Degradation of Oxytetracycline by Persulfate Activation Using a Magnetic Separable Iron Oxide Catalyst Derived from Hand-Warmer Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Catalyst Preparation
2.3. Experimental Procedure
2.4. Analytical Method
2.5. Characterization
3. Results and Discussion
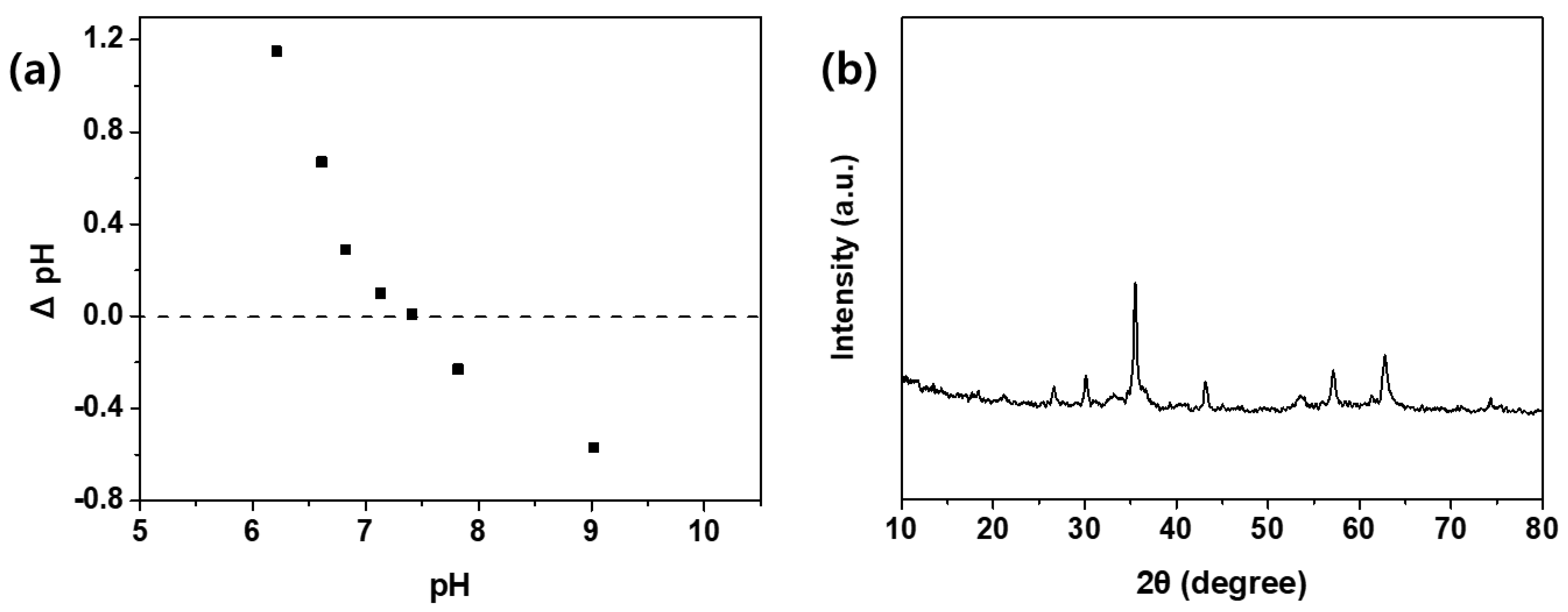
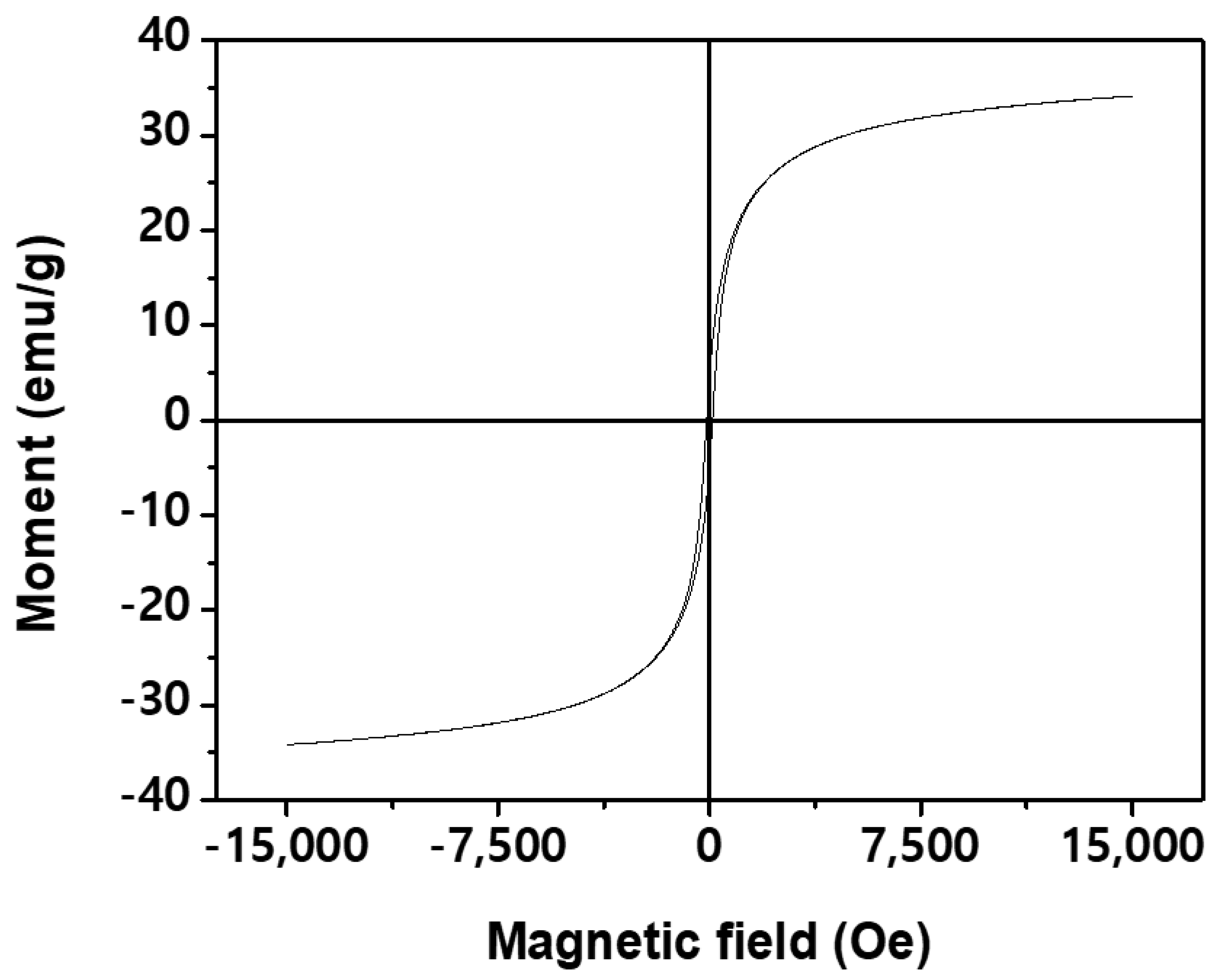
3.1. Crystal Structure, Surface Morphology, and Magnetic Properties of HWWC
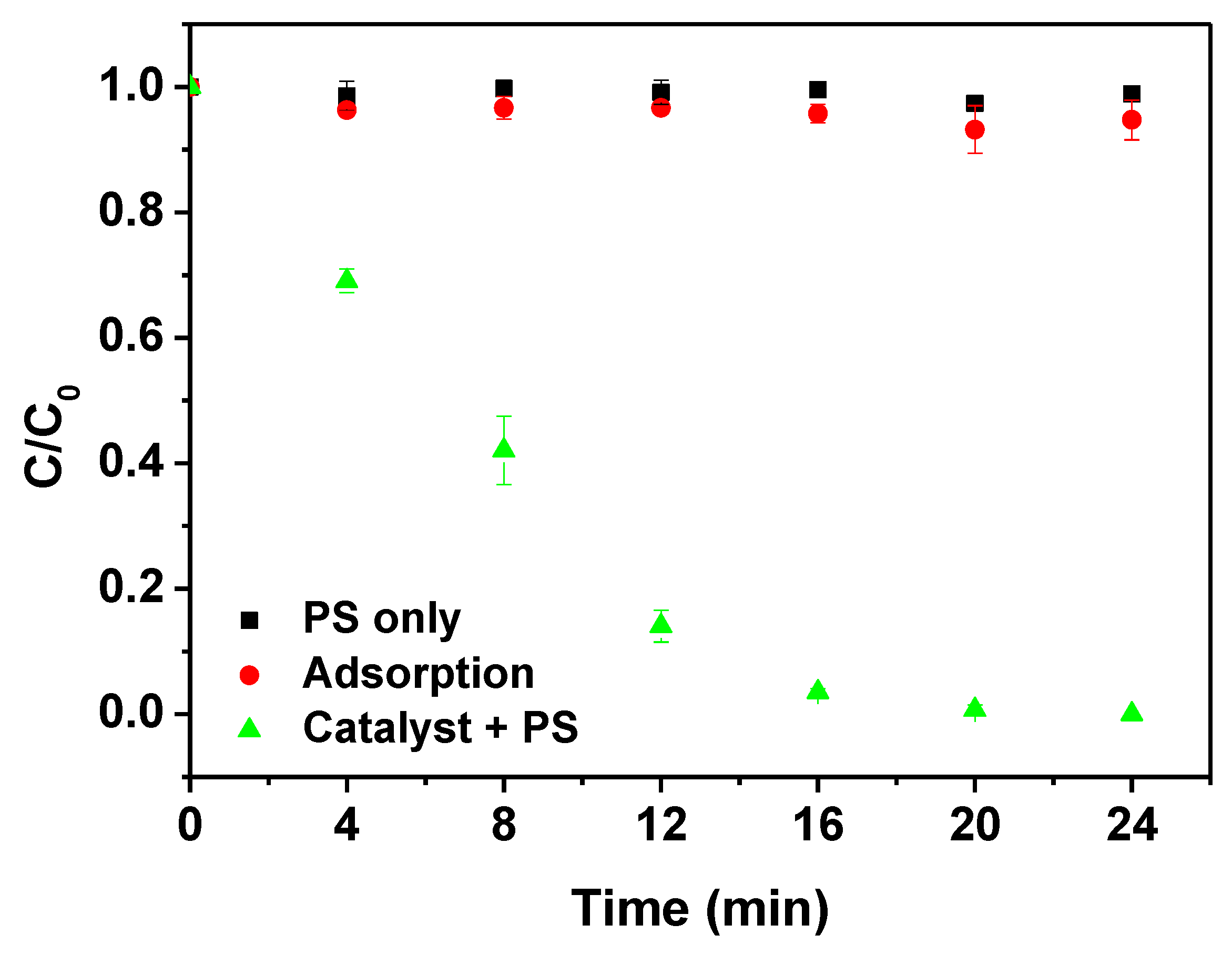
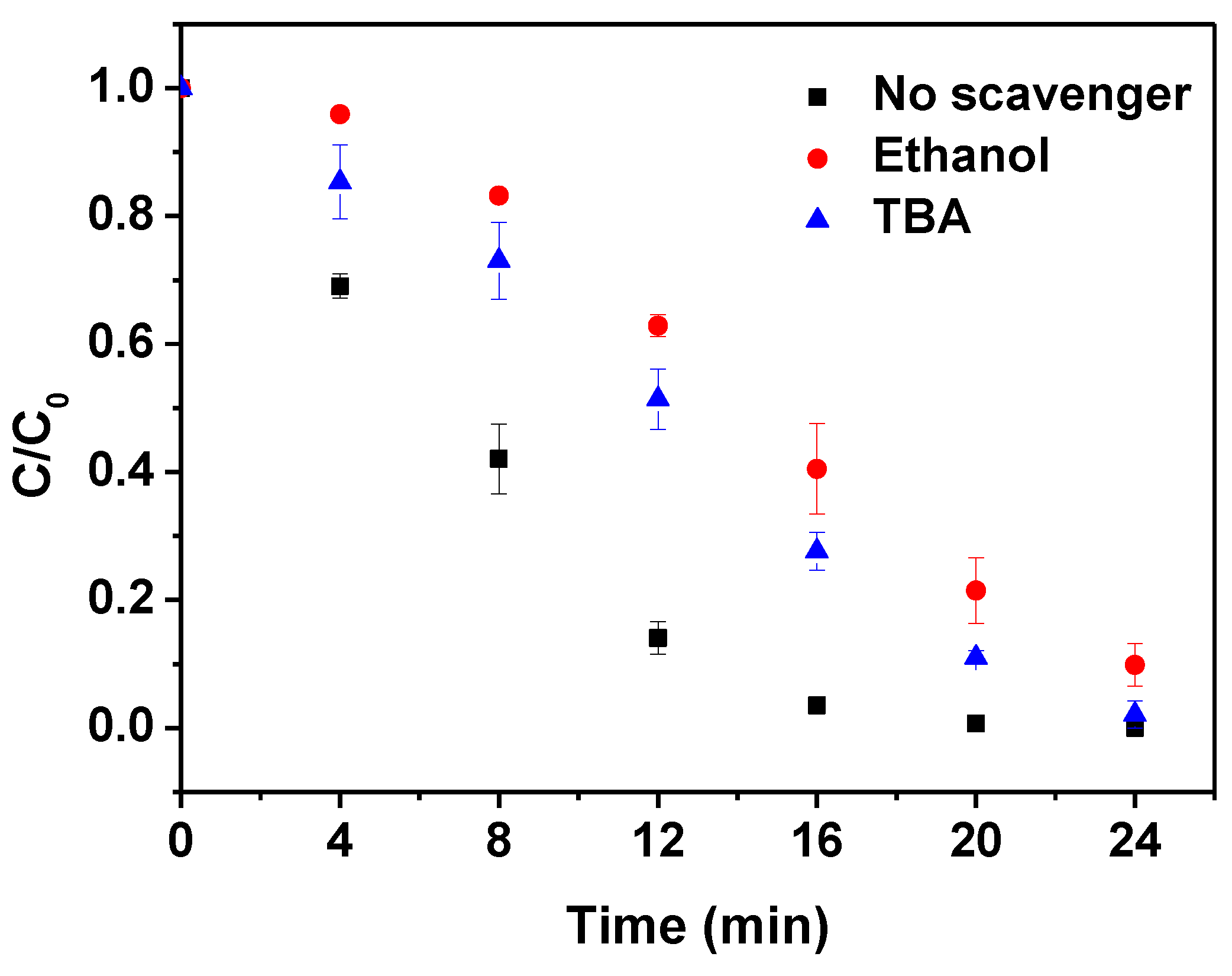
3.2. Control Experiments
3.3. Effects of HWWC Dosage and PS Concentration on OTC Degradation
3.4. Effects of pH on OTC Degradation
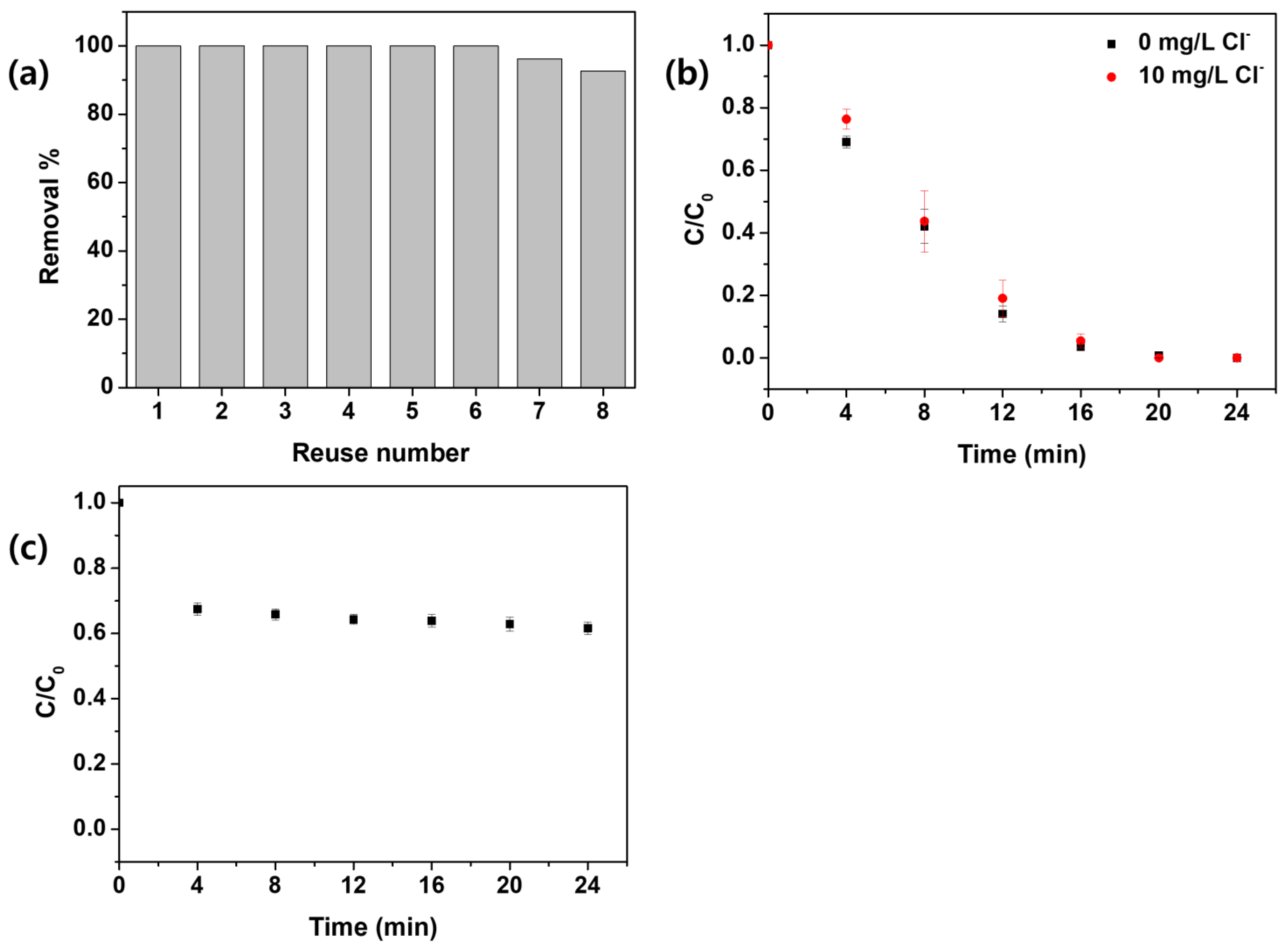
3.5. Applicability of HWWC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, D.; Li, M.; Li, X.; Ren, F.; Sun, P.; Zhou, L. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation. Chem. Eng. J. 2020, 387, 124008. [Google Scholar] [CrossRef]
- Baquero, F.; Martinez, J.L.; Canton, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Zhang, C.; Mo, Y.; Lu, X. Determination of oxytetracycline, tetracycline and chloramphenicol antibiotics in animal feeds using subcritical water extraction and high performance liquid chromatography. Anal. Chim. Acta 2008, 619, 54–58. [Google Scholar] [CrossRef]
