Synergistic Effects and Toxic Mechanism of Phosphine with Ethyl Formate against Citrus Mealybug (Planococcus citri)
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Breeding Conditions
2.2. Chemicals
2.3. Fumigation Bioassay and Calculation of Lethal Concentration-Time (LCT)
- -
- EF: EF fumigation was performed in a concentration range of 1.5 to 40.57 mg/L for 4 h. Gas was sampled at 0.5, 1, 2, and 4 h post fumigation and analyzed using a flame ionization detector.
- -
- PH3: PH3 fumigation was performed in a concentration range of 0.005 to 0.102 mg/L for 20 h. Gas was sampled at 0.5, 1, 2, 4, 18, and 20 h post fumigation and analyzed using a flame photometric detector.
2.4. Synergistic Effect of EF and PH3
2.5. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.6. Protein Extraction and Enzyme Assay
2.7. Proteomic Analysis
2.8. Lipid Extraction and Lipidomics Analysis Using MALDI-TOF MS
3. Results
3.1. Individual Efficacy of EF and PH3 against P. citri
3.2. Enzymatic Responses at Each Stage of Development by Individual Treatment of EF and PH3
3.3. Gene Expression Responses
3.4. Synergistic Effect of EF and PH3 against P. citri
3.5. Omics Analysis Using Proteomics and Metabolomics Techniques
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.; Lee, Y.H.; Kim, G.; Lee, B.H.; Yang, J.O.; Lee, S.E. Ethyl formate and phosphine fumigations on the two-spotted spider mite, Tetranychus urticae and their biochemical responses. Appl. Biol. Chem. 2019, 62, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Yang, J.O.; Sung, J.Y.; Lee, J.Y.; Park, J.S.; Lee, H.S.; Lee, B.H.; Ren, Y.; Lee, D.W.; Lee, S.E. Minimization of energy transduction confers resistance to phosphine in the rice weevil, Sitophilus oryzae. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Park, M.G.; Park, C.G.; Yang, J.O.; Kim, G.H.; Ren, Y.L.; Lee, B.H.; Cha, D.H. Ethyl Formate as a Methyl Bromide Alternative for Phytosanitary Disinfestation of Imported Banana in Korea With Logistical Considerations. J. Econ. Entomol. 2020, 113, 1711–1717. [Google Scholar] [CrossRef]
- De Cal, A.; Martinez-Treceno, A.; Salto, T.; Lopez-Aranda, J.M.; Melgarejo, P. Effect of chemical fumigation on soil fungal communities in Spanish strawberry nurseries. Appl. Soil Ecol. 2005, 28, 47–56. [Google Scholar] [CrossRef]
