Potassium Recovery from Potassium Solution and Seawater Using Different Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbents
2.2. Sorption Experiments with KCl Solutions
2.2.1. Batch Sorption Experiments with KCl Solution
2.2.2. Sorption Isotherm and Kinetic Models
2.3. K Sorption Experiments with Seawater
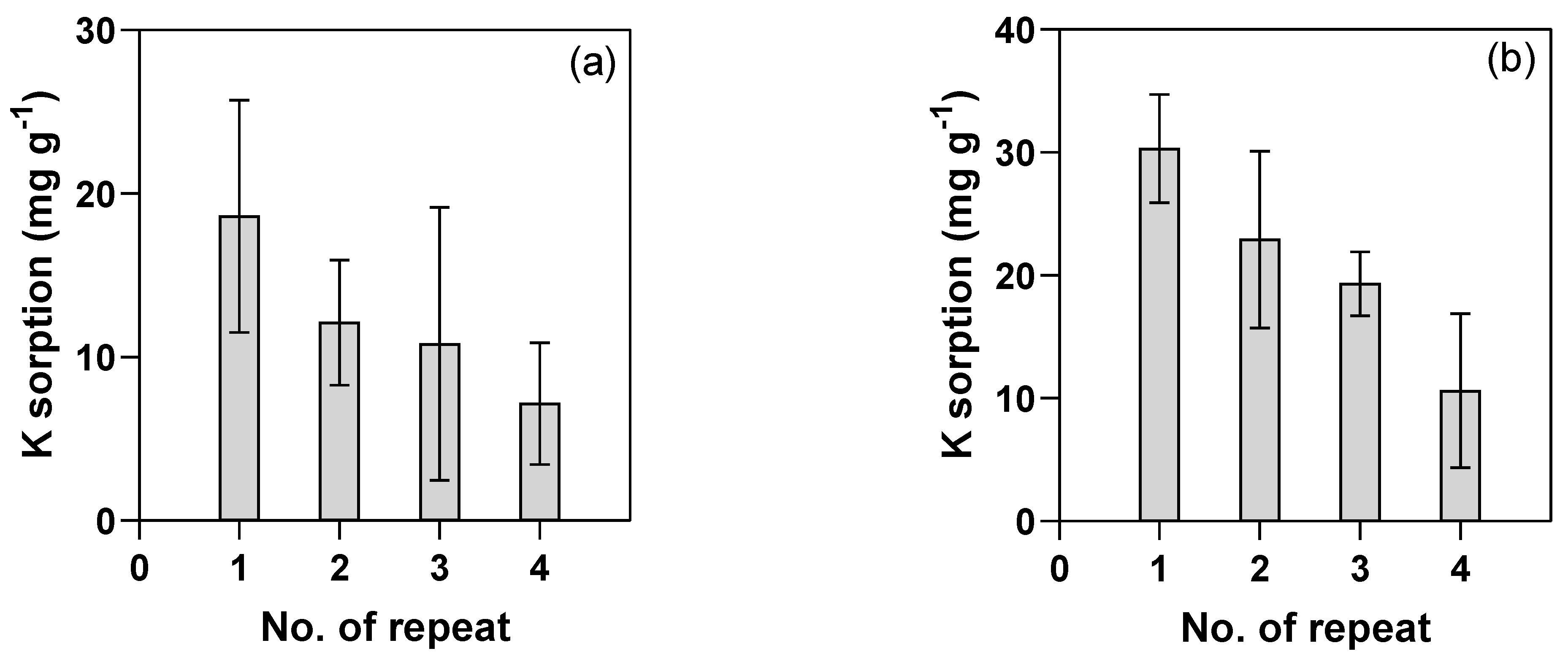
2.4. Regeneration Study Using Natural Zeolite
3. Results and Discussion
3.1. K Sorption by Different Adsorbents
3.1.1. K Sorption Isotherms Using KCl Solutions
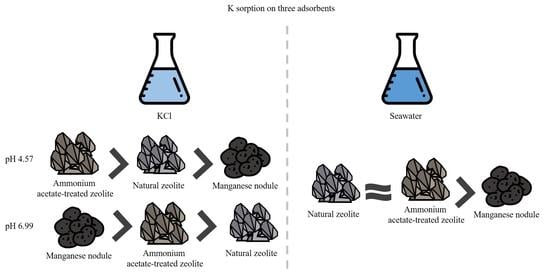
3.1.2. Effect of pH on K Sorption
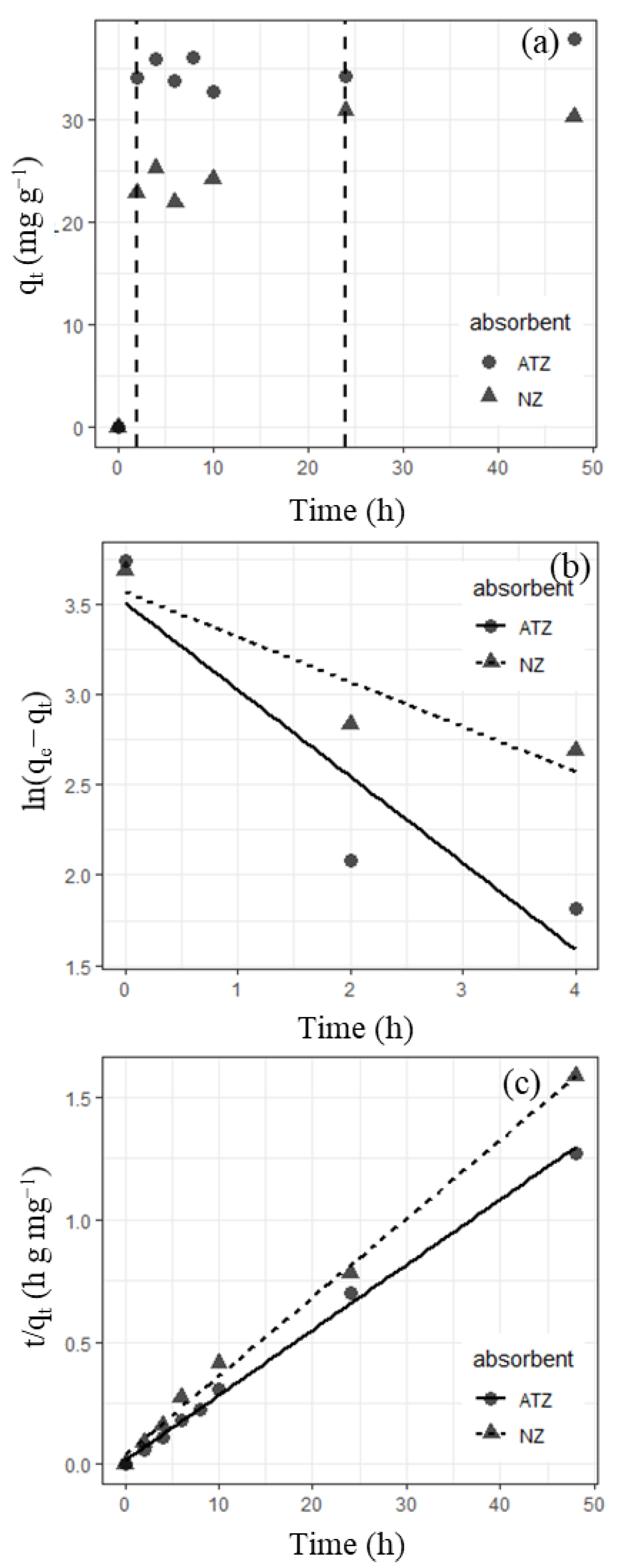
3.2. K Sorption Kinetics
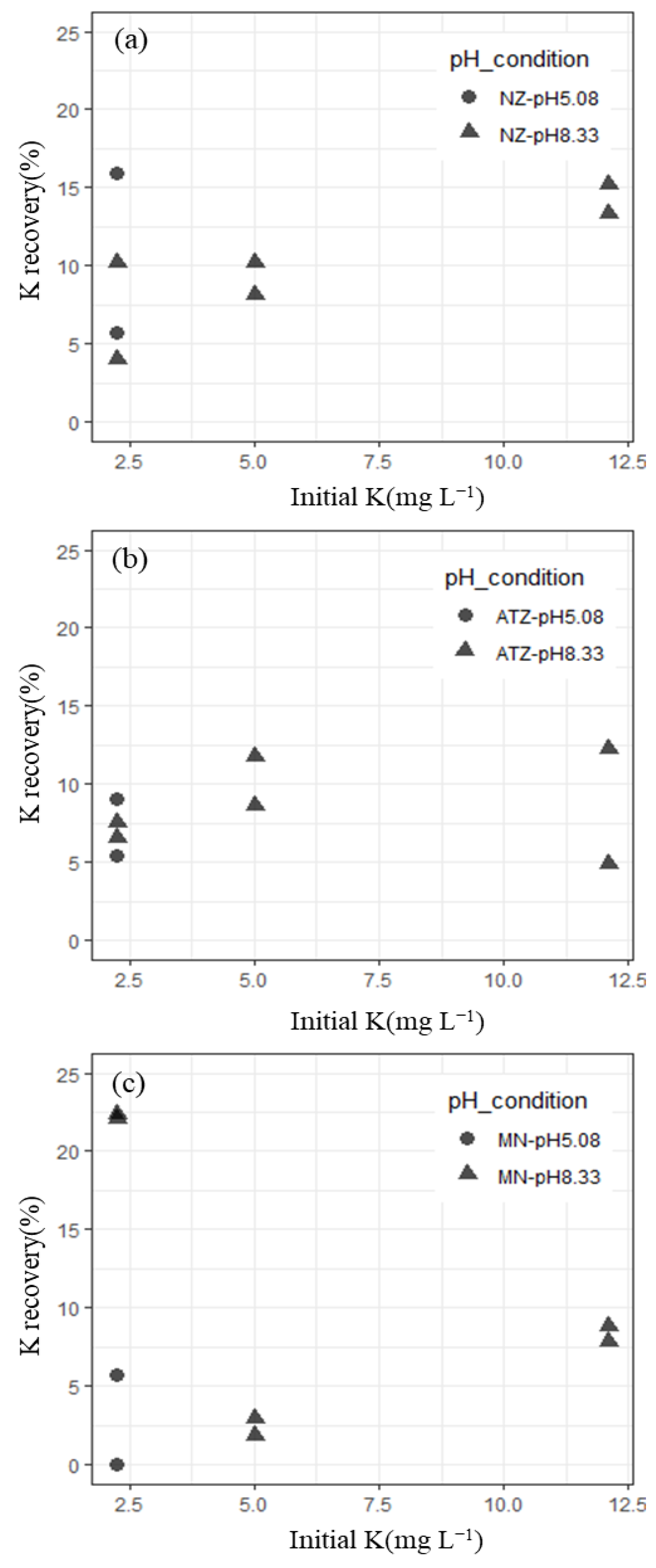
3.3. K Recovery from Seawater
3.4. Regeneration Study with KCl Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.-F.; Ji, Z.-Y.; Yuan, J.-S.; Zhao, Y.-Y.; Liu, J. Recovery of K + from concentrates from brackish and seawater desalination with modified clinoptilolite. Desalin. Water Treat. 2015, 57, 6829–6837. [Google Scholar] [CrossRef]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2016, 3, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Singhal, N.; Jho, E.H. Lithium sorption properties of HMnO in seawater and wastewater. Water Res. 2015, 87, 320–327. [Google Scholar] [CrossRef]
