Abstract
Gingival recessions constitute serious limitations for effective interdisciplinary periodontal, orthodontic, and implant therapy. A proper bone morphology of the alveolar bone and soft tissues that cover it are interdependent. The regeneration procedures known to date are based on the use of autogenous bone, or its allogeneic, xenogeneic, or alloplastic substitutes. These substitutes are characterized by different osteogenesis potentials. No effective procedure for three-dimensional bone reconstruction for cases in which there is dentition with recessions has been described to date, especially in its vertical dimension. This article presents the patented method of the three-dimensional bone reconstruction of the anterior mandible with preserved dentition when using an allogeneic bone block, and also includes a case report with a 2-year follow-up as an example. Based on clinical observations, it was stated that the intended therapeutic effect was achieved. There was no recession, shallowing of the vestibule, signs of inflammation, or pathological mobility of the teeth in the area undergoing reconstruction. The radiographic images revealed the formation of a new layer of cortical bone on the vestibular side and a certain volume of cancellous bone. No radiological demarcation zone of brightening, which indicates an incomplete adaptation, integration, and reconstruction of the bone block, was found.
1. Introduction
Gingival recessions are not only a significant problem of an aesthetic nature, but also constitute serious limitations for effective interdisciplinary periodontal, orthodontic and implant therapy—especially when they are accompanied by an advanced loss of bone [1,2].
Correct bone morphology of the alveolar bone, as well as the soft tissues that cover it (periosteum and gingiva), are interdependent [3]. Khoury et al. highlight the important role of a three-dimensional stable base with periosteum, which is the source of signaling factors for physiological remodeling. A lack of cancellous bone, considered as the primary developmental condition in some patients, or the effect of its pathological loss, results in a thin layer of compact bone in a given area. In such a case, due to the lack of a three-dimensional stable base, there is a consistent loss of bone—even when the periosteum is preserved [3].
In these cases, there is only an avascular base created by exposed surfaces of tooth roots for potential regeneration. This is an unpredictable therapeutic challenge in terms of restoring the correct morphology of a given area [4]. In turn, the abandonment of reconstruction leads to the loss of the support of teeth, their loosening, and as a result their loss. At the same time, there is also a lack of adequate dimensions of the avascular base to conduct the subsequent implantation [5].
The regeneration procedures known to date are based on the use of autogenous bone, or its allogeneic, xenogeneic, or alloplastic substitutes, which exhibit different osteogenesis potentials. Biomaterials are available in the form of blocks or crushed chips and granules [6,7]. Autogenous grafts provide optimal osteoconductive, osteoinductive, and osteogenic properties. However, donor site morbidity, costs, and the fact that stable blocks of autogenous bone are not always sufficient for regenerating an existing defect prompt one to look for alternative biomaterials. Alloplastic and xenogenous materials are mainly osteoconductive without the intrinsic potential for osteogenesis or osteoinduction. Although they are readily available commercially, they do not constitute a “gold standard”. Therefore, an ideal compromise seems to be an allogenic material that exerts both an osteoconductive and osteoinductive potential [7,8,9].
Therefore, in regenerative surgery, the use of techniques aimed to obtain three-dimensional structures of porous scaffolds is gaining increased popularity [10,11]. The use of volumetric tomography scans of bone defects allows for designing and creating a bone block that fits the recipient’s site and adheres to it with its entire surface. It is desirable that not only the macrostructure of the scaffold fits the defect, but also that this microstructure should favor angiogenesis, cell migration, and the diffusion of nutrients. This so-called biomimetism then leads to regeneration within the graft through the adhesion, proliferation, and differentiation of cells [11].
Modern methods enable to obtain an adequate volume of bone tissue within the edentulous alveolar bone. They are described in the literature with reports of their high effectiveness during long-term observation [12,13,14].
However, no effective procedure for three-dimensional bone reconstruction for cases in which there is dentition with recessions has been described to date, especially with regards to the bone’s vertical dimension.
The aim of this case report was to describe the patented method of the three-dimensional bone reconstruction of the anterior mandible with preserved dentition when using an allogeneic bone block. The additional goal was to report the results of the method presenting a case with a 2-year follow-up as an example.
2. Materials and Methods
The reconstruction method is presented based on a case description of a 21-year-old, generally healthy patient who was referred to a dental clinic due to pathological mobility of his front teeth in the mandible. The patient had started orthodontic treatment with fixed appliances three months earlier. The patient has never undergone any surgical or periodontal treatment in a given area of the mandible. He also denied facial injuries.
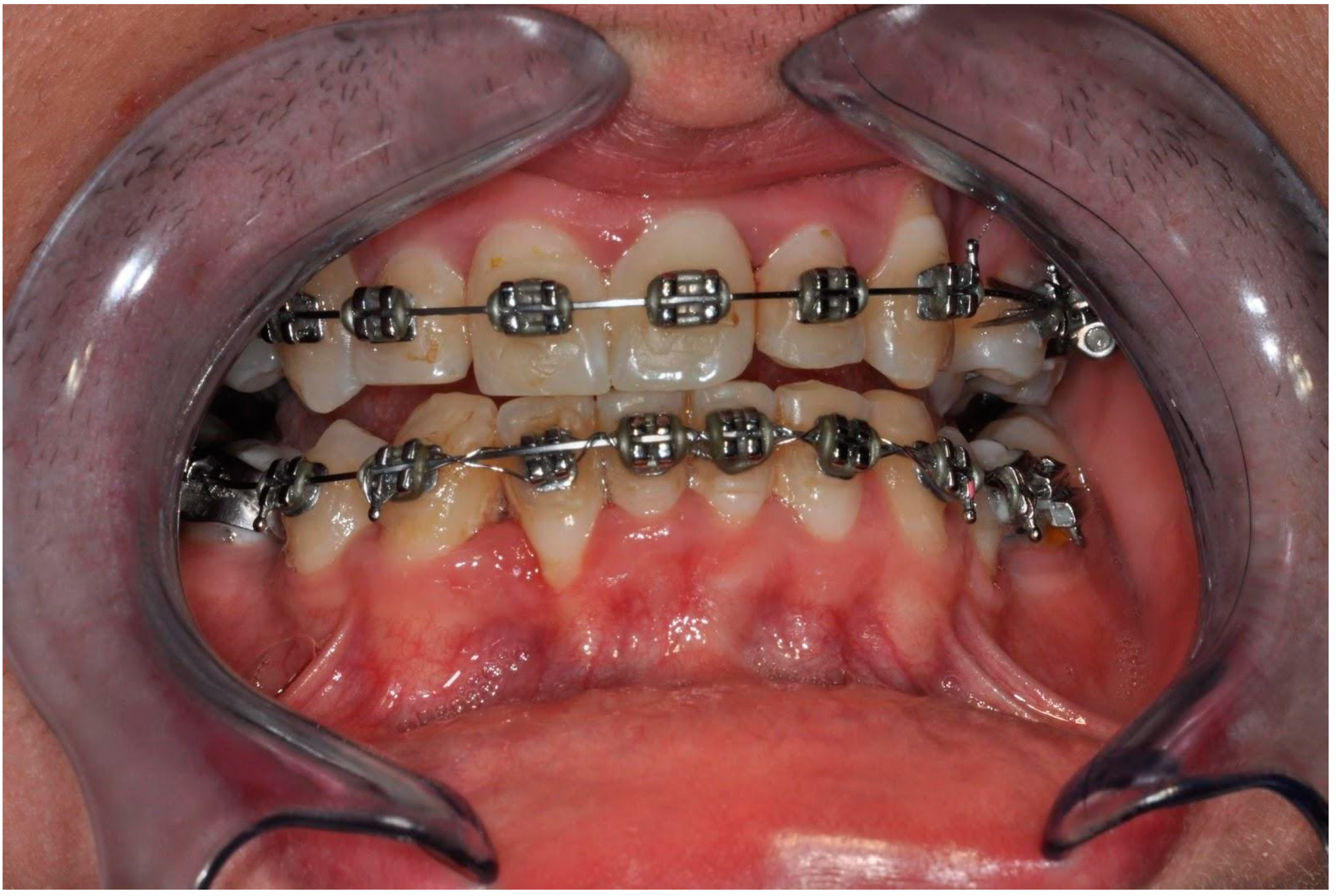
A clinical examination revealed an inadequate amount of keratinized gingiva, Miller grade II gingival recession in the area of tooth 42 (right lateral incisor in the mandible), a thin biotype of the gingiva with the correct vestibule height, as well as marked alveolar concaves and convexities [Figure 1]. Probing depth (PD) was 1–2 mm around lower incisors and canines. Bleeding on probing (BoP) was found in incisors. Attention was also paid to insufficient oral hygiene and plaque and calculus deposits. The patient underwent scaling and root planning. It was also recommended to perform cone-beam computed tomography (CBCT) of the anterior mandible. CBCT scans were captured using Pax Flex3D Vatech Computer Tomography X-ray System (Vatech, Hwaseong-si, Gyeonggi-do, Korea) with FOV 80 mm width and 50 mm height. Images were analyzed using EzDent-i software (Vatech, Hwaseong-si, Gyeonggi-do, Korea).
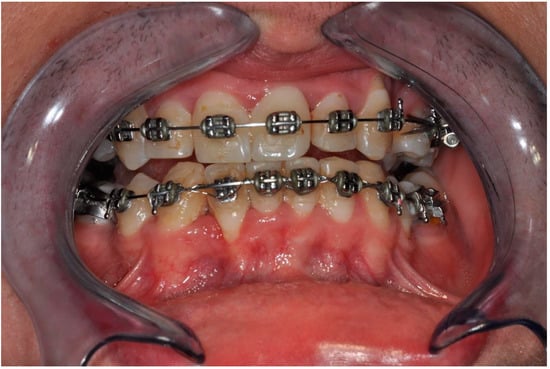
Figure 1.
Clinical picture before treatment showing the inadequate amount of keratinized gingiva, Miller grade II gingival recession in the area of tooth 42, a thin biotype of the gingiva with the correct vestibule height as well as including calculus, gingival recessions, and alveolar concaves and concavities.