- Wood, J.P.; Wendling, M.; Richter, W.; Lastivka, A.; Mickelsen, L. Evaluation of the Efficacy of Methyl Bromide in the Decontamination of Building and Interior Materials Contaminated with Bacillus anthracis Spores. Appl. Environ. Microb. 2016, 82, 2003–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gareau, B.J. Lessons from the Montreal Protocol delay in phasing out methyl bromide. J. Environ. Stud. Sci. 2015, 5, 163–168. [Google Scholar] [CrossRef]
- Yang, J.; Park, Y.; Hyun, I.H.; Kim, G.H.; Kim, B.S.; Lee, B.H.; Ren, Y. A Combination Treatment Using Ethyl Formate and Phosphine to Control Planococcus citri (Hemiptera: Pseudococcidae) on Pineapples. J. Econ. Entomol. 2016, 109, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.G.; White, N.D.G. Alternatives to methyl bromide treatments for stored-product and quarantine insects. Annu. Rev. Entomol. 2002, 47, 331–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opit, G.P.; Phillips, T.W.; Aikins, M.J.; Hasan, M.M. Phosphine Resistance in Tribolium castaneum and Rhyzopertha dominica From Stored Wheat in Oklahoma. J. Econ. Entomol. 2012, 105, 1107–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, P.J.; Falk, M.G.; Nayak, M.K.; Emery, R.N.; Holloway, J.C. Monitoring resistance to phosphine in the lesser grain borer, Rhyzopertha dominica, in Australia: A national analysis of trends, storage types and geography in relation to resistance detections. J. Stored Prod. Res. 2017, 70, 25–36. [Google Scholar] [CrossRef]
- Schlipalius, D.I.; Valmas, N.; Tuck, A.G.; Jagadeesan, R.; Ma, L.; Kaur, R.; Goldinger, A.; Anderson, C.; Kuang, J.J.; Zuryn, S.; et al. A Core Metabolic Enzyme Mediates Resistance to Phosphine Gas. Science 2012, 338, 807–810. [Google Scholar] [CrossRef]
- Agrafioti, P.; Athanassiou, C.G.; Nayak, M.K. Detection of phosphine resistance in major stored-product insects in Greece and evaluation of a field resistance test kit. J. Stored Prod. Res. 2019, 82, 40–47. [Google Scholar] [CrossRef]