- Hou, J.; Yuan, J.; Shang, R. Synthesis and characterization of zeolite W and its ion-exchange properties to K+ in seawater. Powder Technol. 2012, 226, 222–224. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, Y.; Li, Q.; Ji, Z.; Guo, X. Preparation of potassium ionic sieve membrane and its application on extracting potash from seawater. Sep. Purif. Technol. 2012, 99, 55–60. [Google Scholar] [CrossRef]
- Epstein, J.; Altaras, D.; Feist, E.; Rosenzweig, J. The recovery of potassium chloride from Dead Sea brines by precipitation and solvent extraction. Hydrometall 1975, 1, 39–50. [Google Scholar] [CrossRef]
- Ghara, K.K.; Korat, N.; Bhalodia, D.; Solanki, J.; Maiti, P.; Ghosh, P.K. Production of pure potassium salts directly from sea bittern employing tartaric acid as a benign and recyclable K+ precipitant. RSC Adv. 2014, 4, 34706–34711. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Johir, A.H.; Fane, A.G.; Kandasamy, J.; Vigneswaran, S. Rubidium extraction from seawater brine by an integrated membrane distillation-selective sorption system. Water Res. 2017, 123, 321–331. [Google Scholar] [CrossRef]
- Guo, H.; Peng, C.; Kou, C.-J.; Jiang, J.-Y.; Zhang, F.; Yuan, H.-T. Adsorption mechanism of recovering potassium from seawater by modified-clinoptilolite using microwave. J. Water Reuse Desalin. 2017, 8, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Santiago, O.; Walsh, K.; Kele, B.; Gardner, E.; Chapman, J. Novel pre-treatment of zeolite materials for the removal of sodium ions: Potential materials for coal seam gas co-produced wastewater. SpringerPlus 2016, 5, 571. [Google Scholar] [CrossRef] [Green Version]