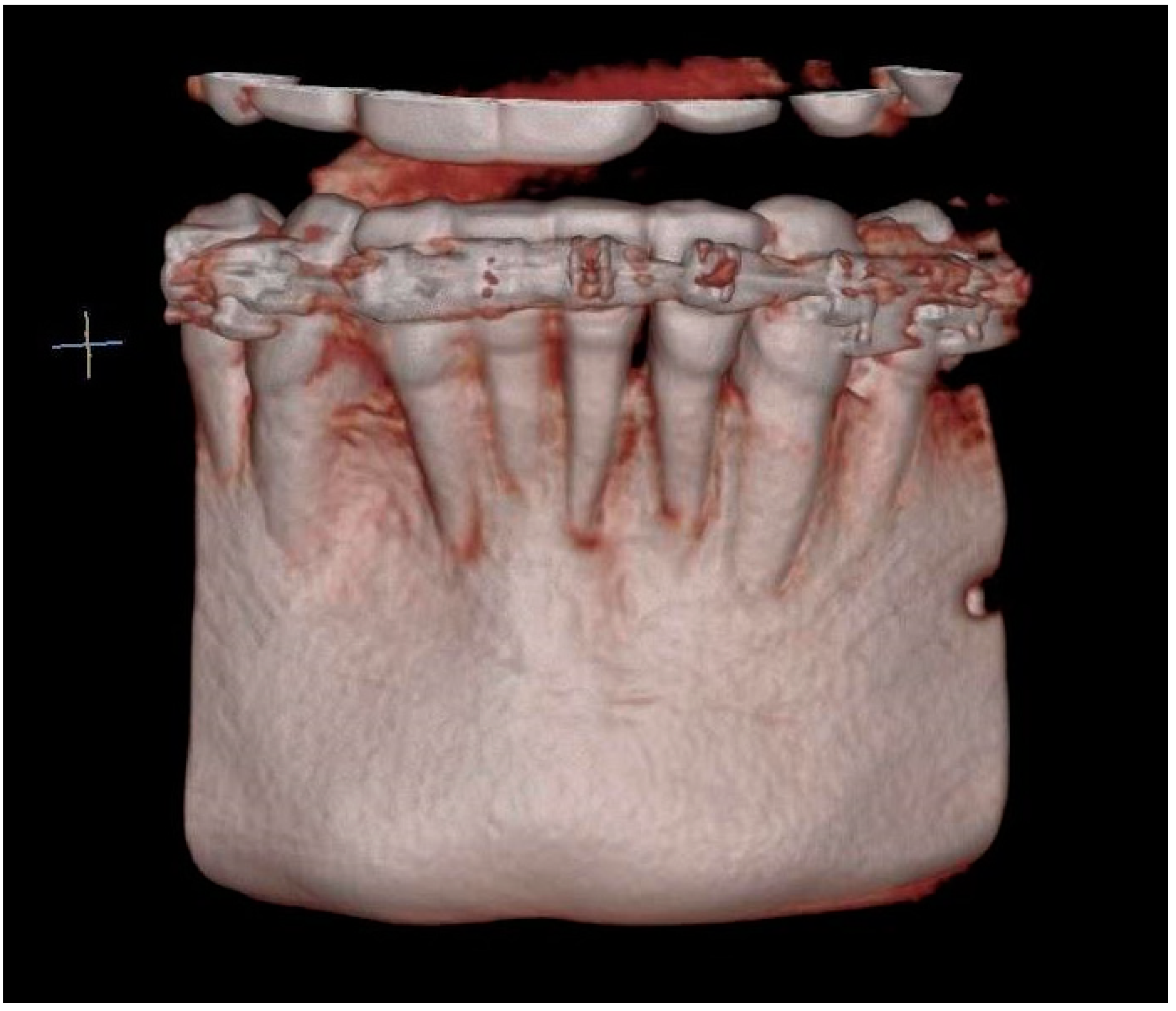
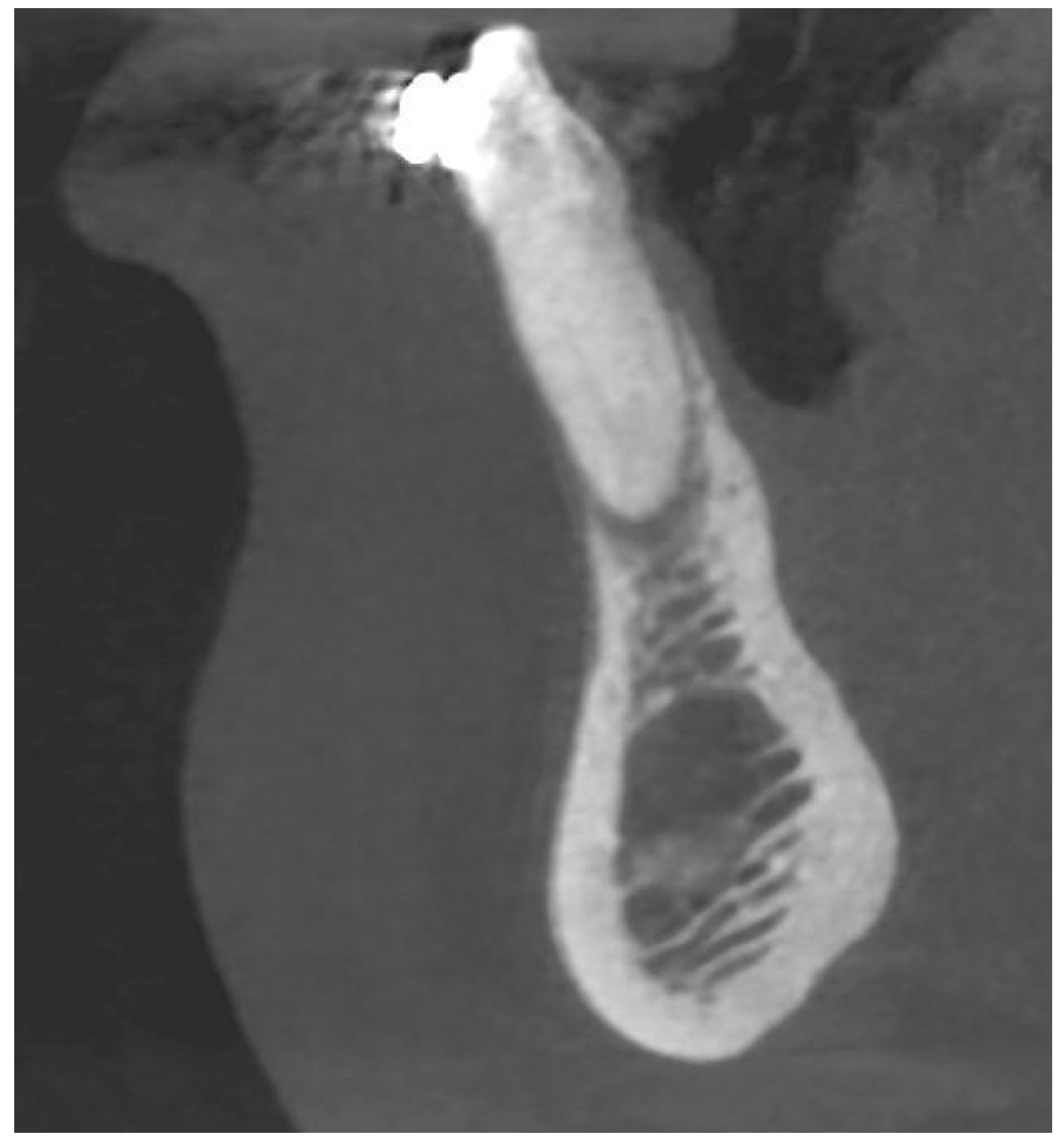
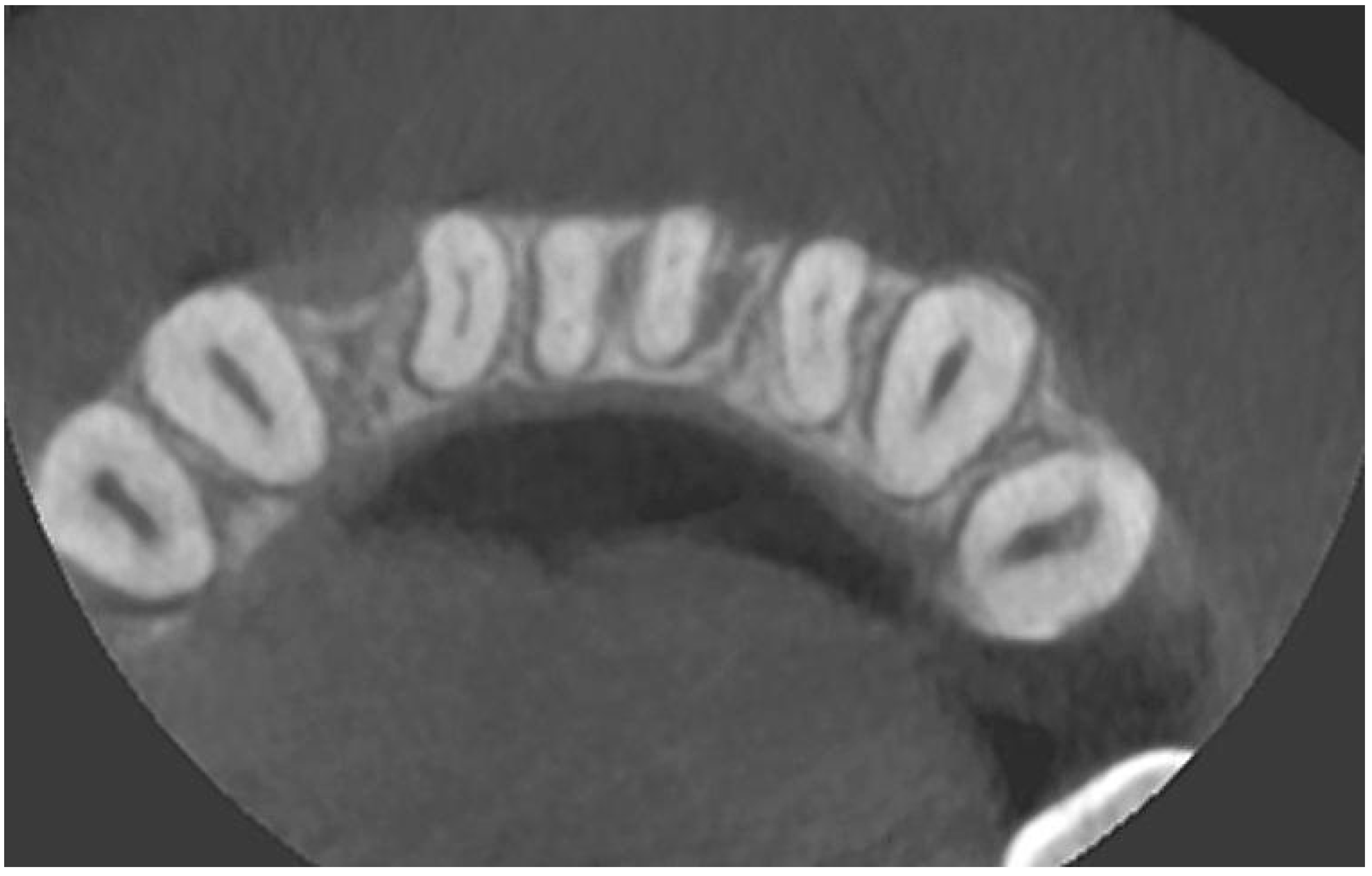
A radiological examination revealed a significant bone deficiency in this area, with clearly marked concavities in the alveolar part of the mandible. Advanced bone dehiscences from the vestibular side, with almost complete exposure of the anterior tooth roots, were also seen [Figure 2, Figure 3 and Figure 4].
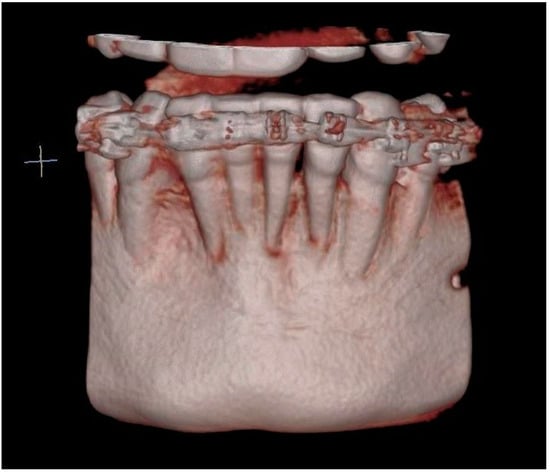
Figure 2.
CBCT image before the treatment showing advanced bone defects—three-dimensional reconstruction.