- Hubhachen, Z.; Jiang, H.B.; Schlipalius, D.; Park, Y.; Guedes, R.N.C.; Oppert, B.; Opit, G.; Phillips, T.W. A CAPS marker for determination of strong phosphine resistance in Tribolium castaneum from Brazil. J. Pest. Sci. 2020, 93, 127–134. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, H.Y. A 90-Day Inhalation Toxicity Study of Ethyl Formate in Rats. Toxicol. Res. 2017, 33, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Zaitoon, A.; Lim, L.T.; Scott-Dupree, C. Synthesis and Characterization of Ethyl Formate Precursor for Activated Release Application. J. Agric. Food Chem. 2019, 67, 13914–13921. [Google Scholar] [CrossRef]
- Lee, B.H.; Park, C.G.; Park, M.G.; Roh, G.H.; Kim, D.; Riddick, E.W.; Chen, J.; Cha, D.H. Ethyl formate fumigation for the disinfestation of red imported fire ants Solenopsis invicta Buren. J. Asia-Pac. Entomol. 2019, 22, 838–840. [Google Scholar] [CrossRef]
- Kim, B.S.; Shin, E.M.; Park, Y.J.; Yang, J.O. Susceptibility of the Cigarette Beetle Lasioderma serricorne (Fabricius) to Phosphine, Ethyl Formate and Their Combination, and the Sorption and Desorption of Fumigants on Cured Tobacco Leaves. Insects 2020, 11, 599. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.K.; Kyung, Y.; Park, G.H.; Lee, B.H.; Yang, J.O.; Koo, H.N.; Kim, G.H. Fumigation Activity of Ethyl Formate and Phosphine Against Tetranychus urticae (Acari: Tetranychidae) on Imported Sweet Pumpkin. J. Econ. Entomol. 2018, 111, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, J.S.; Lee, H.; Kwon, M.; Kim, G.-H.; Kim, J. Identification of a phosphine resistance mechanism in Rhyzopertha dominica based on transcriptome analysis. J. Asia-Pac. Entomol. 2018, 21, 1450–1456. [Google Scholar] [CrossRef]
- Franco, J.C.; Zada, A.; Mendel, Z. Novel Approaches for the Management of Mealybug Pests; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 233–278. [Google Scholar]
- Mansour, R.; Grissa-Lebdi, K.; Suma, P.; Mazzeo, G.; Russo, A. Key Scale Insects (Hemiptera: Coccoidea) of High Economic Importance in a Mediterranean Area: Host Plants, Bio-Ecological Characteristics, Natural Enemies and Pest Management Strategies—A Review. Plant Prot. Sci. 2017, 53, 1–14. [Google Scholar] [CrossRef]
- Bond, E.J.; Food and Agriculture Organization of the United Nations. Manual of Fumigation for Insect Control; Food and Agriculture Organization of the United Nations: Rome, Italy, 1984. [Google Scholar]
