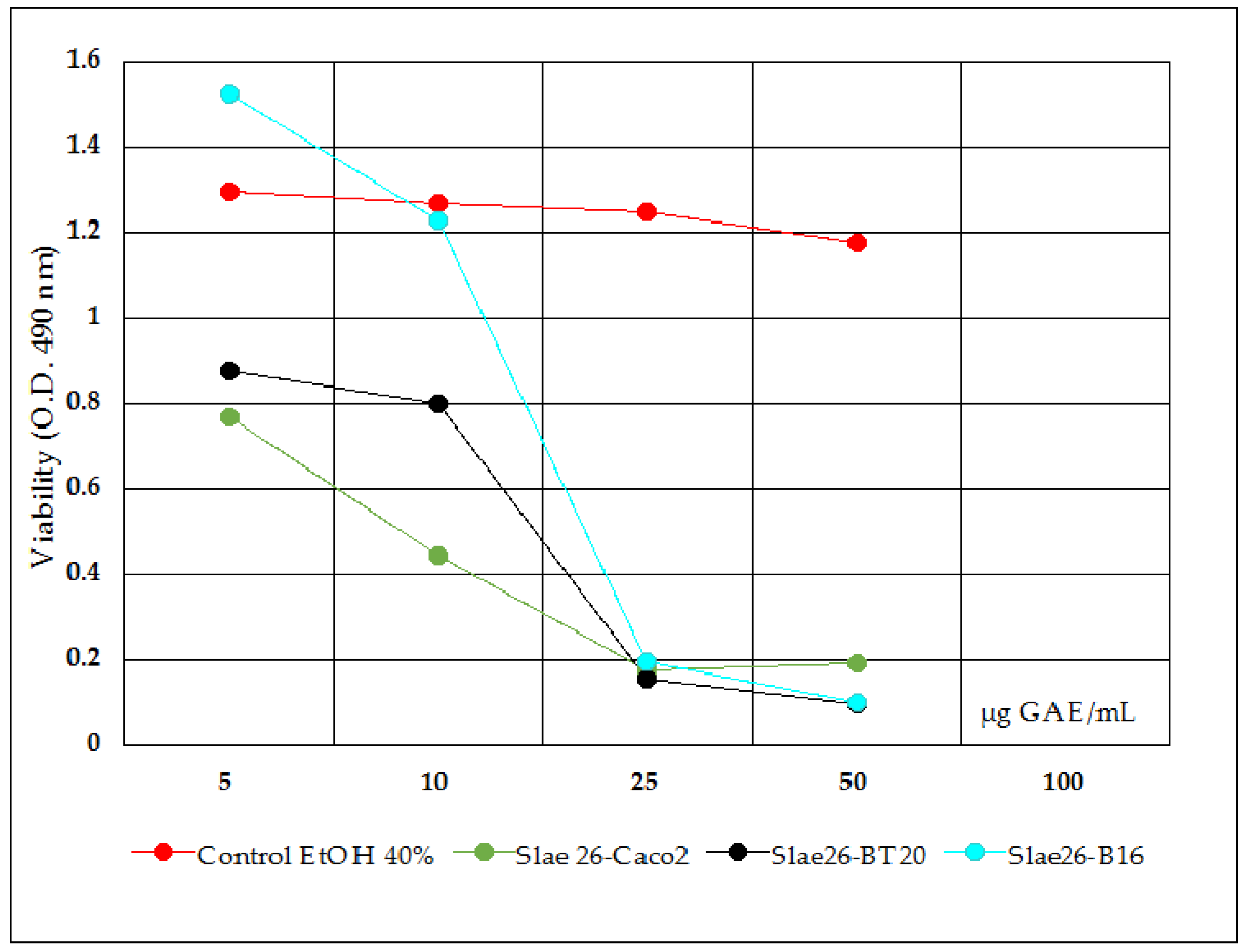
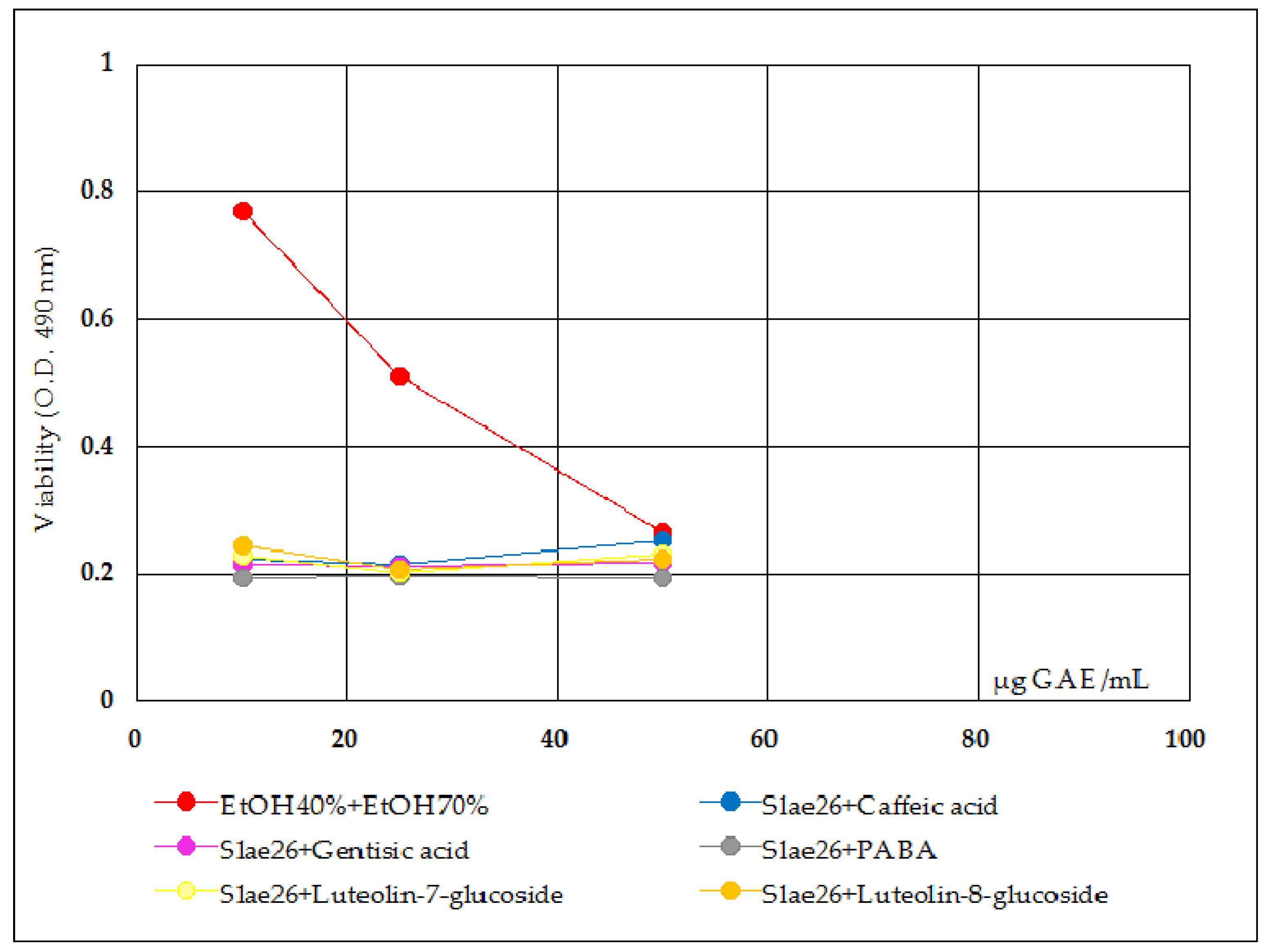
- Antiproliferative activity tests were done on Slae26, on the five polyphenols compounds, and on Slae26 combinations, 1:1 quantitative ratio (w/w), with the five polyphenols selected. Figure 4 presents antiproliferative activity of the five polyphenols compounds (ref.) on Caco-2 cells in comparison with the solvent sample control series (70% ethanol), 48 h after the treatment. Figure 5 presents antiproliferative activity of the test vegetal extract Slae26 on human colon cancer cell line Caco-2, and on human breast cancer cell line BT20 [33] and murine melanoma cell line B16 [34], in comparison with the solvent sample control series (40% ethanol), 48 h after the treatment. Figure 6 presents antiproliferative activity of the combinations of Slae26 with the five polyphenols compounds on human colon cancer cell line Caco-2, in comparison with the solvent sample control series (70% ethanol and 40% ethanol, 1:1, w/w), 48 h after the treatment.