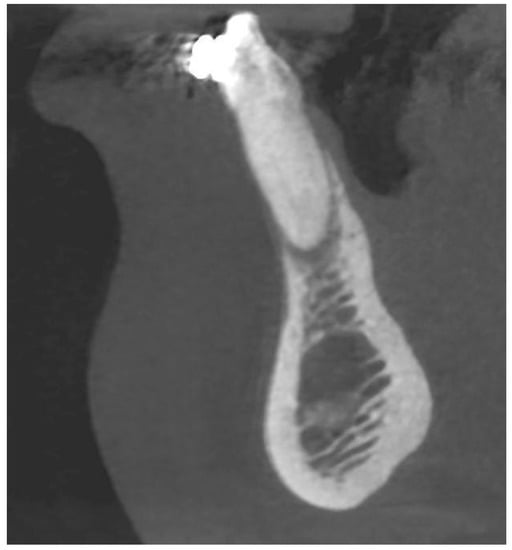
Figure 3.
CBCT image before the treatment showing an almost complete loss of the buccal bone—sagittal section.
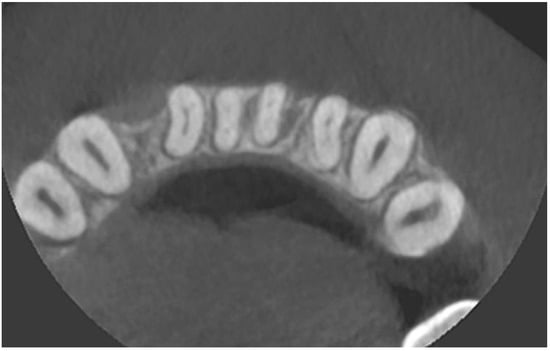
Figure 4.
CBCT image before the treatment—axial section.
The morphology of this region could be classified as C according to the alveolar bone dehiscence classification proposed by Yang et al. [15]. This means that the dehiscence is bilateral. In turn, according to the modification of this classification presented by Dominiak et al., which is more detailed, such a defect could be classified as BDIEII dehiscence from the vestibular side, which reaches an apical foramen, and bone loss from the lingual side is at the level of 1/2 of the root’s length [16].
Due to the inadequate dimensions and volume of soft tissues, as well as the consistent lack of the possibility of adequate bone reconstruction without excessive tension, it was decided to start with gingival augmentation—connective tissue graft (CTG). Tissue grafts are an indispensable stage in the reconstruction of the mucogingival complex.
Under local anesthesia with a 4% solution of articaine with epinephrine 1:100,000 (Ubistesin forte, 3M™ eSPe™, Wroclaw, Poland) the receiving bed in area 32–42 was prepared. A CTG was obtained from the palate and was then sutured into the recipient site. Excessive activity of the mental muscle, which causes the pull of soft tissues, was eliminated by intramuscular injection of botulinum toxin (0.6 mL in the horizontal line; usually, about 24 IU is required (BOTOX® Cosmetic, Allergan, Dublin, Ireland) [17,18].
In the clinical follow-up examination, which was conducted after less than three months, the adequate width and thickness of the soft tissues, as well as the complete coverage of the recession in the area of tooth 42 were recorded. It was therefore decided to start bone reconstruction surgery.
The bone reconstruction procedure with the use of an individualized three-dimensional allogeneic bone block was carried out according to the method patented by Dominiak and Gedrange (EP3287097B1, 2016). First, the block was positioned in relation to skeletal and facial parameters. An orthodontic diagnostic, including CBCT scans, intraoral and extraoral face scans, and a valid lateral cephalogram, was conducted.
In the second stage, the positioning of the block was based on the height, width and thickness of the alveolar bone, as well as the shape of the internal surface of the block. In addition, muscle and soft tissue parameters were evaluated, especially the thickness of the mental muscle (clinically known as the “orange peel” sign). Radiological diagnosis was also conducted, including a cephalometric radiograph, CBCT scans, and elastographic imaging.
The area of the defect was divided into 4 external regions and 1 internal region, and reference points were marked on the recipient field. A personalized bone block has to be highly suitable, thus the internal surface of the block was then assessed to adjust the contact area with the underlying bone to be as wide as possible.
The cephalometric analysis included the measurement of the ANB angle and the definition of the facial type. The correlation between the incisor position and the mandible and the inclination of the mandibular incisors was evaluated. Moreover, the relationship between the upper and lower incisors was considered.
It was extremely important to determine the alveolar bone level. For the prediction of bone resorption with regards to tooth movement, the ALi- CEJ2-B angle was evaluated. For this purpose, three points were set: the deepest concavity on the anterior surface of the mandibular symphysis (point B), the apical point of the most anterior mandibular central incisor (Iia), and the point on the cementoenamel junction of the incisor (CEJ) minus 2 mm, which corresponds to the sulcus depth. CBCT scans were used for shape planning of the whole bone block.
The target values were updated to include the actual reference point values, and a new area with target values was added to the diagnostic defect area. The bone block body was connected with the first analyzed plane of the mandibular segments with the applied bone block. The ANB angle was taken into consideration to ensure face harmony after reconstruction. The shape of the chin and the block’s design were also adjusted. It was important to keep the block thickness within the thickness of the possible physiological bone regeneration. According to the method, the value of the ANB angle should not exceed 4–5° after reconstruction. Moreover, the long axis of the basal symphysis and the alveolar symphysis should be positioned as parallel to each other as possible, and the overall angle created between them should not exceed 10°. The chin’s form and the block’s design were also tweaked. It was critical that the thickness of the block did not surpass the thickness of feasible physiological bone regeneration. After reconstruction, the ANB angle should not exceed 4–5 degrees, according to the procedure. Furthermore, the long axis of the basal symphysis and the alveolar symphysis should be as parallel to each other as feasible, with an overall angle of no more than 10° between them. The shape and the position of the basal symphysis cannot be influenced by the tooth movement of the lower incisors.
The width of the bone block depended on the size of the bone defect. The assessment was carried out by the surgeon based on the analysis of the horizontal cross-section of the alveolar process, as well as objectively with the use of orthodontic analysis. The width was assessed from the right to the left canine to ensure it always matches the shape of the mandible in all dimensions. The incisor crowns were measured, and the measured value determined the arch shape between the canines. The position of the teeth and the alveolar process were drawn on CBCT scans and supplemented with the perfect size of the arch.
The height of the bone block was assessed using two measurements: in the direction of the crown and the apex, while the level of the crestal bone was positioned at the CEJ level minus 2–3 mm which is the biological width of teeth with healthy periodontium. The gingival biotype evaluation was performed using the puncture technique, which was followed by reading the values from the periodontal probe scale.
Then came the phase of computer-aided design and manufacturing (CAD/CAM). A virtual model of the mandible was created by converting CBCT scans and intraoral scans into digital models. A bone block design was placed on the model, and the actual and target value points were placed into one unit. A suitable live donor was selected according to predetermined aspects: an adequate amount of cancellous and/or cortical bone (in this case only cancellous bone), proper bone density-D2-D3 according to Misch. After milling, the block was cleaned, packed, sterilized, and sent to the surgeon. A detailed description of bone block planning, including diagrams of the procedure, has been previously described [16].
The bone reconstruction was performed under local anesthesia with a 4% solution of articaine and epinephrine 1: 100,000 (Ubistesin forte, 3M™ eSPe™, Wroclaw, Poland), and also with an antibiotic—0.6 g of clindamycin administered p.o. 1 h before the procedure (Clindamycin MIP, MIP Pharma GmbH, Blieskastel, Germany). The entire surgical procedure was carried out following a previously established scheme. Photos of the key moments of the surgery show the procedure of another patient who had the same operation. The only difference is the type of bone block fixation. In the presented case, resorbable pins made of amorphous poly-(d,l)-lactide were used, while the photos show a procedure that uses titanium screws. Both variants are acceptable and effective for fixing the block. When titanium screws are used, they are gently removed after 6–8 weeks.
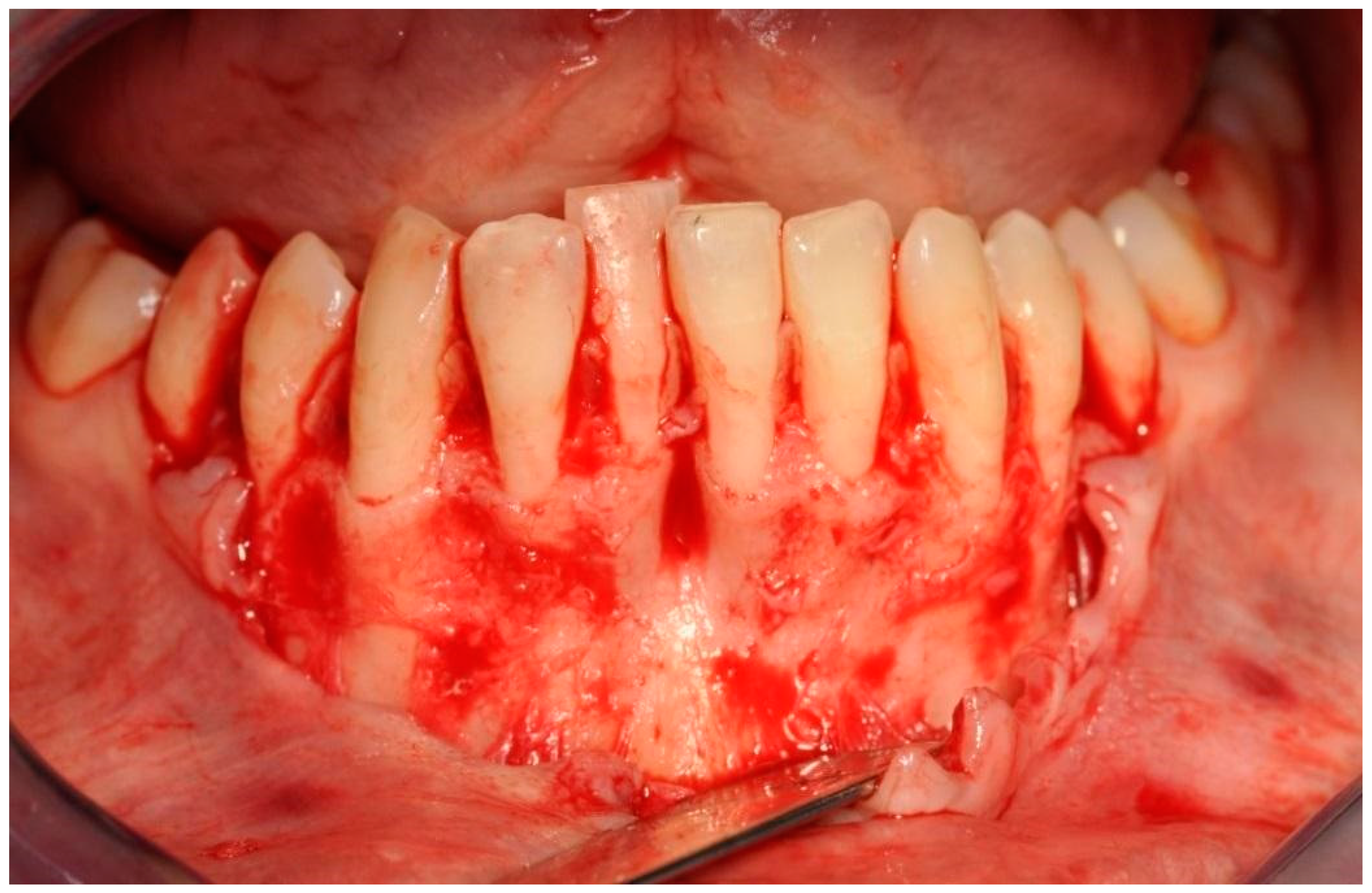
First, an intrasulcular incision was made two spaces mesially and distally wider than the planned treatment area and the envelope mucoperiosteal flap was elevated beyond the mental eminence to create a receiving bed without an excessive tension of the flap. Due to this, bone dehiscences and concavities of the mandibular alveolar part were exposed [Figure 5].