- Plackett, R.L.; Hewlett, P.S. A Unified Theory for Quantal Responses to Mixtures of Drugs: The Fitting to Data of Certain Models for Two Non-Interactive Drugs with Complete Positive Correlation of Tolerances. Biometrics 1963, 19, 517–531. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Campian, J.L.; Gao, X.; Qian, M.; Eaton, J.W. Cytochrome C oxidase activity and oxygen tolerance. J. Biol. Chem. 2007, 282, 12430–12438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [CrossRef]
- Mackness, M.I.; Walker, C.H.; Rowlands, D.G.; Price, N.R. Esterase activity in homogenates of three strains of the rust red flour beetle Tribolium castaneum (Herbst). Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1983, 74, 65–68. [Google Scholar] [CrossRef]
- Searle, B.C. Scaffold: A bioinformatic tool for validating MS/MS-based proteomic studies. Proteomics 2010, 10, 1265–1269. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Franco, J.C.; Suma, P.; da Silva, E.B.; Blumberg, D.; Mendel, Z. Management strategies of mealybug pests of citrus in mediterranean countries. Phytoparasitica 2004, 32, 507. [Google Scholar] [CrossRef]
- Mansour, R.; Belzunces, L.P.; Suma, P.; Zappala, L.; Mazzeo, G.; Grissa-Lebdi, K.; Russo, A.; Biondi, A. Vine and citrus mealybug pest control based on synthetic chemicals. A review. Agron. Sustain. Dev. 2018, 38, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Q.; Ren, Y.L.; Emery, R.; Newman, J.; Agarwal, M. Evaluation of Ethyl Formate, Phosphine, and Their Combination to Disinfest Harvested Celery against Purple Scum Springtails. Horttechnology 2018, 28, 492–501. [Google Scholar] [CrossRef]
- Kim, K.; Lee, B.H.; Park, J.S.; Yang, J.O.; Lee, S.E. Biochemical mechanisms of fumigant toxicity by ethyl formate towards Myzus persicae nymphs. J. Appl. Biol. Chem. 2017, 60, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Brandt, M.; Silva, A.X.; Trionnaire, G.L.; Tagu, D.; Figueroa, C.C. Transcriptomic responses of the aphid Myzus persicae nicotianae Blackman (Hemiptera: Aphididae) to insecticides: Analyses in the single Chilean clone of the tobacco aphid. Chil. J. Agric. Res. 2014, 74, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Del Duca, S.; Riccardi, C.; Vassallo, A.; Fontana, G.; Castronovo, L.M.; Chioccioli, S.; Fani, R. The Histidine Biosynthetic Genes in the Superphylum Bacteroidota-Rhodothermota-Balneolota-Chlorobiota: Insights into the Evolution of Gene Structure and Organization. Microorganisms 2021, 9, 1439. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Salazar, A.; Becerra, A.; Lazcano, A. Evolutionary convergence in the biosyntheses of the imidazole moieties of histidine and purines. PLoS ONE 2018, 13, e0196349. [Google Scholar] [CrossRef] [Green Version]