- Uslu, M.O.; Balcioglu, I.A. Simultaneous removal of oxytetracycline and sulfamethazine antibacterials from animal waste by chemical oxidation processes. J. Agric. Food Chem. 2009, 57, 11284–11291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Kinetics and mechanism investigation on the destruction of oxytetracycline by UV-254nm activation of persulfate. J. Hazard. Mater. 2016, 305, 229–239. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Zheng, S.; Qiu, X.; Chen, B.; Yu, X.; Liu, Z.; Zhong, G.; Li, H.; Chen, M.; Sun, G.; Huang, H.; et al. Antibiotics pollution in Jiulong River estuary: Source, distribution and bacterial resistance. Chemosphere 2011, 84, 1677–1685. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere 2011, 83, 1284–1289. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence and removal of antibiotics in a municipal wastewater reclamation plant in Beijing, China. Chemosphere 2013, 92, 435–444. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Huang, W.; Liu, Y.; Tang, M.; Zhang, L.; Sun, J. Biodegradation of oxytetracycline and electricity generation in microbial fuel cell with in situ dual graphene modified bioelectrode. Bioresour. Technol. 2018, 270, 482–488. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, D.; Liao, C.; Deng, Y.; Zhang, T.; Shih, K. Red mud powders as low-cost and efficient catalysts for persulfate activation: Pathways and reusability of mineralizing sulfadiazine. Sep. Purif. Technol. 2016, 167, 136–145. [Google Scholar] [CrossRef]