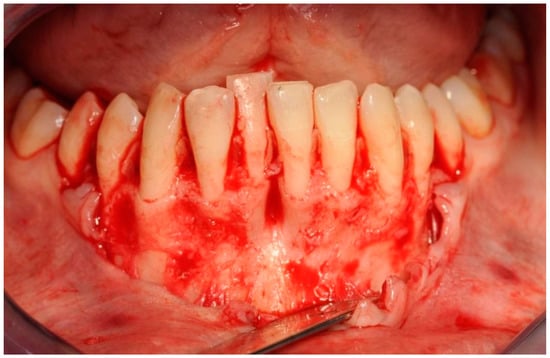
Figure 5.
Exposed tooth roots and bone dehiscences. Picture taken after mucoperiosteal flap elevation in the area of planned bone reconstruction.
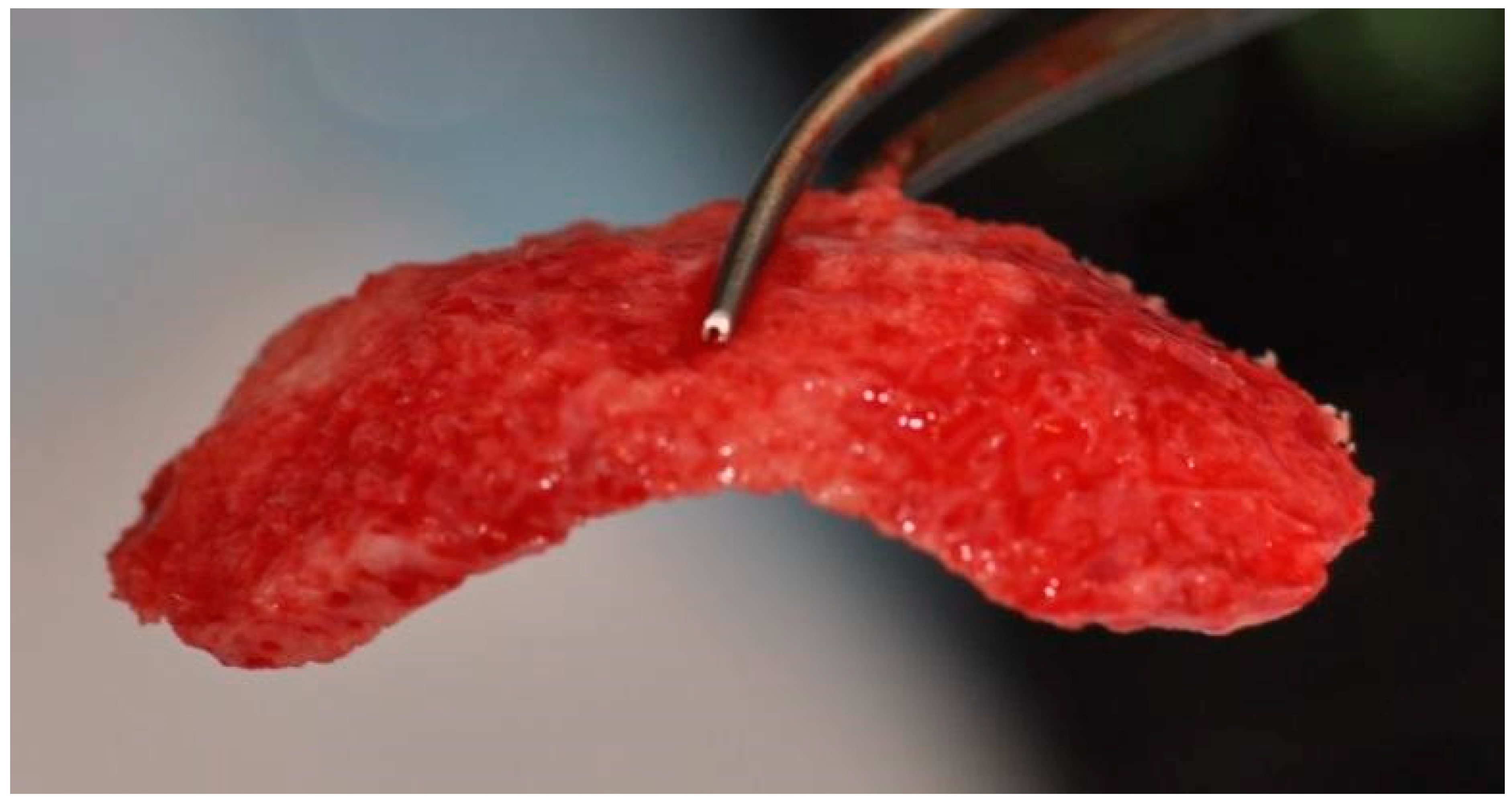
Root surfaces were then mechanically cleaned of deposits, without the use of additional substances. Afterward, a drill was used to decorticate the cortical layer in the interdental spaces to improve vascularization, and advanced platelet-rich fibrin (A-PRF) membranes were prepared from then peripheral patient’s blood and placed on its surface (PRF Duo, Process for PRF, Nice, France) [19]. Allogeneic bone particles obtained from the Bone Bank in Poland were introduced into the deepest bone defects, followed by the placement of an individualized three-dimensional block of allogeneic bone [Figure 6].
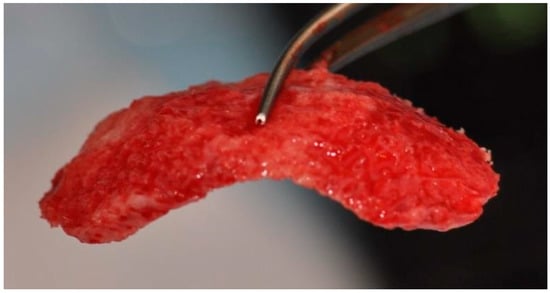
Figure 6.
Individualized allogeneic bone block soaked in injectable platelet-rich fibrin before placement.
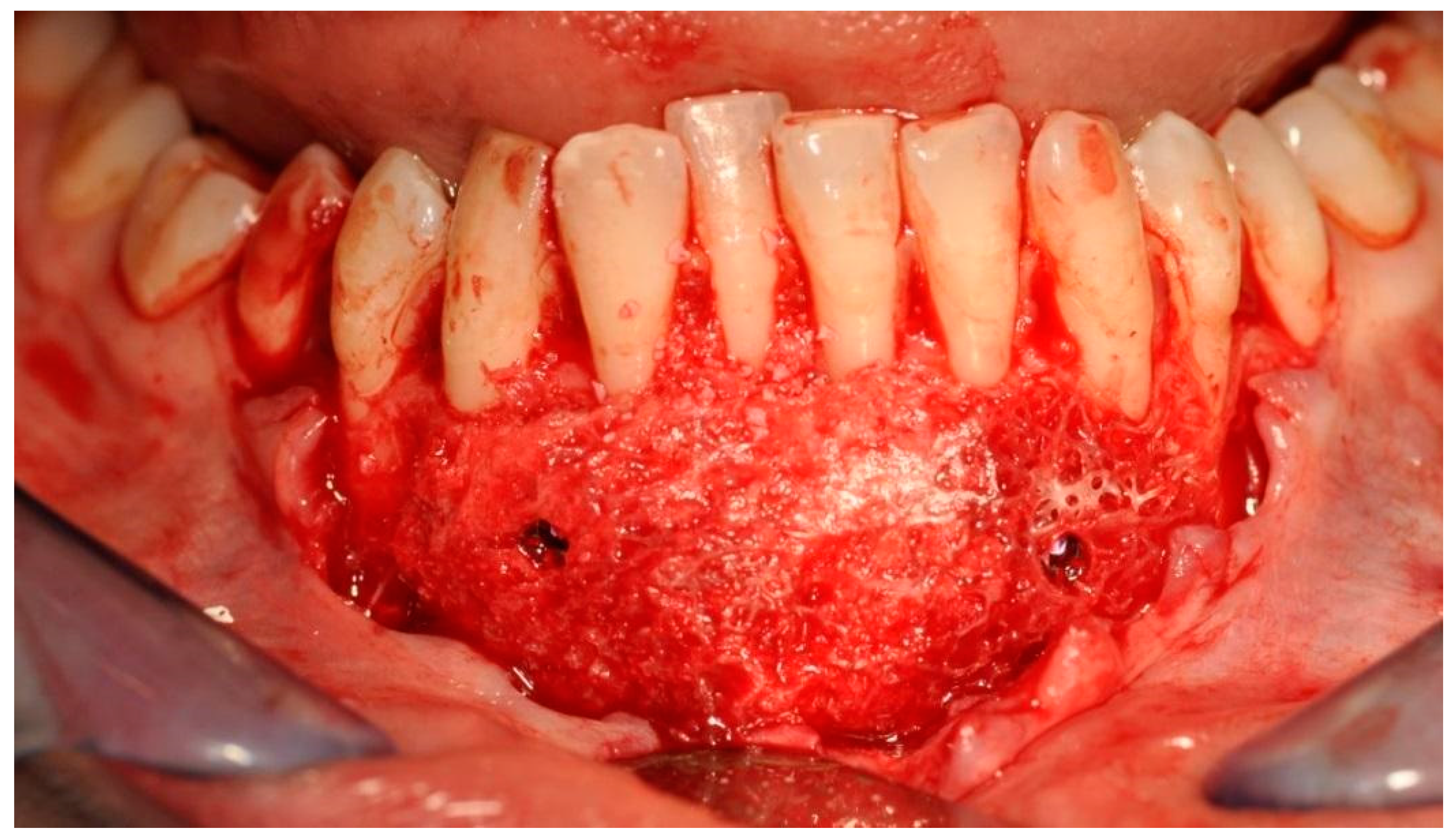
In order to immobilize the bone block, and for its appropriate adaptation to the base, two 11 mm long resorbable polylactaic pins were ultrasonically introduced using the SonicWeld Rx® technique (KLS Martin, Jacksonville, FL, USA). Titanium screws can be used instead of resorbable pins, but should be removed after 6–8 weeks. Screws of about 8 mm long are usually used, i.e., Surgident Co (Daegu, Korea) [Figure 7].
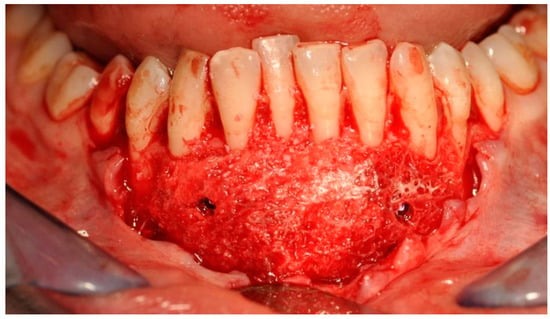
Figure 7.
Bone block fixed to the mandibular bone—a recipient site with titanium screws.
The A-PRF membranes were placed on the outer surface [Figure 8], and a resorbable collagen barrier membrane Geistlich Bio-Gide (Geistlich Pharma AG, Wolhusen, Switzerland), which was previously soaked in a liquid fraction of injectable platelet-rich fibrin (i-PRF) (PRF Duo, Process for PRF, Nice, France) was placed apically.