- Warner, T.E.; Klokker, M.G.; Nielsen, U.G. Synthesis and Characterization of Zeolite Na–Y and Its Conversion to the Solid Acid Zeolite H–Y. J. Chem. Educ. 2017, 94, 781–785. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K. Kinetic and isotherm studies of cadmium adsorption on manganese nodule residue. J. Hazard. Mater. 2006, 137, 915–924. [Google Scholar] [CrossRef]
- Mallick, S.; Dash, S.; Parida, K. Adsorption of hexavalent chromium on manganese nodule leached residue obtained from NH3–SO2 leaching. J. Colloid Interface Sci. 2006, 297, 419–425. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, A.-B.; Sun, J.; Ye, Y.; Chen, X.-G.; Xia, M.-S. Application of ocean manganese nodules for the adsorption of potassium ions from seawater. Miner. Eng. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Park, K.-H.; Nam, C.-W.; Kim, H.-I.; Park, J.-T. Treatment of metal wastes with manganese nodules. J. Korean Inst. Res. Recycl. 2005, 14, 17–21. [Google Scholar]
- Kabwadza-Corner, P.; Johan, E.; Matsue, N. pH Dependence of Lead Adsorption on Zeolites. J. Environ. Prot. 2015, 06, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Kithome, M.; Paul, J.W.; Lavkulich, L.M.; Bomke, A.A. Effect of pH on ammonium adsorption by natural Zeolite clinoptilolite. Commun. Soil Sci. Plant Anal. 1999, 30, 1417–1430. [Google Scholar] [CrossRef]