- Liang, C.J.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Thermally Activated Persulfate Oxidation of Trichloroethylene (TCE) and 1,1,1-Trichloroethane (TCA) in Aqueous Systems and Soil Slurries. Soil Sediment Contam. Int. J. 2010, 12, 207–228. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croue, J.P. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Amr, S.S.; Aziz, H.A.; Adlan, M.N. Optimization of stabilized leachate treatment using ozone/persulfate in the advanced oxidation process. Waste Manag. 2013, 33, 1434–1441. [Google Scholar] [CrossRef]
- Peng, L.; Deng, D.; Guan, M.; Fang, X.; Zhu, Q. Remediation HCHs POPs-contaminated soil by activated persulfate technologies: Feasibility, impact of activation methods and mechanistic implications. Sep. Purif. Technol. 2015, 150, 215–222. [Google Scholar] [CrossRef]
- Liu, C.S.; Shih, K.; Sun, C.X.; Wang, F. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci. Total Environ. 2012, 416, 507–512. [Google Scholar] [CrossRef]
- Teel, A.L.; Ahmad, M.; Watts, R.J. Persulfate activation by naturally occurring trace minerals. J. Hazard. Mater. 2011, 196, 153–159. [Google Scholar] [CrossRef]
- Fang, G.-D.; Dionysiou, D.D.; Al-Abed, S.R.; Zhou, D.-M. Superoxide radical driving the activation of persulfate by magnetite nanoparticles: Implications for the degradation of PCBs. Appl. Catal. B: Environ. 2013, 129, 325–332. [Google Scholar] [CrossRef]