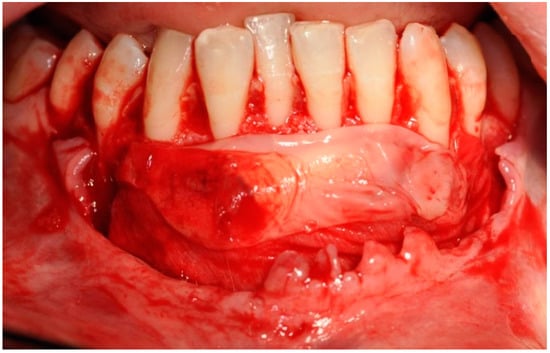
Figure 8.
Advanced platelet-rich fibrin and collagen membranes placed on the surface of the bone block.
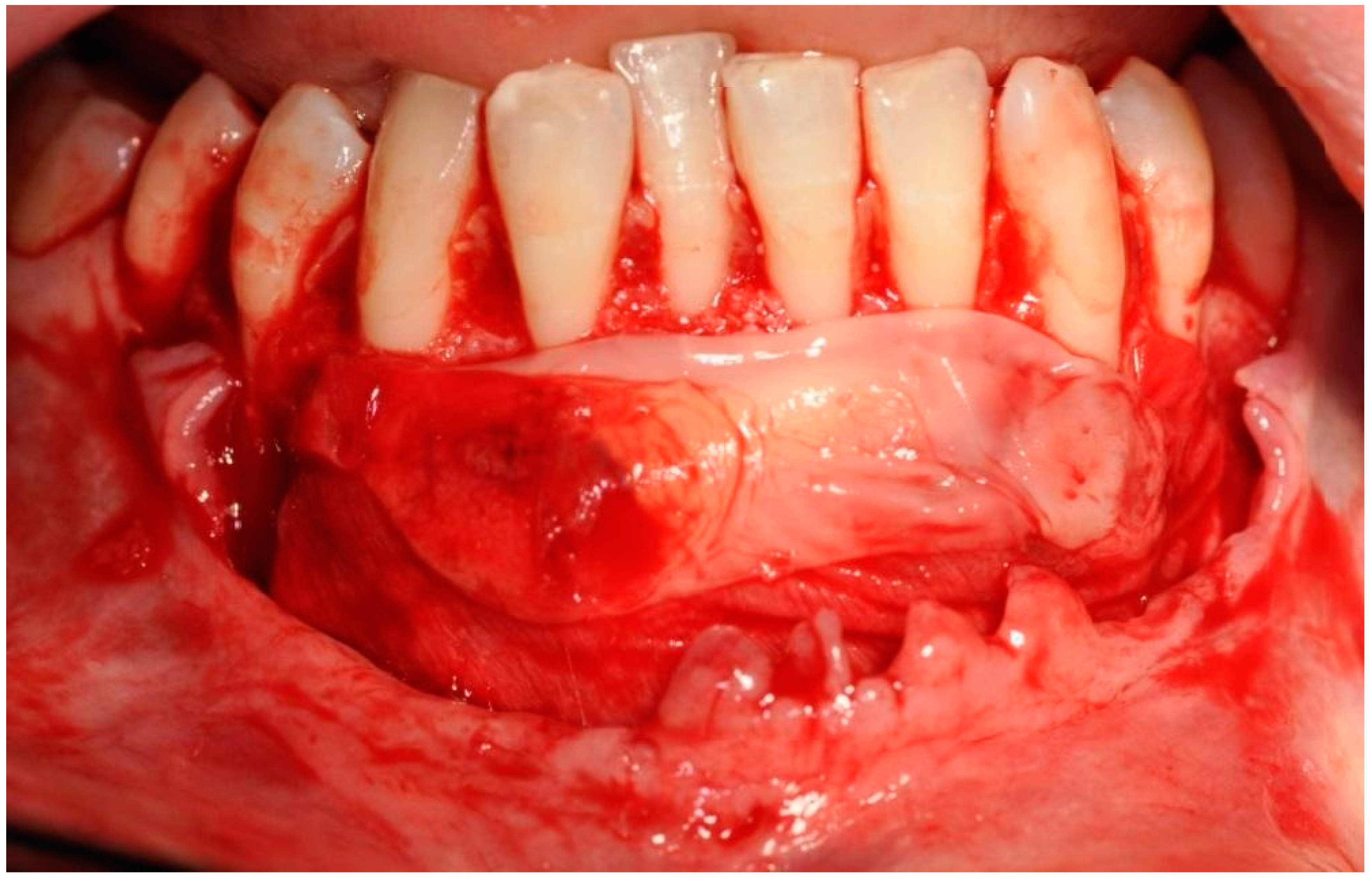
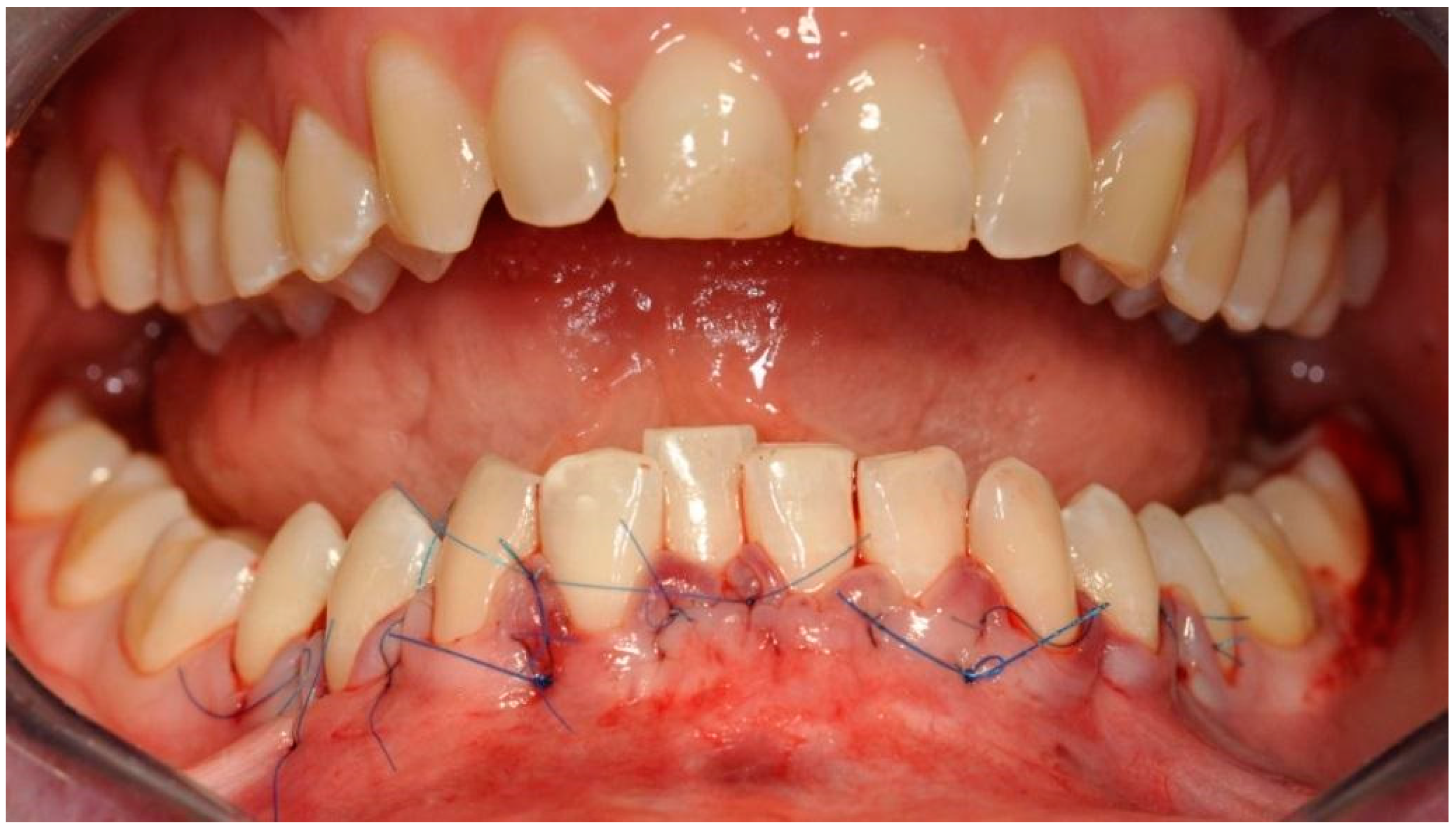
The selected barrier membrane consists of pure non-cross-linked collagen. Its purpose is to prevent the penetration of connective tissue under the block in the chin. It cannot significantly alter the vascularization of the bone block, and therefore it must be of rapid resorption. The last stage involved suturing using the technique of suspended and mattress sutures, which prevented excessive tension of soft tissues (Figure 9).
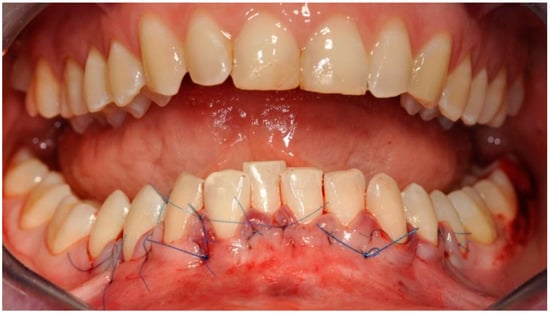
Figure 9.
Reposition and suturing of the soft tissue without extensive tension.
An antibiotic, 0.6 g clindamycin, twice daily and an analgesic and anti-swelling agent for three days were prescribed. The patient was instructed to maintain appropriate oral hygiene using an antiseptic foam preparation and also to use a soft post-treatment brush. Immediately after the procedure, biostimulation with the Nd: YAG laser (10 Hz, 0.5 W) was performed (TwinLight®, Fotona, Ljubljana, Slovenia). The sutures were removed two weeks after the surgery.
Written informed consent was obtained from the patient. The study was conducted according to the guidelines of the Declaration of Helsinki, and the Bioethics Committee’s approval was obtained (KB-530/2021) (Bioethics Committee of Wroclaw Medical University).