- Oppold, A.; Kress, A.; Vanden Bussche, J.; Diogo, J.B.; Kuch, U.; Oehlmann, J.; Vandegehuchte, M.B.; Muller, R. Epigenetic alterations and decreasing insecticide sensitivity of the Asian tiger mosquito Aedes albopictus. Ecotoxicol. Environ. Saf. 2015, 122, 45–53. [Google Scholar] [CrossRef]
- Hart, R.J.; Abraham, A.; Aly, A.S.I. Genetic Characterization of Coenzyme A Biosynthesis Reveals Essential Distinctive Functions during Malaria Parasite Development in Blood and Mosquito. Front. Cell Infect. Microbiol. 2017, 20, 260. [Google Scholar] [CrossRef] [Green Version]

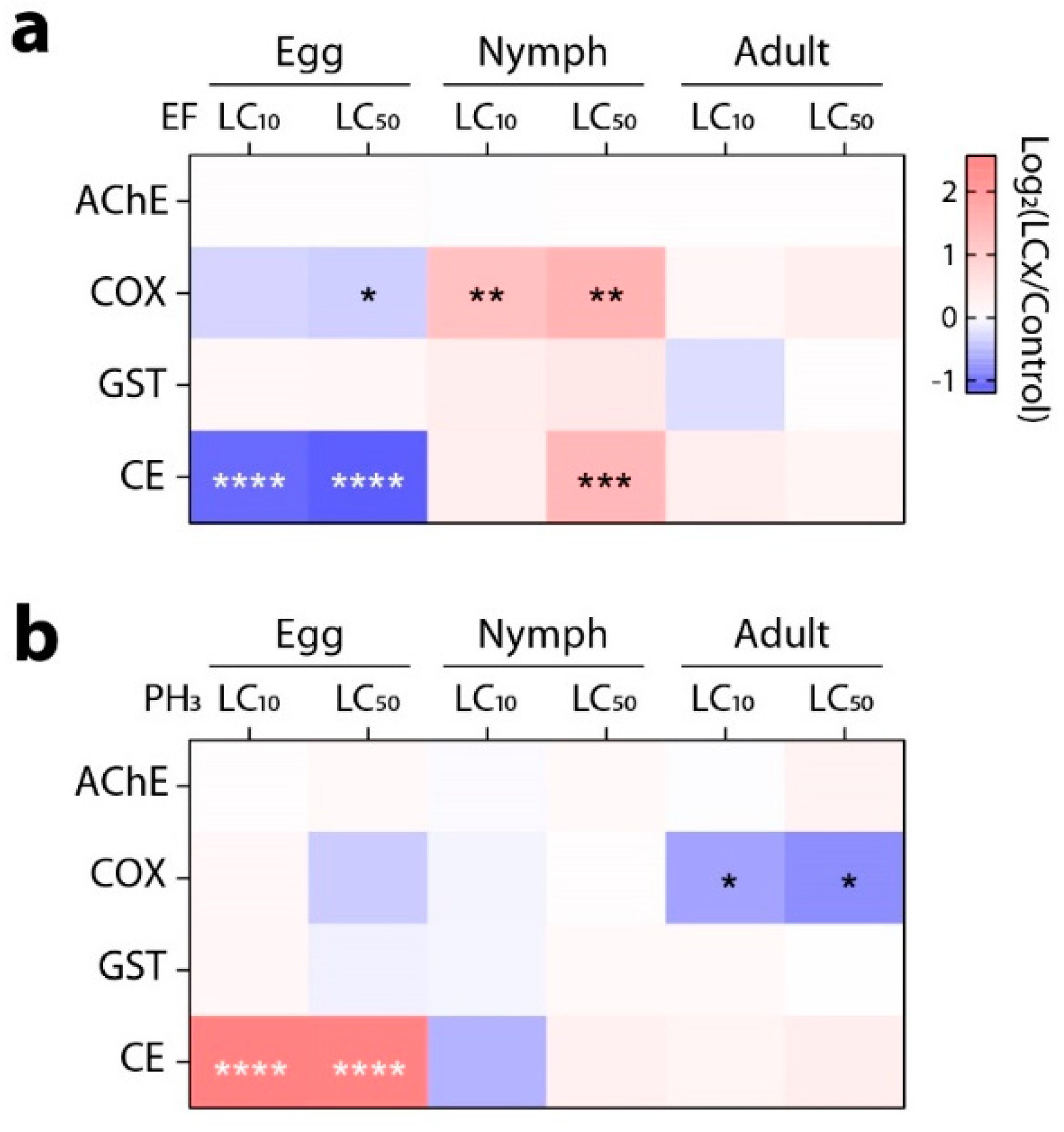
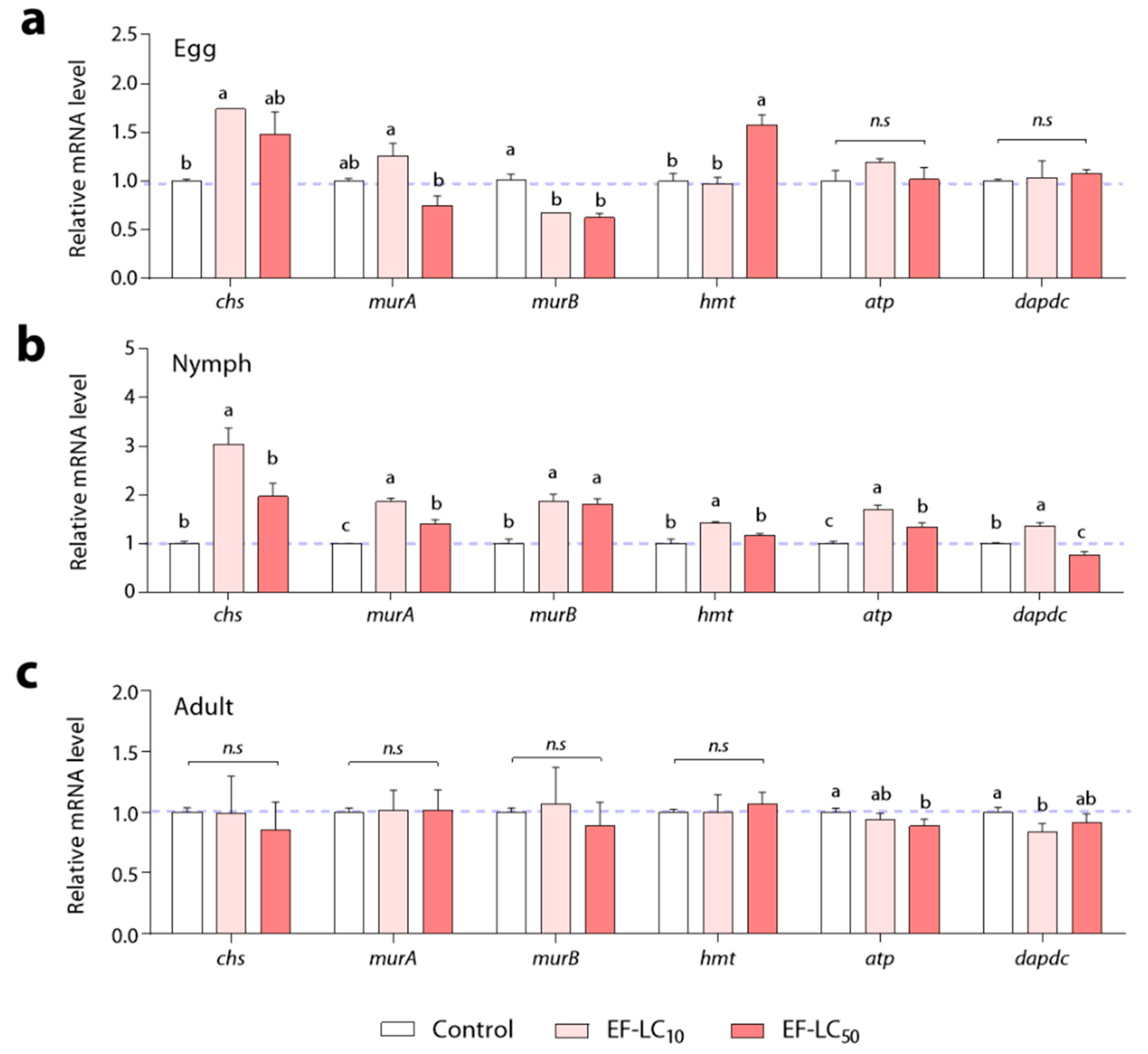

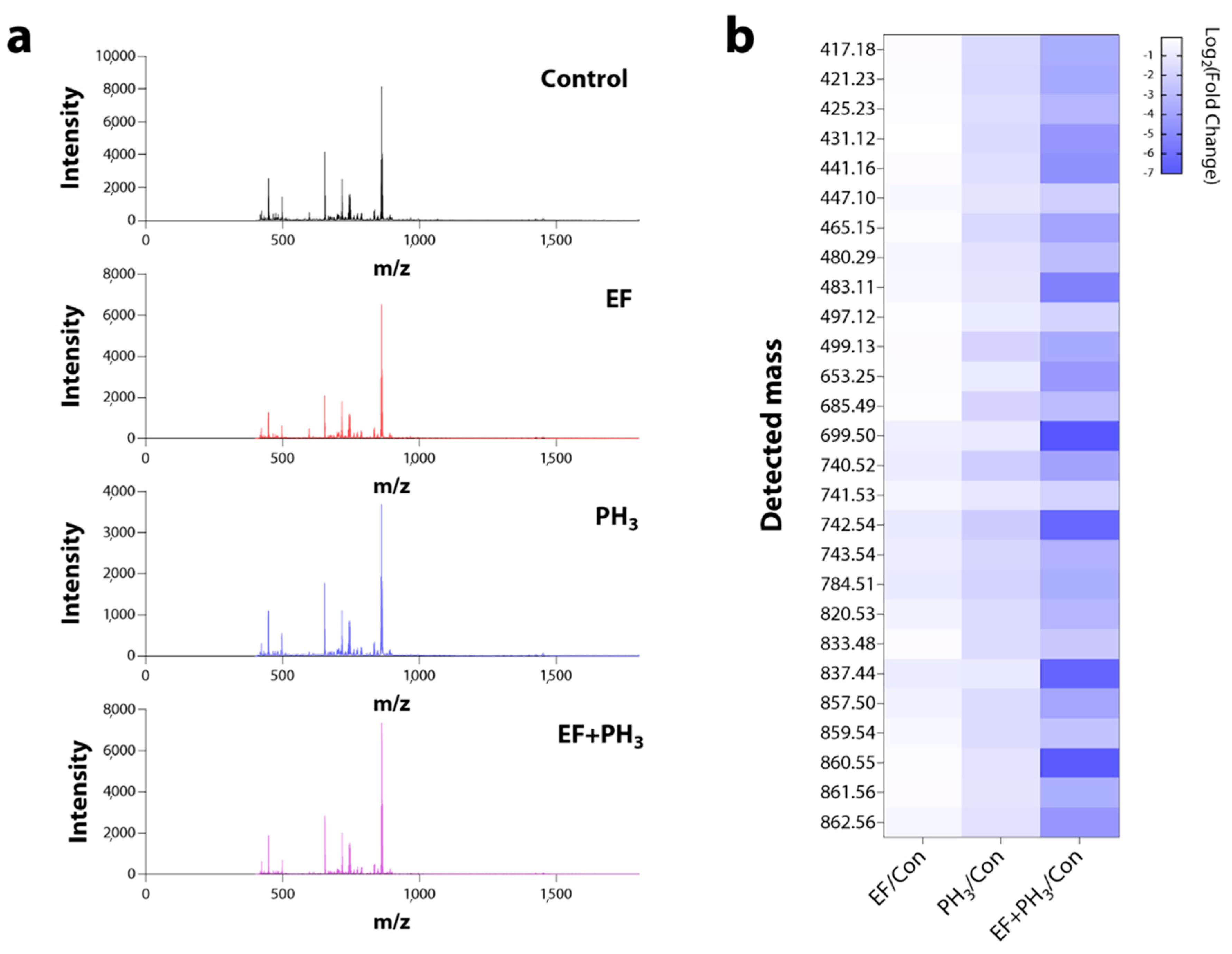
| Fumigant | Time (h) | Stages | LCTx (mg∙h/L, 95% CL a) | Slope ± SE | df | χ2 | |||
|---|---|---|---|---|---|---|---|---|---|
| LCT10 | LCT25 | LCT50 | LCT99 | ||||||
| EF | 4 | Egg | 9.49 (5.24–12.78) | 13.13 (8.63–16.62) | 18.84 (14.43–23.12) | 65.43 (46.43–132.60) | 4.30 ± 0.52 | 5 | 8.29 |
| Nymph | 6.83 (0.23–12.05) | 9.41 (0.82–15.05) | 13.44 (3.09–20.33) | 45.85 (28.23–627.17) | 4.37 ± 1.28 | 7 | 13.77 | ||
| Adult | 14.06 (6.91–18.03) | 17.23 (10.39–20.91) | 21.57 (15.98–25.19) | 46.90 (37.04–91.26) | 6.90 ± 1.13 | 5 | 0.44 | ||
| PH3 | 20 | Egg | 0.20 (0.14–0.25) | 0.26 (0.20–0.31) | 0.33 (0.28–0.42) | 0.83 (0.60–1.71) | 5.92 ± 0.77 | 5 | 2.55 |
| Nymph | 0.07 (0.01–0.11) | 0.07 (0.01–0.12) | 0.08 (0.02–0.13) | 0.13 (0.05–0.18) | 12.67 ± 2.15 | 5 | 0.00 | ||
| Adult | 0.14 (0.07–0.20) | 0.17 (0.09–0.22) | 0.20 (0.12–0.25) | 0.36 (0.29–0.41) | 8.95 ± 0.97 | 5 | 0.10 | ||
| PH3 | 4 | Egg | - b | - | - | - | – | – | – |
| Nymph and Adult | 0.14 (0.10–0.16) | 0.25 (0.21–0.28) | 0.36 (0.32–0.40) | 1.32 (1.05–1.82) | 4.12 ± 0.42 | 22 | 9.01 | ||
| Combination No. | Chemicals | LCTx a | Dose b | CTP | Mortality (Mean ± SE) | SR c |
|---|---|---|---|---|---|---|
| Adults and Nymphs | ||||||
| 1 | Control | - | 0 | 0 | 3.3 ± 3.3 | |
| EF | LCT10 | 6 | 21.3 | 14.7 ± 2.6 | ||
| PH3 | LCT25 | 0.1 | 0.29 | 32.9 ± 4.8 | ||