- Therefore, referring to the antiproliferative potency of the five reference compounds tested (Figure 4), conclusions can be drawn only in the range of 10–25 μg/mL sample; after the threshold of 25 μg/mL sample, the effects being sensible alike to those of the control 70% ethanol sample series; at concentrations smaller than 25 μg/mL sample, gentisic acid, caffeic acid, p-aminobenzoic acid and luteolin-7-O-glucoside indicated certain inhibitory effects upon Caco-2 cells viability, therefore an antiproliferative activity against human tumor cancer cells Caco-2.
- Slae26 test sample in comparison with 40% ethanol sample control series (Figure 5) indicated certain inhibitory activity upon the viability of human tumor cancer cell Caco-2 (IC50 = 36 μg GAE/mL extract), similar to that previously found for human tumor breast cell BT20 (IC50 = 42 μg GAE/mL extract) [33] and murine melanoma cell line B16 (IC50 = 39 μg GAE/mL extract) [34].
- The antiproliferative MTS tests made on the combinations of Slae26 with the five reference compounds (Figure 6), and also in comparison with the control ethanol sample series (represented by 1:1 mixture between 40% ethanol from Slae26 sample and 70% ethanol from reference compounds samples) indicated their massive inhibitory effects upon the viability of Caco-2 cells and an IC50 value towards 5 μg active compounds per 1 mL sample. The results on Slae26 combinations with the five plant phenolics support the possibility of boosting the antiproliferative activity of plant-derived products with IC50 values < 50 μg active compounds/mL sample.
3.3. In Silico Evaluation of Reference Compounds by Docking Simulations
- Results of molecular docking study (Table 1) on human tankyrase 1 (PDB ID: 4W6E) have revealed the greatest inhibitory score (−104.15) for the Co-crystallized 3J5A ligand: four hydrogen bond formations with GLU1291 (x2), SER1221 and GLY1185 amino acid residues (see Supplementary Figure S1) were counted. It is noticed that luteolin-7-O-glucoside strongly interacted with SER1221, and GLY1185 by hydrogen bonding and additionally with GLY1196 and ASP1198, achieving a docking score value of −80.49, the best among the investigated ligands. Except for Luteolin-8-C-glcoside, all molecules interact with GLY1185 and SER1221 (see Supplementary Figures S2–S6), meaning satisfactory docking scores.
- Molecular docking simulations on the human tankyrase 2 (PDB ID: 4HKI) (Table 2) have revealed luteolin-7-O-glucoside the highest inhibiting potency, as suggests its docking score (−85.17), greater than that obtained for the native PDB ligand FLN (−76.97); punctually, luteolin-7-O-glucoside interacts by eleven hydrogen bonds with the amino acids residues SER1068 and GLY1032 on chain A (see Supplementary Figure S7), and additionally, amino acid GLU1138 on chain C (see Supplementary Figure S8). Furthermore, the results place the other screened ligands in order: caffeic acid (−57.17) > gentisic acid (−51.66) > p-aminobenzoic acid (−50.42) > luteolin-8-C-glcoside (−43.47); their interactions within the active binding site of tankyrase 2 are given in Supplementary Figures S9–S12. To better identify and depict their interactions within the complexes, in Figure S13, the atomic labeling schemes for investigated structures, as arbitrarily provided by Spartan software upon energy minimization, are given.