3. Results
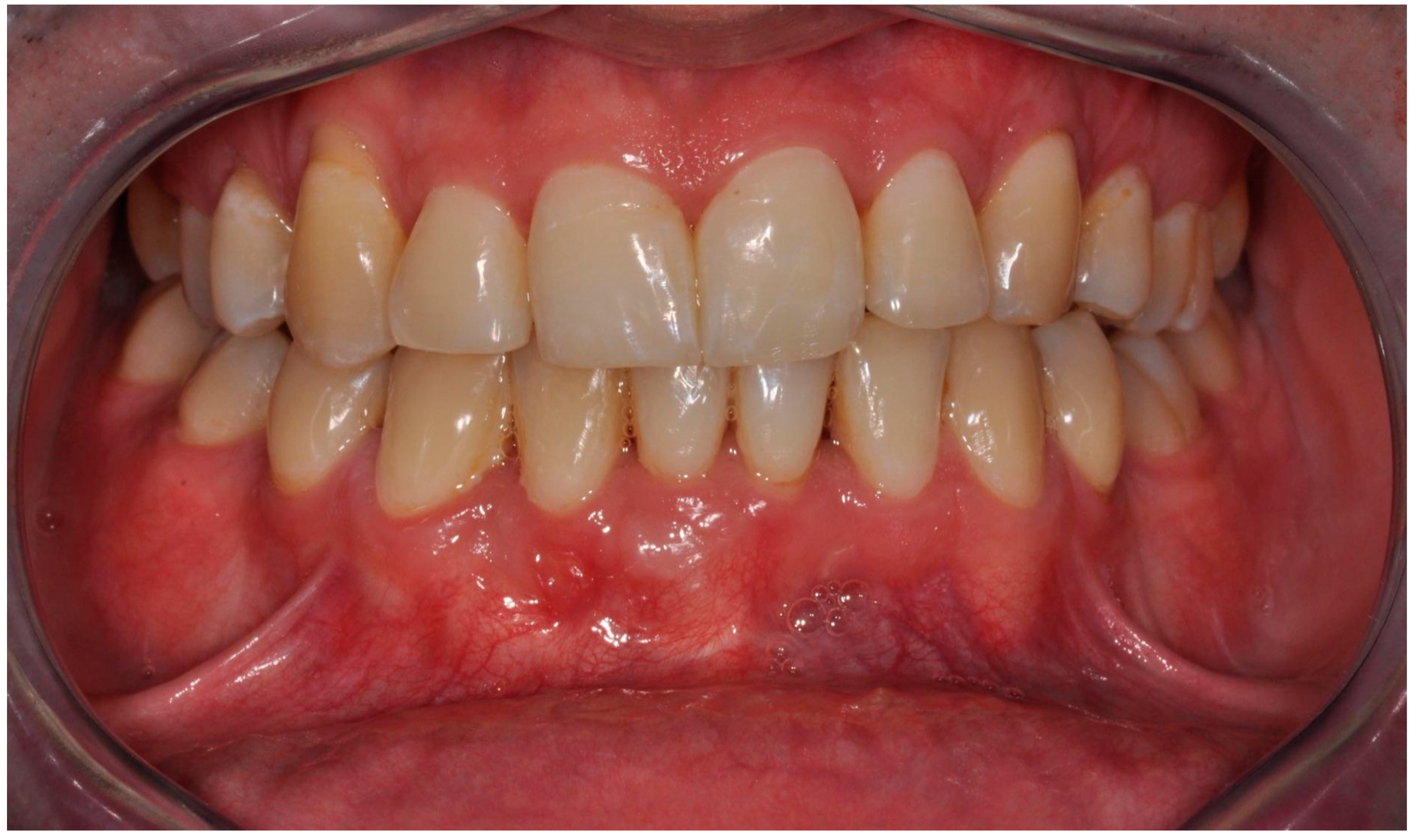
The first follow-up CBCT examination was performed after twelve months. A progressive remodeling of the bone block with its perfect adherence to the bone was visible. The correct arch curvature was also restored, and a significant increase in the volume of the bone covering the roots of the teeth was achieved. The clinical examination showed no signs of inflammation or recession at that time. Probing depth did not change significantly. There was no calculus and no bleeding on probing (Figure 10).
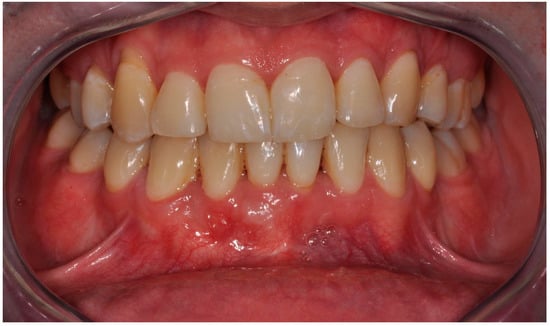
Figure 10.
Clinical follow-up picture showing proper occlusion, the adequate amount of keratinized gingiva, effect of gingival recession coverage with the correct vestibule height as well as lack of calculus and advanced alveolar concaves and concavities. There was only a slight reddening of the gingiva in area 42 associated with a small loose bone fragment resulting from the remodeling of the graft.
Another follow-up radiographic examination two years after the reconstruction procedure revealed the complete remodeling of the allogeneic material, as well as the formation of the vestibular cortical layer, despite the fact that the surgical procedure used a block that only consisted of cancellous bone. The correct morphology of the mandibular alveolar part was restored with a significant reduction in dehiscence. Clinically, there were no signs of inflammation or gingival recession, and the correct depth of the oral vestibule was maintained. The lower incisors and canines did not show excessive mobility.
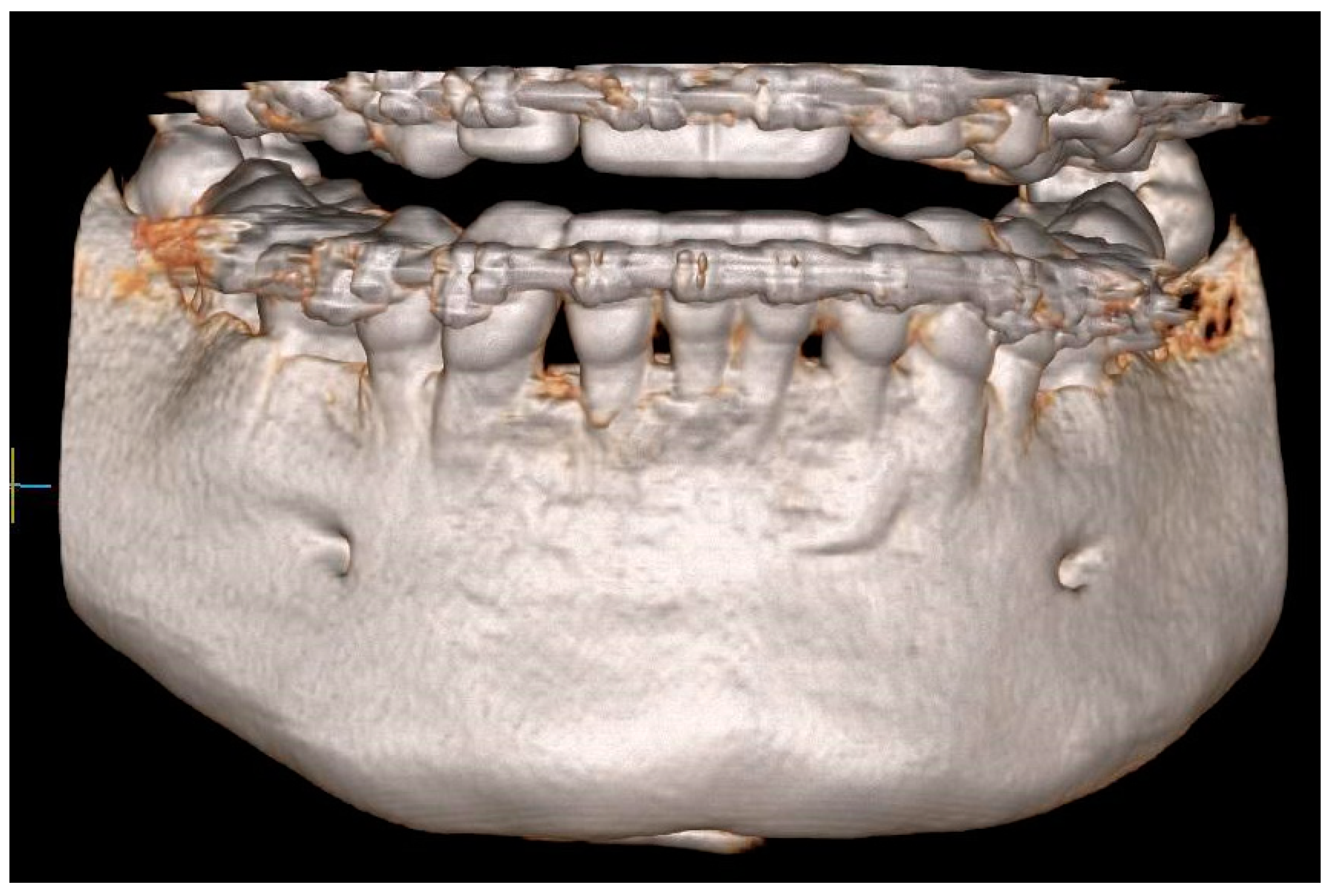
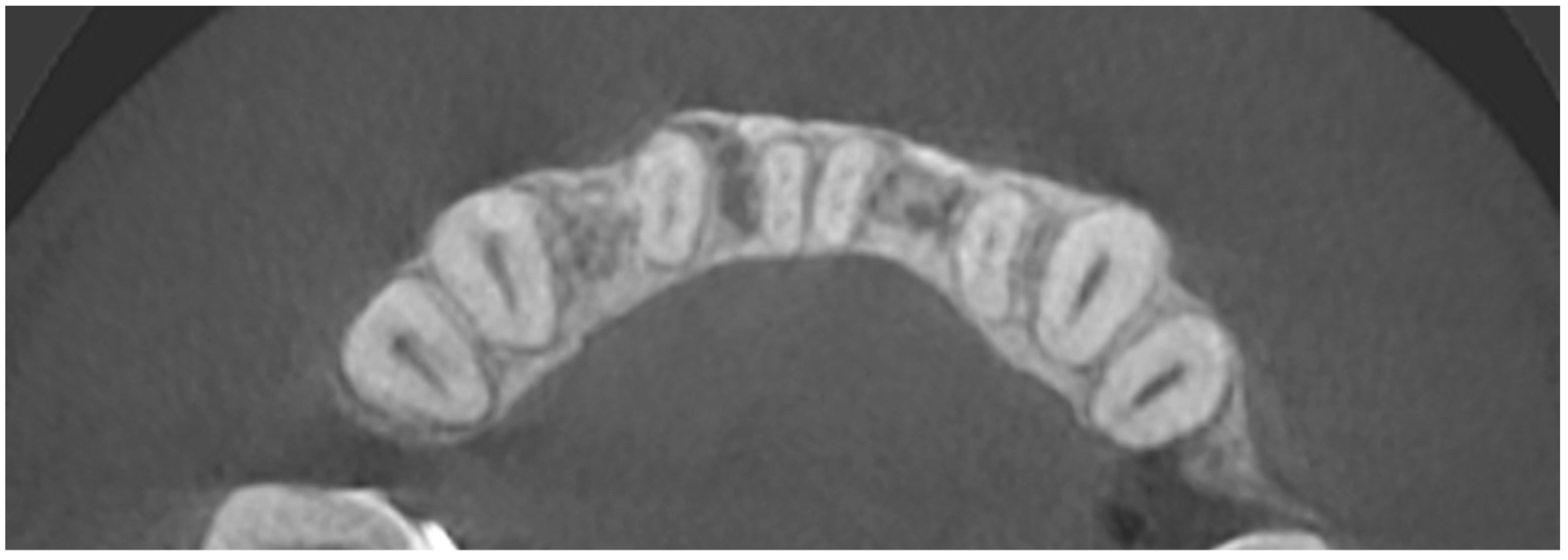
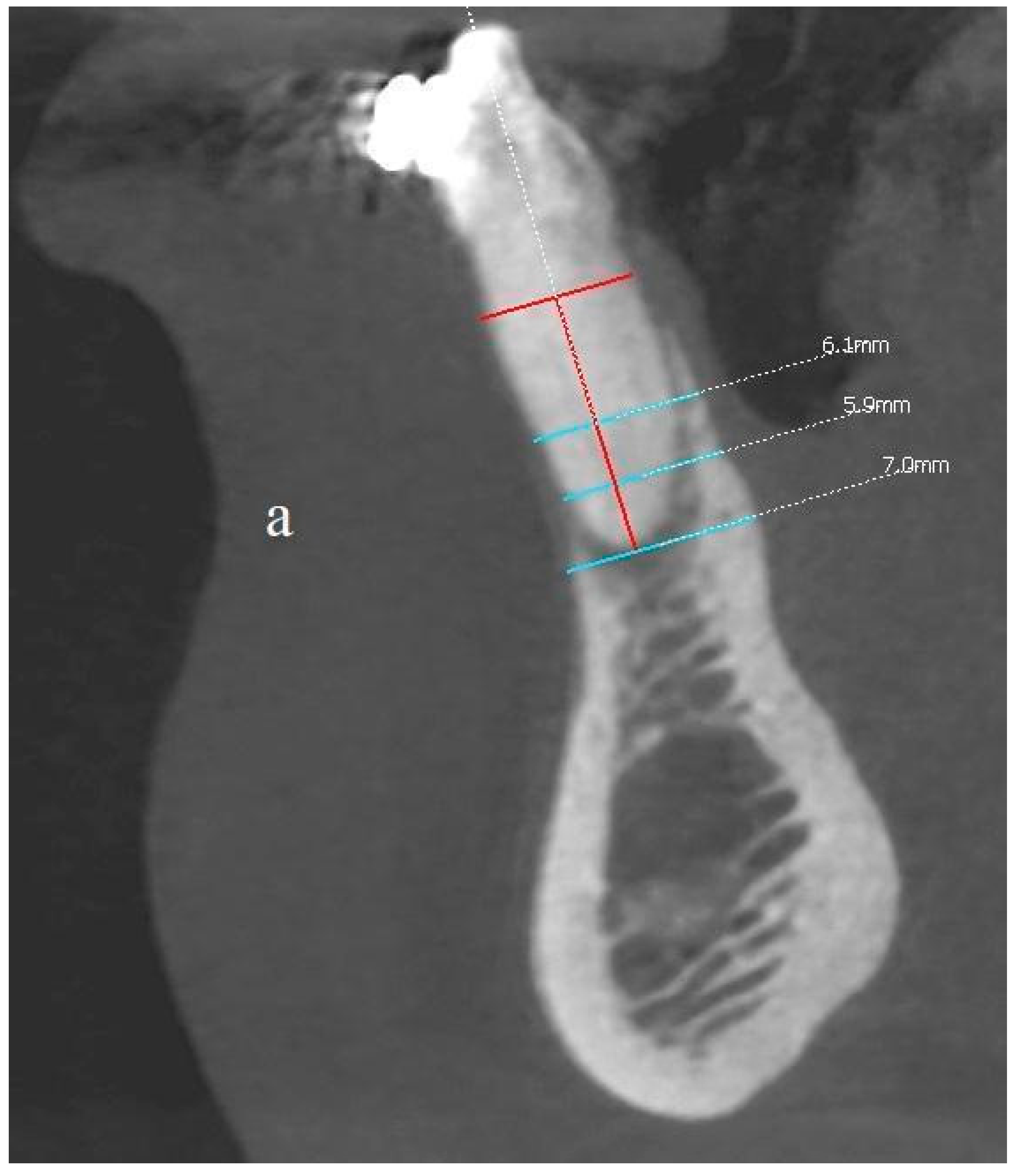
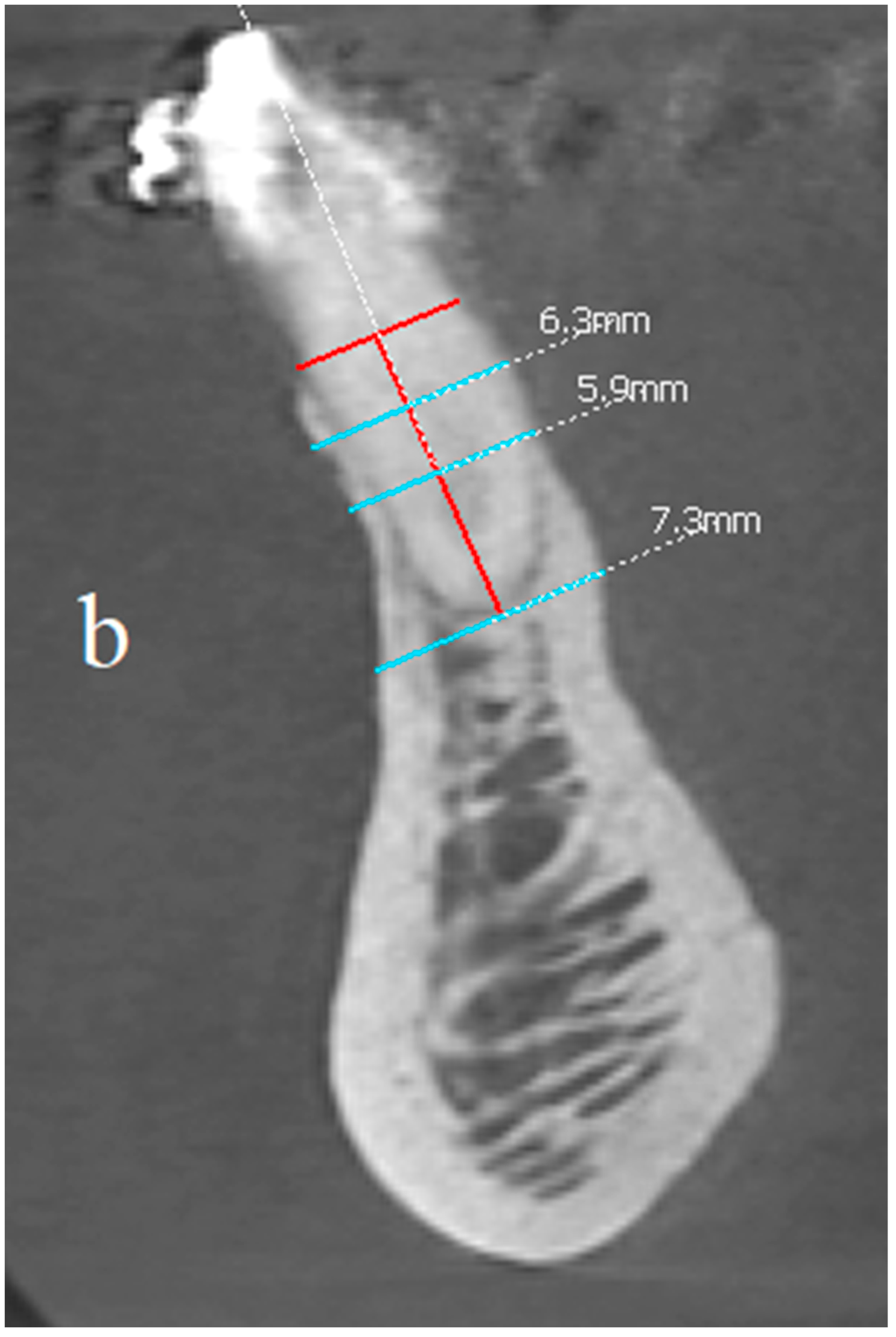
In order to assess the scope of the reconstruction, radiological measurements of the width of the mandibular alveolar bone were performed in the sagittal plane both before the reconstruction procedure and after two years (Figure 11, Figure 12 and Figure 13). Both CBCT examinations were performed using the same equipment and the same imaging parameters. Measurements were taken at certain adopted reference points within the area of tooth 31 (medial left incisor in the mandible). The reference lines were set perpendicular to each other, with one of them being along the long axis of the tooth’s root.
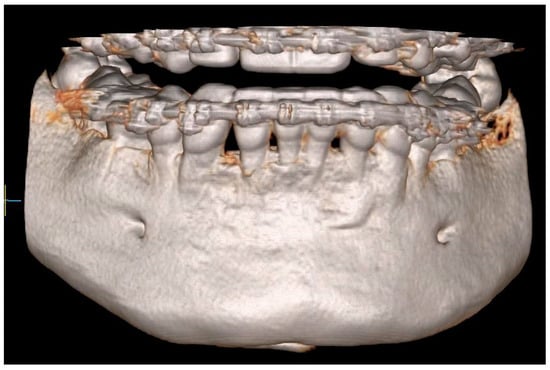