- Kyziol-Komosinska, J.; Rosik-Dulewska, C.; Franus, M.; Antoszczyszyn-Szpicka, P.; Czupiol, J.; Krzyżewska, I. Sorption Capacities of Natural and Synthetic Zeolites for Cu (II) Ions. Pol. J. Environ. Stud. 2015, 24, 1111–1123. [Google Scholar] [CrossRef]
- Tong, Y.; Yuan, D.; Zhang, W.; Wei, Y.; Liu, Z.; Xu, Y. Selective exchange of alkali metal ions on EAB zeolite. J. Energy Chem. 2021, 58, 41–47. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungl. Svenska. Ventenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Na, C.-K.; Han, M.-Y.; Park, H.-J. Applicability of Theoretical Adsorption Models for Studies on Adsorption Properties of Adsorbents (1). J. Korean Soc. Environ. Eng. 2011, 33, 606–616. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process. Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55. [Google Scholar] [CrossRef] [Green Version]
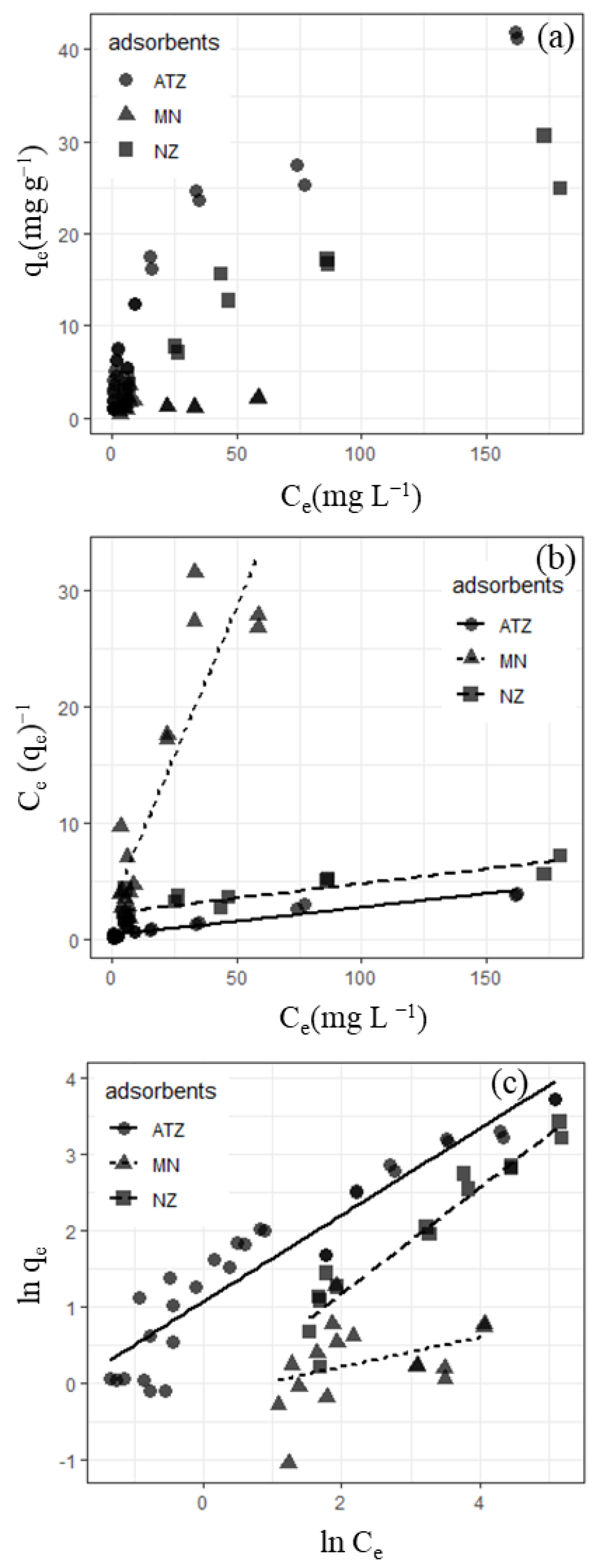



| pH | Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|---|
| qm (mg g−1) | Kd | R2 | KF | n | R2 | ||
| 4.57 | Natural zeolite | 40 | 0.011 | 0.77 | 0.80 | 1.4 | 0.92 |
| Ammonium acetate-treated zeolite | 42 | 0.057 | 0.93 | 2.9 | 1.8 | 0.90 | |
| Manganese nodule | 2.0 | 0.17 | 0.80 | 0.84 | 5.2 | 0.14 | |