| EF + PH3 | LCT10 + LCT25 | 6 + 0.1 | 19.7 + 0.3 | 56.0 ± 7.8 | 1.17 | |
| 2 | Control | - | 0 | 0 | 0.0 ± 0.0 | |
| EF | LCT25 | 10 | 27.70 | 25.0 ± 5.0 | ||
| PH3 | LCT25 | 0.1 | 0.29 | 30.0 ± 7.6 | ||
| EF + PH3 | LCT25 + LCT25 | 10 + 0.1 | 28.3 + 0.3 | 63.3 ± 7.3 | 1.15 | |
| 3 | Control | - | 0 | 0 | 3.3 ± 1.7 | |
| EF | LCT50 | 17 | 44.1 | 46.7 ± 6.0 | ||
| PH3 | LCT25 | 0.1 | 0.28 | 28.3 ± 4.4 | ||
| EF + PH3 | LCT50 + LCT25 | 17 + 0.1 | 41.6 + 0.3 | 85.0 ± 2.9 | 1.13 | |
| Eggs | ||||||
| 4 | Control | - | 0 | 0 | 0.0 ± 0.0 | |
| EF | LCT10 | 5 | 16.1 | 13.2 ± 3.4 | ||
| PH3 | 1 mg/L | 1 | 3.4 | 42.9 ± 7.1 | ||
| EF + PH3 | LCT10 + 1 mg/L | 5 + 1 | 16.5 + 3.2 | 75.0 ± 5.8 | 1.33 | |
| 5 | Control | - | 0 | 0 | 0.0 ± 0.0 | |
| EF | LCT25 | 10 | 30.2 | 26.4 ± 3.4 | ||
| PH3 | 1 mg/L | 1 | 3.4 | 41.7 ± 1.7 | ||
| EF + PH3 | LCT25 + 1 mg/L | 10 + 1 | 31.6 + 3.4 | 84.7 ± 3.4 | 1.24 | |
| 6 | Control | - | 0 | 0 | 1.7 ± 1.7 | |
| EF | LCT50 | 15 | 38.4 | 45.0 ± 2.9 | ||
| PH3 | 1 mg/L | 1 | 3.4 | 46.7 ± 4.4 | ||
| EF + PH3 | LCT50 + 1 mg/L | 15 + 1 | 40.6 + 3.4 | 98.3 ± 1.7 | 1.07 | |
| No | Identified Proteins | ANOVA | Average Quantitative Value 1 (Normalized Total Spectra) | Biological Process | |||
|---|---|---|---|---|---|---|---|
| Con | EF | PH3 | EF + PH3 | ||||
| 1 | Pyruvate kinase | <0.0001 | 6.71 a | 7.31 a | 2.59 b | 3.55 b | Glycolysis |
| 2 | Putative dihydrolipoyl lysine-residue acetyltransferase | <0.0001 | 1.35 a | 0 b | 0 b | 0 b | Glycolysis |
| 3 | Putative 50S ribosomal protein L11 | 0.0006 | 1.33 a | 0.48 b | 0 b | 0 b | Translation |
| 4 | Putative co-chaperonin GroES | 0.0009 | 6.75 b | 6.35 b | 10.6 a | 10.9 a | Stress response |
| 5 | ATP synthase subunit alpha | 0.0012 | 3.65 ab | 4.82 a | 1.28 c | 2.52 bc | ATP synthesis |
| 6 | Homocysteine S-methyltransferase | 0.0014 | 1.35 ab | 0.92 bc | 1.97 a | 0.28 c | Amino acid biosynthesis |
| 7 | ATP synthase beta subunit | 0.0016 | 20.8 b | 21.5 ab | 22.2 a | 18.9 b | ATP synthesis |
| 8 | Mitochondrial aconitase 1, isoform B | 0.0050 | 1.12 a | 0.23 b | 0 b | 0.78 ab | TCA cycle |
| 9 | Calmodulin | 0.0058 | 3.99 a | 1.71 b | 5.00 a | 3.02 ab | Host–virus interaction |
| 10 | Tryptophan 2-monooxygenase oxidoreductase | 0.0064 | 25.0 a | 26.4 a | 17.9 b | 20.1 ab | Tryptophan metabolism |