- Ioannidi, A.; Oulego, P.; Collado, S.; Petala, A.; Arniella, V.; Frontistis, Z.; Angelopoulos, G.N.; Diaz, M.; Mantzavinos, D. Persulfate activation by modified red mud for the oxidation of antibiotic sulfamethoxazole in water. J. Environ. Manag. 2020, 270, 110820. [Google Scholar] [CrossRef] [PubMed]
- Naim, S.; Ghauch, A. Ranitidine abatement in chemically activated persulfate systems: Assessment of industrial iron waste for sustainable applications. Chem. Eng. J. 2016, 288, 276–288. [Google Scholar] [CrossRef]
- Lian, J.-Z.; Tsai, C.-T.; Chang, S.-H.; Lin, N.-H.; Hsieh, Y.-H. Iron waste as an effective depend on TiO2 for photocatalytic degradation of dye waste water. Optik 2017, 140, 197–204. [Google Scholar] [CrossRef]
- Yu, Y.; Paul Chen, J. Key factors for optimum performance in phosphate removal from contaminated water by a Fe-Mg-La tri-metal composite sorbent. J. Colloid. Interface Sci. 2015, 445, 303–311. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, R.; Li, M.; Jiang, N. Preparation and characterization of porous ultrafine Fe2O3 particles. Mater. Res. Bull. 2005, 40, 1968–1975. [Google Scholar] [CrossRef]
- Molodtsova, T.; Gorshenkov, M.; Saliev, A.; Vanyushin, V.; Goncharov, I.; Smirnova, N. One-step synthesis of γ-Fe2O3/Fe3O4 nanocomposite for sensitive electrochemical detection of hydrogen peroxide. Electrochim. Acta 2021, 370, 137723. [Google Scholar] [CrossRef]