| 6.99 | Natural zeolite | 5.8 | 0.045 | 0.54 | 0.55 | 2.0 | 0.77 |
| Ammonium acetate-treated zeolite | 8.1 | 0.037 | 0.66 | 2.2 | 4.9 | 0.30 | |
| Manganese nodule | 9.7 | 0.018 | 0.11 | 2.1 | 3.8 | 0.14 | |
| Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| k1 (h−1) | qe (mg g−1) | R2 | k2 (g mg−1 h−1) | qe (mg g−1) | h (mg g−1 h−1) | R2 | |
| Natural zeolite | 0.249 | 39.9 | 0.857 | 0.0266 | 31.0 | 25.7 | 0.996 |
| Ammonium acetate-treated zeolite | 0.480 | 42.0 | 0.852 | 4.46 | 3.75 | 62.6 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Jho, E.H.; Park, H.; Lee, S.; Kim, J.H. Potassium Recovery from Potassium Solution and Seawater Using Different Adsorbents. Appl. Sci. 2021, 11, 8660. https://doi.org/10.3390/app11188660
Shin S, Jho EH, Park H, Lee S, Kim JH. Potassium Recovery from Potassium Solution and Seawater Using Different Adsorbents. Applied Sciences. 2021; 11(18):8660. https://doi.org/10.3390/app11188660
Chicago/Turabian StyleShin, Sora, Eun Hea Jho, HyunJu Park, Sungjong Lee, and Joon Ha Kim. 2021. "Potassium Recovery from Potassium Solution and Seawater Using Different Adsorbents" Applied Sciences 11, no. 18: 8660. https://doi.org/10.3390/app11188660
APA StyleShin, S., Jho, E. H., Park, H., Lee, S., & Kim, J. H. (2021). Potassium Recovery from Potassium Solution and Seawater Using Different Adsorbents. Applied Sciences, 11(18), 8660. https://doi.org/10.3390/app11188660