| 11 | Dihydrolipoyl dehydrogenase | 0.0077 | 1.33 a | 0.46 ab | 0.28 b | 0 b | Glycolysis/TCA cycle |
| 12 | 26S proteasome regulatory subunit 8 | 0.0082 | 1.14 a | 0.97 a | 0 b | 1.22 a | Protein catabolic process |
| 13 | Acetohydroxyacid isomeroreductase | 0.0087 | 2.43 b | 3.42 b | 6.6 a | 5.19 ab | Amino acid biosynthesis |
| 14 | 30S ribosomal protein S7 | 0.011 | 1.14 ab | 1.71 a | 0 b | 0.82 ab | Translation |
| 15 | Translation elongation factor Tu | 0.012 | 7.86 a | 7.52 ab | 4.49 b | 9 a | Translation |
| 16 | Putative 50S ribosomal protein L9 | 0.012 | 0.9 a | 0 b | 0 b | 0 b | Translation |
| 17 | Putative 50S ribosomal protein L7/L12 | 0.014 | 1.13 ab | 0.92 ab | 0.27 b | 1.65 a | Translation |
| 18 | Aspartate aminotransferase | 0.019 | 4.25 a | 3.46 ab | 4.52 a | 2.18 b | Lipid transport |
| 19 | Putative phosphoribosyl formimino-5-aminoimidazole carboxamideribotide isomerase | 0.027 | 1.13 a | 0.25 ab | 0 b | 0.54 ab | Amino acid biosynthesis |
| 20 | Cysteine synthase | 0.028 | 3.15 ab | 3.64 ab | 2.28 b | 3.81 a | Amino acid biosynthesis |
| 21 | Putative heat shock protein IbpA | 0.028 | 0.46 b | 1.13 ab | 1.71 ab | 2.47 a | Stress response |
| 22 | 26S proteasome regulatory subunit 4 | 0.028 | 2.54 ab | 2.77 a | 0.29 b | 1.51 ab | Protein catabolic process |
| 23 | Calcium pump | 0.029 | 1.56 ab | 2.07 a | 0.35 b | 0.75 ab | Ion transporter |
| 24 | Putative cold shock-like protein CspD | 0.031 | 3.81 ab | 3.00 b | 3.14 ab | 5.18 a | Regulation of transcription |
| 25 | Heat shock protein 82 | 0.032 | 2.50 ab | 2.50 ab | 2.25 b | 3.23 a | Stress response |
| 26 | beta-actin, partial | 0.034 | 15.5 b | 16.5 ab | 20.3 a | 16.1 ab | Skeleton |
| 27 | Dihydroxyacid dehydratase | 0.037 | 3.80 ab | 3.44 ab | 3.16 b | 5.45 a | Amino acid biosynthesis |
| 28 | Histone H4 replacement, isoform A | 0.037 | 2.14 ab | 1.68 ab | 0.77 b | 2.25 a | Regulation of transcription |
| 29 | Vacuolar H[+]-ATPase 55kD subunit, isoform A | 0.038 | 1.14 ab | 1.58 ab | 0.24 b | 1.96 a | Ion transporter |
| 30 | ATP-dependent chaperone protein ClpB | 0.045 | 2.45 ab | 1.81 ab | 0.59 b | 1.36 a | Stress response |
| No | m/z Value | Delta | Assignment [M-H]- | Average Intensity Value | |||
|---|---|---|---|---|---|---|---|
| Con | PH3 | EF | EF + PH3 | ||||