- Khoshnam, M.; Salimijazi, H. Synthesis and characterization of magnetic-photocatalytic Fe3O4/SiO2/a-Fe2O3 nano core-shell. Surf. Interfaces 2021, 26, 101322. [Google Scholar] [CrossRef]
- Chu, J.-H.; Kang, J.-K.; Park, S.-J.; Lee, C.-G. Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. J. Water Process Eng. 2020, 37, 101455. [Google Scholar] [CrossRef]
- Chu, J.-H.; Kang, J.-K.; Park, S.-J.; Lee, C.-G. Enhanced sonocatalytic degradation of bisphenol A with a magnetically recoverable biochar composite using rice husk and rice bran as substrate. J. Environ. Chem. Eng. 2021, 9, 105284. [Google Scholar] [CrossRef]
- Zouanti, M.; Bezzina, M.; Dhib, R. Experimental study of degradation and biodegradability of oxytetracycline antibiotic in aqueous solution using Fenton process. Environ. Eng. Res. 2019, 25, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Zhang, X.; Guo, R.; Zhang, H.; Cheng, Q.; Xie, M.; Cheng, X. Persulfate activation by magnetic γ-Fe2O3/Mn3O4 nanocomposites for degradation of organic pollutants. Sep. Purif. Technol. 2019, 210, 335–342. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Ren, Y.M.; Liu, Y.L.; Xiao, J.Y.; Lin, L.Q.; Zhang, C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Zhang, Y.-Q.; Huang, S.-B.; Hussain, I. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chem. Eng. J. 2013, 218, 384–391. [Google Scholar] [CrossRef]