- Table 3 depicts Lipinski’s parameters for druggability assessment of the two native PDB inhibitors (3J5A and FLN), and of the five phenolics studied (ref.). The data indicate that the major difference between the two native ligands consists in the number of hydrogen bond acceptors (e.g., hydroxyl and amino -groups), HBA = 7 for 3J5 structure and HBA = 2 for FLN structure, partly explaining their significant difference in score’s magnitude and inhibition effectiveness.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harborne, J.B. Classes and Functions of Secondary Products from Plants. Chemicals from Plants, Perspectives on Secondary Plant Products; Walton, N.J., Brown, D.E., Eds.; Imperial College Press: London, UK, 1999; pp. 1–25. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Carrington, Y.; Guo, J.; Le, C.H.; Fillo, A.; Kwon, J.; Tran, L.T.; Ehlting, J. Evolution of a secondary metabolic pathway from primary metabolism: Shikimate and quinate biosynthesis in plants. Plant J. 2018, 95, 823–833. [Google Scholar] [CrossRef]
- Weng, J.K. The evolutionary paths towards complexity: A metabolic perspective. New Phytol. 2014, 201, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallarino, J.G.; de Abreu e Lima, F.; Soria, C.; Tong, H.; Pott, D.M.; Willmitzer, L.; Fernie, A.R.; Nikoloski, Z.; Osorio, S. Genetic diversity of strawberry germplasm using metabolomic biomarkers. Sci. Rep. 2018, 8, 14386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defense mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Kroymann, J. Natural diversity and adaptation in plant secondary metabolism. Curr. Opin. Plant. Biol. 2011, 14, 246–251. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Grabley, S.; Thiericke, R. Bioactive agents from natural sources: Trends in discovery and application. Adv. Biochem. Eng. Biotechnol. 1999, 64, 101–154. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Macarron, R. Critical review of the role of HTS in drug discovery. Drug Discov. Today 2006, 11, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.H.; Pichota, A.; Yin, Z. A practical view of ’druggability. Curr. Opin. Chem. Biol. 2006, 10, 357–361. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Neveu, V.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S-242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Williamson, G. Dietary intake, and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Microbial metabolites of ingested caffeic acid are absorbed by the monocarboxylic acid transporter (MCT) in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Neilson, A.P.; Ferruzzi, M.G. Influence of formulation and processing on absorption and metabolism of flavan-3-ols from tea and cocoa. Annu. Rev. Food Sci. Technol. 2011, 2, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Parine, N.R.; Govindan, N. Evaluation of in-vitro antioxidant and antibacterial properties of Commelina nudiflora L. extracts prepared by different polar solvents. Saudi J. Biol. Sci. 2015, 22, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkari, H.; Hajaji, S.; B’chir, F.; Rekik, M.; Gharbi, M. Correlation of polyphenolic content with radical-scavenging capacity and anthelmintic effects of Rubus ulmifolius (Rosaceae) against Haemonchus contortus. Vet. Parasitol 2016, 221, 46–53. [Google Scholar] [CrossRef]
- Bartmanska, A.; Walecka-Zacharska, E.; Tronina, T.; Poplonski, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef] [Green Version]
- Rasoanaivo, P.; Wright, C.V.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malaria J. 2011, 10, S4. [Google Scholar] [CrossRef] [Green Version]
- Pirvu, L.; Neagu, G.; Terchescu, I.; Albu, B.; Stefaniu, A. Comparative studies on two vegetal extracts from Stokesia laevis and Geranium pratense; polyphenols profile, cytotoxic effect and antiproliferative activity. Open Chem. 2020, 18, 488–502. [Google Scholar] [CrossRef]
- Neagu, G.; Stefaniu, A.; Albu, B.; Terchescu, I.; Pintilie, L.; Pirvu, L. Stokesia aster ethanolic extract activity on the normal and malignant murine cell line viability L969 and B16. Chem. Proc. 2021, 3, 318. [Google Scholar] [CrossRef]
- Romanian Pharmacopoeia, 10th ed.; Medicala: Bucharest, Romania, 1993.