| 1 | 699.4970 | 0.000038 | PA(O-16:0) | 551.7 | 454.9 | 151.2 | 41.4 |
| 2 | 740.5180 | 0.005587 | PC(P-20:0) | 569.6 | 460.0 | 237.0 | 50.7 |
| 3 | 742.5392 | 0.000037 | PC(O-20:0) | 1618.6 | 1189.4 | 678.5 | 39.8 |
| 4 | 784.5134 | 0.000017 | PC(14:0) | 237.8 | 212.0 | 81.1 | 26.0 |
| 5 | 820.5265 | 0.008047 | PS(18:0) | 94.1 | 84.2 | 48.7 | 24.5 |
| 6 | 833.4822 | 0.000022 | PG(16:1) | 678.2 | 552.7 | 245.8 | 26.0 |
| 7 | 837.4407 | 0.000008 | PI(20:0) | 100.6 | 77.7 | 41.9 | 23.4 |
| 8 | 857.4976 | 0.000167 | PG(20:3) | 571.8 | 497.8 | 294.7 | 27.0 |
| 9 | 859.5425 | 0.008287 | PI(O-16:0) | 3970.8 | 3389.5 | 1630.5 | 34.3 |
| 10 | 860.5447 | 0.000017 | PC(18:2) | 2484.6 | 2088.0 | 778.9 | 203.6 |
| 11 | 862.5604 | 0.000033 | PC(18:1) | 5549.8 | 4745.0 | 1654.6 | 408.9 |
| 12 | 699.4970 | 0.000038 | PA(O-16:0) | 551.7 | 454.9 | 151.2 | 41.4 |
| 13 | 740.5180 | 0.005587 | PC(P-20:0) | 569.6 | 460.0 | 237.0 | 50.7 |
| 14 | 742.5392 | 0.000037 | PC(O-20:0) | 1618.6 | 1189.4 | 678.5 | 39.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Park, M.-G.; Lee, Y.H.; Jeon, H.-J.; Kwon, T.H.; Kim, C.; Park, J.; Lee, B.-H.; Yang, J.O.; Lee, S.-E. Synergistic Effects and Toxic Mechanism of Phosphine with Ethyl Formate against Citrus Mealybug (Planococcus citri). Appl. Sci. 2021, 11, 9877. https://doi.org/10.3390/app11219877
Kim K, Park M-G, Lee YH, Jeon H-J, Kwon TH, Kim C, Park J, Lee B-H, Yang JO, Lee S-E. Synergistic Effects and Toxic Mechanism of Phosphine with Ethyl Formate against Citrus Mealybug (Planococcus citri). Applied Sciences. 2021; 11(21):9877. https://doi.org/10.3390/app11219877
Chicago/Turabian StyleKim, Kyeongnam, Min-Goo Park, Yong Ho Lee, Hwang-Ju Jeon, Tae Hyung Kwon, Chaeeun Kim, Jungeun Park, Byung-Ho Lee, Jeong Oh Yang, and Sung-Eun Lee. 2021. "Synergistic Effects and Toxic Mechanism of Phosphine with Ethyl Formate against Citrus Mealybug (Planococcus citri)" Applied Sciences 11, no. 21: 9877. https://doi.org/10.3390/app11219877
APA StyleKim, K., Park, M.-G., Lee, Y. H., Jeon, H.-J., Kwon, T. H., Kim, C., Park, J., Lee, B.-H., Yang, J. O., & Lee, S.-E. (2021). Synergistic Effects and Toxic Mechanism of Phosphine with Ethyl Formate against Citrus Mealybug (Planococcus citri). Applied Sciences, 11(21), 9877. https://doi.org/10.3390/app11219877