- Norzaee, S.; Taghavi, M.; Djahed, B.; Kord Mostafapour, F. Degradation of Penicillin G by heat activated persulfate in aqueous solution. J. Environ. Manag. 2018, 215, 316–323. [Google Scholar] [CrossRef]
- Liang, C.; Lee, I.L. In situ iron activated persulfate oxidative fluid sparging treatment of TCE contamination--a proof of concept study. J. Contam. Hydrol. 2008, 100, 91–100. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, J.; Ouyang, D.; Qian, L.; Han, L.; Chen, M. Heterogeneously catalyzed persulfate by CuMgFe layered double oxide for the degradation of phenol. Appl. Catal. A: Gen. 2017, 538, 19–26. [Google Scholar] [CrossRef]
- Long, M.; Brame, J.; Qin, F.; Bao, J.; Li, Q.; Alvarez, P.J. Phosphate Changes Effect of Humic Acids on TiO2 Photocatalysis: From Inhibition to Mitigation of Electron-Hole Recombination. Environ. Sci. Technol. 2017, 51, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Z.; Xiang, Y.; Ling, L.; Fang, J.; Shang, C.; Dionysiou, D.D. Bromate formation in bromide-containing water through the cobalt-mediated activation of peroxymonosulfate. Water Res. 2015, 83, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.-H.; Kang, J.-K.; Park, S.-J.; Lee, C.-G. Ultrasound-activated peroxydisulfate process with copper film to remove bisphenol A: Operational parameter impact and back propagation-artificial neural network modeling. J. Water Process Eng. 2021, 44, 102326. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Jiang, X.; Xie, Z.; Li, X.; Chen, C.; Chen, H. Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water. Chemosphere 2018, 190, 296–306. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Lee, C.-G.; Park, S.-J.; Jho, E.H. Degradation of Oxytetracycline by Persulfate Activation Using a Magnetic Separable Iron Oxide Catalyst Derived from Hand-Warmer Waste. Appl. Sci. 2021, 11, 10447. https://doi.org/10.3390/app112110447
Lee Y-J, Lee C-G, Park S-J, Jho EH. Degradation of Oxytetracycline by Persulfate Activation Using a Magnetic Separable Iron Oxide Catalyst Derived from Hand-Warmer Waste. Applied Sciences. 2021; 11(21):10447. https://doi.org/10.3390/app112110447
Chicago/Turabian StyleLee, Youn-Jun, Chang-Gu Lee, Seong-Jik Park, and Eun Hea Jho. 2021. "Degradation of Oxytetracycline by Persulfate Activation Using a Magnetic Separable Iron Oxide Catalyst Derived from Hand-Warmer Waste" Applied Sciences 11, no. 21: 10447. https://doi.org/10.3390/app112110447
APA StyleLee, Y.-J., Lee, C.-G., Park, S.-J., & Jho, E. H. (2021). Degradation of Oxytetracycline by Persulfate Activation Using a Magnetic Separable Iron Oxide Catalyst Derived from Hand-Warmer Waste. Applied Sciences, 11(21), 10447. https://doi.org/10.3390/app112110447