Figure 11.
CBCT image two years after the surgical treatment—three-dimensional reconstruction.
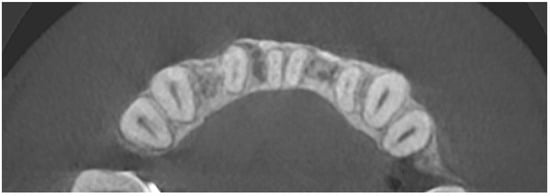
Figure 12.
CBCT image two years after the surgical treatment—axial view.
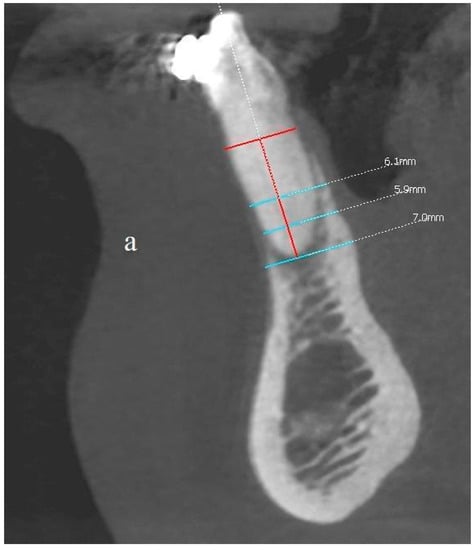
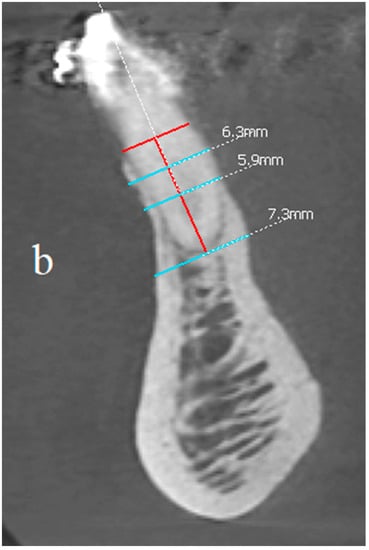
Figure 13.
(a,b) Comparison of the CBCT scans (sagittal view) before the procedure and two years after the surgical treatment. Before and after images at the same location, the same threshold level is used.
The comparison of the baseline dimensions and those at the end of the follow-up were as follows:
- The bone width at the half-length from the cementoenamel junction, minus 2 mm, (CEJ-2) to the apex: 6.1 mm before surgery, 6.3 mm after surgery; difference +0.2 mm;
- The bone width at the quarter-length from the CEJ-2 to the apex: 5.9 mm before surgery, 5.9 mm after surgery; difference 0 mm;
- Bone width measured at the height of the apex: 7 mm before the procedure, 7.3 mm after the procedure; difference + 0.3 mm;
- Bone dehiscence from the vestibular side (calculated from the CEJ-2 point): 7.5 mm before the procedure, 1 mm after the procedure; difference −6.5 mm.
The final dimensions after the observation period were not only influenced by the reconstruction procedure, but also by the orthodontic movement of the teeth (Figure 13a,b).
4. Discussion
Asa’ad et al., when focusing on the topic of bone tissue engineering, emphasized the significant benefits of introducing scaffolds with a three-dimensional structure, which imitate the extracellular matrix. By design, this promotes the adhesion, proliferation, and differentiation of cells. They also mentioned that the used biomaterials must be compatible with the native environment, and consequently must lead to regeneration, not repair in which scar tissue is formed. The authors describe scaffolds combined with stem cells and/or osteoprogenitor cells, which are placed on the prefabricated scaffold or incorporated through encapsulation during the production of the scaffold. This is performed by using a hydrogel polymer matrix and by capturing the cells within a semipermeable membrane. They paid attention to the fact that the regeneration process begins peripherally in the places where biomaterials come into contact with the bone, which emphasizes the essence of the perfect adhesion of the implant to the recipient site [11].