- Wagner, H.; Bladt, S. Plant Drug Analysis. A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- Reikh, E.; Schibli, A. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants; Thieme Medical Pub.: Stuttgart, Germany, 2008. [Google Scholar] [CrossRef]
- Protocols & Applications Guide. Available online: www.promega.com (accessed on 2 August 2021).
- Pinto, D.; Gregorieff, A.; Begthel, H.; Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003, 17, 1709–1713. [Google Scholar] [CrossRef] [Green Version]
- Johannes, J.W.; Almeida, L.; Barlaam, B.; Boriack-Sjodin, P.A.; Casella, R.; Croft, R.A.; Dishington, A.P.; Gingipalli, L.; Gu, C.; Hawkins, J.L.; et al. Pyrimidinone Nicotinamide Mimetics as Selective Tankyrase and Wnt Pathway Inhibitors Suitable for in Vivo Pharmacology. ACS Med. Chem. Lett. 2015, 6, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Narwal, M.; Haikarainen, T.; Fallarero, A.; Vuorela, P.M.; Lehtio, L. Screening and structural analysis of flavones inhibiting tankyrases. J. Med. Chem. 2013, 56, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Molnar, L.F.; Jung, Y. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Haikarainen, T.; Krauss, S.; Lehtio, L. Tankyrases: Structure, Function and Therapeutic Implications in Cancer. Curr. Pharm. Des. 2014, 20, 6472–6488. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Emilsson, L.; Bozorg, S.R.; Nguyen, L.H.; Joshi, A.D.; Staller, K.; Nayor, J.; Chan, A.T.; Ludvigsson, J.F. Risk of colorectal cancer incidence and mortality after polypectomy: A Swedish record-linkage study. Lancet Gastroenterol. Hepatol. 2020, 5, 537–547. [Google Scholar] [CrossRef]
- Zamudio-Martinez, E.; Herrera-Campos, A.B.; Munoz, A.; Rodriguez-Vargas, J.M.; Olivier, F.V. Tankyrases as modulators of pro-tumoral functions: Molecular insights and therapeutic opportunities. J. Exp. Clin. Cancer Res. 2021, 40, 144. [Google Scholar] [CrossRef] [PubMed]
- Korb, O.; Stutzle, T.; Exner, T.E. Empirical Scoring Functions for Advanced Protein-Ligand Docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.A.; Ignacimuthu, S.; Michel, G.O.; Al Numair, K.S. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos Linn. Nutr. Cancer 2011, 63, 130–138. [Google Scholar] [CrossRef]
- Goodarzi, S.; Tabatebaei, M.J.; Jafari, R.M.; Shemirani, F.; Tavakoli, S.; Mofasseri, M.; Tofighi, Z. Cuminum cyminum fruits as source of luteolin- 7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2020, 34, 1602–1606. [Google Scholar] [CrossRef]
- Palombo, R.; Caporali, S.; Falconi, M.; Iacovelli, F.; Della Roca, B.M.; Lo Surdo, A.; Campione, E.; Candi, E.; Melino, G.; Bernardini, S.; et al. Luteolin-7-O-β-d-Glucoside Inhibits Cellular Energy Production Interacting with HEK2 in Keratinocytes. Int. J. Mol. Sci. 2019, 20, 2689. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Kim, J.H.; Lee, D.H.; Kang, J.W.; Song, H.H.; Oh, S.R.; Yoon, D.Y. Luteolin 8-C-β-fucopyranoside inhibits invasion and suppresses TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-κB signaling in MCF-7 breast cancer cells. Biochimie 2013, 95, 2082–2090. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.H.; Lee, J.; Chun, H.; Choi, M.K.; Yoon, J.H.; Pham, T.H.; Kim, K.H.; Kwon, T.; Ryu, H.W.; et al. Differential effects of luteolin and its glycosides on invasion and apoptosis in MDA-MB-231 triple-negative breast cancer cells. EXCLI J. 2019, 18, 750–763. [Google Scholar] [CrossRef]
- Devipriya, N.; Sudheer, A.R.; Menon, V.P. Caffeic acid protects human peripheral blood lymphocytes against gamma radiation-induced cellular damage. J. Biochem. Mol. Toxicol. 2008, 22, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sana, N.; Sheikh, T.A.; Wani, A.W.; Summya, R.; Nemat, A.; Sarwat, S. Modulatory effects of gentisic acid against genotoxicity and hepatotoxicity induced by cyclophosphamide in Swiss albino mice. J. Pharm. Pharmacol. 2012, 64, 259–267. [Google Scholar] [CrossRef]
- Joshi, R.; Gangabhagirathi, R.; Venu, S.; Adhikari, S.; Mukherjee, T. Antioxidant activity and free radical scavenging reactions of gentisic acid: In-Vitro and pulse radiolysis studies. Free Radic. Res. 2012, 46, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef]
- Daeschel, M.S. Antimicrobial substance in lactic acid bacteria for use as food preservatives. Food Technol. 1997, 1, 164–167. [Google Scholar]
- Kalpana, V.N.; Devi Rajeswari, V. Chapter 1—Preservatives in Beverages: Perception and Needs. In Preservatives and Preservation Approaches in Beverages, Volume 15: The Science of Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 1–30. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoo, M.Y.; Paik, H.D.; Lim, S.D. Production of benzoic acid as a natural compound in fermented skim milk using commercial cheese starter. J. Dairy Sci. 2017, 100, 4269–4275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitarek, P.; Merecz-Sadowska, A.; Sliwinnski, T.; Zajdel, R.; Kowalczyk, T. An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines. Cancer 2020, 12, 2957. [Google Scholar] [CrossRef]
- Donadu, M.G.; Usai, D.; Mazzarello, V.; Molicotti, P.; Cannas, S.; Bellardi, M.G.; Zanetti, S. Change in Caco-2 cells following treatment with various lavender essential oils. Nat. Prod. Res. 2017, 31, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- Jenab, A.; Roghanian, R.; Emtiazi, G. Bacterial Natural Compounds with Anti-Inflammatory and Immunomodulatory Properties (Mini Review). Drug Des. Dev. Ther. 2020, 14, 3787–3801. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Hsieh, M.J.; Wu, C.R.; Peng, W.H.; Hsieh, M.T.; Hsieh, C.C. The Synergistic Effect of Antioxidant Interaction between Luteolin and chlorogenic Acid in Lonicera japonica. bioRxiv 2018. [Google Scholar] [CrossRef]