Rasperini et al. presented a therapeutic method that aimed to regenerate periodontium using a three-dimensional resorbable polymer scaffold in the course of an aggressive form of periodontitis. Their design included the conducting of perforations to immobilize the scaffold, the internal port for the delivery of recombinant human platelet-derived growth factor-BB (rhPDGF-BB), and the protruding perpendicular elements to produce periodontal ligament. During a 12-month observation, when the scaffold was completely covered with soft tissues, the authors observed a 3 mm improvement in the connective tissue attachment, and also partial coverage of the previously exposed root. However, after 13 months, the scaffold was exposed to the oral environment. Fragments of the scaffold were removed and replaced by amelogenin, and then the pocket was sealed with surgical cyanoacrylate gel. Due to this, the graft was uncovered 3 mm below the gingival margin, which required the removal of the exposed fragment. Afterward, there was an increase in dehiscence, which resulted in the need to completely remove the scaffold. Finally, it was found after 14 months that 75.9% of the scaffold’s molecular weight remained and that the healing mainly concerned the connective tissue—with minimal evidence of bone repair. Ultimately, the therapy was not proven to be successful. This procedure also required several preparatory steps, a meticulous three-dimensional design, and involved the use of polymers and a recombinant growth factor. However, the authors confirmed the correctness of using biomaterials with a three-dimensional structure, but at the same time raised doubts about the use of a polycaprolactone scaffold. The failure to achieve the desired effect was also explained by the fact that the used material was high in volume and slowly resorbing [20].
An implant material should not only be easy to use but also be perfectly biocompatible for it to be able to integrate, remodel and provide an appropriate scaffold for osteogenic cells. Allogeneic bone blocks fulfill these criteria [7,8]. However, the collection of autogenous blocks and the use of allogeneic blocks supplied from tissue banks require shaping and fitting when the traditional intra-procedure approach is used. In turn, the use of CAD/CAM technology significantly improves this process. The use of allogeneic bone is also associated with reduced surgical intervention and blood loss, a lack of donor site complications, and negligible antigenicity [21].
The success of treatments with the use of this type of biomaterial is estimated at about 90% in the literature, but an increased risk of its contamination and, consequently, of infectious complications when compared to autogenous blocks cannot be omitted. However, it has been determined that these complications either occur when matching the block to the bone defect or are the result of improper management of soft tissues, which results in the exposure of the graft, the opening of the wound, and perforation of the mucosa [22,23]. It was also shown that these complications are more common in the mandible due to its lower vascularization, as well as the fact that the flap is more difficult to manage [12]. Thus, proper evaluation of soft tissues is essential in planning bone augmentation procedures. In cases of an insufficient amount of soft tissues and an increased risk of the inability to close the wound without excessive tension of the flap, implementation of correction/augmentation procedures preceding the actual bone reconstruction surgery is recommended. Such a procedure was carried out in the presented case.
The performed procedures conducted on soft tissues allowed for obtaining their sufficient width and thickness and avoiding the primary closure of the wound with excessive tension of the flap. In turn, the use of a fully individualized three-dimensional allogeneic bone block allowed for the restoration of the correct morphology of the anterior mandibular segment of the alveolar part, which had dentition, and which was characterized with existing advanced soft tissue dehiscences and recessions. The perfect fit of the block to the recipient site minimized the risk of graft contamination when adjusting it in the shortest possible contact time, and at the same time ensuring its stability and the lack of mobility towards the base.
After two years, based on clinical observations, it was stated that the intended therapeutic effect was achieved. There was no recession, shallowing of the vestibule, signs of inflammation, or pathological mobility of the teeth in the area undergoing reconstruction. The radiographic images revealed the formation of a new layer of cortical bone on the vestibular side and a certain volume of cancellous bone. It is worth noting that the block was prepared only from the spongy bone. This is probably related to the way bone blocks are remodeled, which depends on the force and physiological loads acting on them [24]. Moreover, there was no radiological demarcation zone of brightening, which could indicate an incomplete adaptation, integration and reconstruction of the bone block.
Similar findings in CBCT studies were reported by Blume et al., who also indicated the formation of a compact bone layer after a 10-month observation. Their histological analysis confirmed that the augmented tissues showed a high degree of mineralization, without signs of inflammation. Moreover, the ongoing reconstruction process was noticed. Concavities, which are characteristic of remodeling, were also observed. They concluded that the area of nonmineralized tissue located apically to the specimen probably resulted from soft tissue parts being attached to the outer surface. Soft tissue was also observed at the apical part of the specimen. The authors concluded that the accumulation of soft tissue represents the border zone between the graft and the native bone. They also emphasized that an area with the increased lining of connective tissue at the distal part of the biopsy specimen indicates the border zone between native bone and augmented bone [22].
The limitation of this method is the cost of making the bone block, which requires many stages, both in terms of planning and production. Due to the fact that this is a patented method not yet in commercial use, it increases the costs further. However, we hope that the detailed presentation of the method, both in the technical and clinical aspects, along with showing its positive results, will allow for commercialization and widespread use in specific patients’ indications. To confirm the effectiveness of the reconstruction and to show the bone remodeling, patients are subjected to CBCT scans every six months (up to 2–3 years). However, we plan to reduce the frequency and number of control radiological examinations after testing a large group of patients, proving the correct method of remodeling, and obtaining the final results. In the future, we also plan to focus on additional activities and materials that added to the described and developed method, would allow for an even better degree of reconstruction. These include, for example, growth factors, laser therapy.
5. Conclusions
In conclusion, our observation of treatments with the use of an individualized three-dimensional bone block allows us to distinguish the many advantages resulting from this method. Careful planning, considering clinical and radiological examination, as well as orthodontic and periodontal evaluation with the precise fabrication of such a bone block contributes to the providing of a three-dimensional structure that facilitates cell adhesion and angiogenesis with a low risk of complications and immune reactions. For this process, tissue banks that allow obtaining a graft are of importance. Using tailored blocks adjusted to each patient individually makes the procedure shorter and easier, without the need for intraoperative modeling of their shape and surfaces. It is possible to achieve perfect adhesion along the entire surface and to avoid micromovements. The great success of this method is the effect of the bone reconstruction in the area of the teeth in their vertical dimensions.
Author Contributions
Conceptualization, M.D. and T.G.; methodology, M.D. and T.G.; investigation, M.D., S.H., C.O. and T.G.; resources, M.D.; data curation, M.D., S.H., C.O., S.D. and T.G.; writing—original draft preparation, M.D., S.H. and C.O.; writing—review and editing, M.D., S.H., C.O. and S.D.; supervision, M.D. and T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Authors.
Conflicts of Interest
The authors declare no conflict of interest.
Patents
T. Gedrange and M. Dominiak, “Verwendung einer Kenngröße eines Individuums zur Beurteilung des Risikos eines Zahnfleisch- und/oder Knochenschwundes an einem Schneidezahn”, European Patent Office, Munich, Germany, 2016, EP3287097B1.
References
- Dominiak, M.; Gedrange, T. New Perspectives in the Diagnostic of Gingival Recession. Adv. Clin. Exp. Med. 2014, 23, 857–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puzio, M.; Błaszczyszyn, A.; Hadzik, J.; Dominiak, M. Ultrasound assessment of soft tissue augmentation around implants in the aesthetic zone using a connective tissue graft and xenogeneic collagen matrix-1-year randomised follow-up. Ann. Anat. 2018, 217, 129–141. [Google Scholar] [CrossRef]
- Khoury, F.; Hanser, T. Mandibular bone block harvesting from the retromolar region: A 10-year prospective clinical study. Int. J. Oral Maxillofac. Implant. 2015, 30, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Esteibar, J.R.; Zorzano, L.A.; Cundín, E.E.; Blanco, J.D.; Medina, J.R. Complete root coverage of Miller Class III recessions. Int. J. Periodontics Restor. Dent 2011, 31, e1–e7. [Google Scholar]
- Warmuz, J.; Puszkiel, P.; Botzenhart, U.; Gedrange, T.; Dominiak, M. Practical application of a method for assessing the progression of gingival recessions in orthodontically treated patients—A pilot study. Oral Health Dent. Manag. 2014, 13, 772–778. [Google Scholar] [PubMed]
- Khoury, F.; Hanser, T. Three-Dimensional Vertical Alveolar Ridge Augmentation in the Posterior Maxilla: A 10-year Clinical Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Dorosz, N.; Dominiak, M. Mandibular ridge reconstruction: A review of contemporary methods. Adv. Clin. Exp. Med. 2018, 27, 1159–1168. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef] [Green Version]
- Laino, L.; Iezzi, G.; Piattelli, A.; Lo Muzio, L.; Cicciù, M. Vertical ridge augmentation of the atrophic posterior mandible with sandwich technique: Bone block from the chin area versus corticocancellous bone block allograft—Clinical and histological prospective randomized controlled study. BioMed Res. Int. 2014, 2014, 982104. [Google Scholar] [CrossRef] [Green Version]
- Simion, M.; Jovanovic, S.A.; Tinti, C.; Benfenati, S.P. Long-term evaluation of osseointegrated implants inserted at the time or after vertical ridge augmentation. A retrospective study on 123 implants with 1–5 year follow-up. Clin. Oral Implant. Res. 2001, 12, 35–45. [Google Scholar] [CrossRef]
- Khoury, F.; Doliveux, R. The Bone Core Technique for the Augmentation of Limited Bony Defects: Five-Year Prospective Study with a New Minimally Invasive Technique. Int. J. Periodontics Restor. Dent. 2018, 38, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, H.; Pan, H.; Xu, J.; Hu, T. Evaluation and New Classification of Alveolar Bone Dehiscences Using Cone-beam Computed Tomography in vivo. Int. J. Morphol. 2015, 33, 361–368. [Google Scholar] [CrossRef]
- Dominiak, M.; Hnitecka, S.; Olchowy, C.; Olchowy, A.; Gedrange, T. Analysis of alveolar ridge width in an area of central lower incisor using cone-beam computed tomography in vivo. Ann. Anat. 2021, 236, 151699. [Google Scholar] [CrossRef]
- Dominiak, M.; Dominiak, S.; Targonska, S.; Gedrange, T. Three-Dimensional Bone Block Planning for Mandibular Sagittal Bone Defect Reconstruction. J. Healthc. Eng. 2020, 2020, 8829288. [Google Scholar] [CrossRef]
- Dressler, D.; Saberi, F.A. Botulinum toxin: Mechanisms of action. Eur Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Stimmelmayr, M.; Allen, E.P.; Gernet, W.; Edelhoff, D.; Beuer, F.; Schlee, M.; Iglhaut, G. Treatment of gingival recession in the anterior mandible using the tunnel technique and a combination epithelialized-subepithelial connective tissue graft-a case series. Int. J. Periodontics Restor. Dent. 2011, 31, 165–173. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 37–44. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloss, F.R.; Offermanns, V.; Kloss-Brandstätter, A. Comparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects-A 12-month retrospective radiographic evaluation. Clin. Oral Implant. Res. 2018, 29, 1163–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume, O.; Donkiewicz, P.; Back, M.; Born, T. Bilateral maxillary augmentation using CAD/CAM manufactured allogenic bone blocks for restoration of congenitally missing teeth: A case report. J. Esthet. Restor. Dent. 2019, 31, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, G.; Mardinger, O.; Peleg, M.; Ghelfan, O.; Nissan, J. Analysis of complications following augmentation with cancellous block allografts. J. Periodontol. 2010, 81, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F. Bone Augmentation in Oral Implantology; Quintessence Publishing Co Ltd.: London, UK, 2007. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).