- Borse, S.P.; Singh, D.P.; Upadhyay, D.; Nivsarkar, M. Potential synergistic effects of quercetin with other phytoconstituents of Costus pictus (insulin plant) extract in the control of hyperglycemia and prevention of NSAID-induced gastroenteropathy in diabetic rats. Food. Chem. Toxicol. 2018, 120, 448–461. [Google Scholar] [CrossRef]

| Ligand | Score * | RMSD | Hydrogen Bond | Length (Å) |
|---|---|---|---|---|
| Co-crystallized 3J5 (2-(4-{6-[(3S)-3,4-dimethylpiperazin-1-yl]-4-methylpyridin-3-yl}phenyl)-8-(hydroxymethyl)quinazolin-4(3H)-one) | −104.15 | 0.16 | O1 sp3–Osp2 GLU1291 | 3.393 |
| O1 sp3–Osp2 GLU1291 | 3.250 | |||
| O sp2–Osp3 SER1221 | 2.778 | |||
| O sp2–Nsp2GLY1185 | 2.895 | |||
| Luteolin-7-O-glucoside | −80.49 | 0.69 | O10 sp3–Osp2 GLY1196 | 3.284 |
| O10 sp3–Nsp2 ASP1198 | 2.729 | |||
| O11 sp3–Nsp2 ASP1198 | 2.741 | |||
| O11 sp3–Osp2 ASP1198 | 3.067 | |||
| O5 sp3–Osp2 GLY1185 | 2.456 | |||
| O3 sp3–Osp2 GLY1185 | 3.090 | |||
| O3 sp3–Nsp2 GLY1185 | 2.647 | |||
| O3 sp3–Osp3 SER1221 | 3.040 | |||
| O4 sp3–Osp3 SER1221 | 3.087 | |||
| O4 sp3–Nsp2 SER1221 | 3.298 | |||
| Luteolin-8-C-glucoside | −61.88 | 0.02 | O11 sp3–Nsp2 ASP1198 | 2.992 |
| O9 sp2–Nsp2 TYR1213 | 3.034 | |||
| O4 sp3–Nsp2 HIS1201 | 2.920 | |||
| O3 sp3–Osp2 ALA1202 | 3.129 | |||
| O6 sp3–Nsp2 HIS1201 | 2.817 | |||
| Caffeic acid | −58.55 | 0.15 | O3 sp3–Osp3 TYR1224 | 3.050 |
| O3 sp3–Osp2 GLU1291 | 2.912 | |||
| O2 sp3–Osp2 TYR1213 | 3.364 | |||
| O2 sp3–Nsp2 HIS1184 | 3.089 | |||
| O2 sp3–Nsp2 GLY1185 | 2.925 | |||
| O2 sp3–Osp2 GLY1185 | 3.022 | |||
| Gentisic acid | −51.02 | 0.01 | O1 sp3–Osp3 TYR1224 | 3.142 |
| O0 sp3–Osp2 TYR1213 | 3.212 | |||
| O0 sp3–Osp3 SER1221 | 2.880 | |||
| O2 sp3–Nsp2 HIS1184 | 3.196 | |||
| O2 sp3–Nsp2 GLY1185 | 3.123 | |||
| O2 sp3–Osp2 GLY1185 | 2.829 | |||
| p-aminobenzoic acid | −49.16 | 0.17 | N2 sp3–Osp3 TYR1224 | 3.092 |
| O0 sp3–Osp2 TYR1213 | 3.087 | |||
| O0 sp3–Nsp2 ALA1225 | 3.270 | |||
| O0 sp3–Osp3 SER1221 | 2.678 | |||
| O0 sp3–Osp2 PHE1183 | 2.989 | |||
| O1 sp2–Nsp2 HIS1184 | 3.037 | |||
| O1 sp2–Nsp2 GLY1185 | 3.001 |
| Ligand | Score | RMSD | Hydrogen Bond | Length (Å) |
|---|---|---|---|---|
| Co-crystallized FLN (2-phenyl-4h-chromen-4-one) | −76.97 | 0.02 | O4 sp2–Osp3 SER1068(A) | 2.850 |
| O4 sp2–Nsp2 GLY1032(A) | 3.029 | |||
| Luteolin-7-O-glucoside | −85.17 | 0.55 | O11 sp3–Osp2 ALA1049(A) | 3.203 |
| O10 sp3–Osp2 HIS1048(A) | 3.088 | |||
| O10 sp3–Osp3 SER1033(A) | 3.075 | |||
| O9 sp2–Nsp2 GLY1032(A) | 3.176 | |||
| O9 sp2–Osp3 SER1068(A) | 3.007 | |||
| O8 sp3–Osp3 SER1068(A) | 3.016 | |||
| O2 sp3–Osp3 GLU1138(C) | 3.189 | |||
| O2 sp3–Nsp3 LYS1067(A) | 2.522 | |||
| O6 sp3–Nsp2 MET1054(A) | 3.202 | |||
| O6 sp3–Osp2 GLY1052(A) | 3.397 | |||
| O6 sp3–Osp3 TYR1050(A) | 3.104 | |||
| Luteolin-8-C-glucoside | −43.47 | 0.67 | O11 sp3–Osp2 GLY1032(A) | 2.535 |
| O9 sp2–Nsp2 ILE1051(A) | 3.075 | |||
| O2 sp3–Nsp2 ILE1075(A) | 2.875 | |||
| O3 sp3–Osp2 TYR1073(A) | 3.256 | |||
| Caffeic acid | −57.17 | 0.05 | O3 sp3–Osp2 TYR1071(A) | 3.003 |
| O4 sp2–Osp3 GLU1138(C) | 2.651 | |||
| O1 sp3–Nsp2 ALA1062(A) | 3.217 | |||
| O1 sp3–Osp2 PHE1030(A) | 3.016 | |||
| O1 sp3–Osp3 SER1068(A) | 2.397 | |||
| O2 sp3–Nsp2 HIS1031(A) | 2.988 | |||
| O2 sp3–Nsp2 GLY1032(A) | 2.636 | |||
| O2 sp3–Osp2 GLY1032(A) | 3.005 | |||
| Gentisic acid | −51.66 | 0.02 | O1 sp3–Osp3 TYR1071(A) | 3.118 |
| O2 sp3–Osp2 GLY1032(A) | 2.873 | |||
| O2 sp3–Nsp2 GLY1032(A) | 3.044 | |||
| O2 sp3–Nsp2 HIS1031(A) | 3.158 | |||
| O0 sp3–Osp3 SER1068(A) | 2.800 | |||
| O0 sp3–Osp2 PHE1030(A) | 3.258 | |||
| O0 sp3–Osp2 TYR1060(A) | 3.1817 | |||
| p-aminobenzoic acid | −50.42 | 0.16 | O0 sp3–Osp3 GLU1138(C) | 2.848 |
| O1 sp2–Osp3 TYR1071(A) | 3.048 | |||
| N2 sp3–Nsp2 HIS1031(A) | 2.987 | |||
| N2 sp3–Nsp2 GLY1032(A) | 2.650 | |||
| N2 sp3–Osp2 GLY1032(A) | 2.829 |
| Ligand | HBD | HBA | LogP | LV | rb |
|---|---|---|---|---|---|
| Co-crystallized 3J5A (PDB ID: 4W6E) | 1 | 7 | 4.66 | 0 | 4 |
| Co-crystallized FLN (PDB ID: 4HKI) | 0 | 2 | 4.80 | 0 | 1 |
| Luteolin-7-O-glucoside | 7 | 11 | 1.57 | 2 | 4 |
| Luteolin-8-C-glucoside | 8 | 11 | −0.70 | 2 | 3 |
| Caffeic acid | 3 | 4 | 1.58 | 0 | 2 |
| Gentisic acid | 3 | 4 | 1.49 | 0 | 1 |
| p-aminobenzoic acid | 3 | 3 | 0.97 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, G.; Stefaniu, A.; Albulescu, A.; Pintilie, L.; Pirvu, L.C. Antiproliferative Activity of Stokesia laevis Ethanolic Extract in Combination with Several Food-Related Bioactive Compounds; In Vitro (Caco-2) and In Silico Docking (TNKS1 and TNKS2) Studies. Appl. Sci. 2021, 11, 9944. https://doi.org/10.3390/app11219944
Neagu G, Stefaniu A, Albulescu A, Pintilie L, Pirvu LC. Antiproliferative Activity of Stokesia laevis Ethanolic Extract in Combination with Several Food-Related Bioactive Compounds; In Vitro (Caco-2) and In Silico Docking (TNKS1 and TNKS2) Studies. Applied Sciences. 2021; 11(21):9944. https://doi.org/10.3390/app11219944
Chicago/Turabian StyleNeagu, Georgeta, Amalia Stefaniu, Adrian Albulescu, Lucia Pintilie, and Lucia Camelia Pirvu. 2021. "Antiproliferative Activity of Stokesia laevis Ethanolic Extract in Combination with Several Food-Related Bioactive Compounds; In Vitro (Caco-2) and In Silico Docking (TNKS1 and TNKS2) Studies" Applied Sciences 11, no. 21: 9944. https://doi.org/10.3390/app11219944
APA StyleNeagu, G., Stefaniu, A., Albulescu, A., Pintilie, L., & Pirvu, L. C. (2021). Antiproliferative Activity of Stokesia laevis Ethanolic Extract in Combination with Several Food-Related Bioactive Compounds; In Vitro (Caco-2) and In Silico Docking (TNKS1 and TNKS2) Studies. Applied Sciences, 11(21), 9944. https://doi.org/10.3390/app11219944







