Abstract
The genus Zingiber consists of about 85 species and many of these species are used as food, spices, and medicines. One of the species, Zingiber montanum (J. Koenig) Link ex A. Dietr. is native to Southeast Asia and has been extensively used as traditional medicines and food. The aim of this review was to collect and critically analyze the scientific information about the bioactive compounds and pharmacological activities of Z. montanum with focus on one of the main components, zerumbone (ZER). Various studies have reported the analysis of volatile constituents of the essential oils from Z. montanum. Similarly, many phenylbutanoids, flavonoids and terpenes were also isolated from rhizomes. These essential oils, extracts and compounds showed potent antimicrobial, anti-inflammatory and antioxidant activities among others. Zerumbone has been studied widely for its anticancer, anti-inflammatory, and other pharmacological activities. Future studies should focus on the exploration of various pharmacological activities of other compounds including phenylbutanoids and flavonoids. Bioassay guided isolation may result in the separation of other active components from the extracts. Z. montanum could be a promising source for the development of pharmaceutical products and functional foods.
1. Introduction
The Zingiberaceae family consists of about 50 genera and more than 1500 species which are distributed all over the world and most of them are found in Asia, Central America, and Africa. Plants belonging to various genera of Zingiberaceae family are used as food, spice and medicines in many parts of the world [1]. One of the must studied genera of this family, Zingiber consists of about 85 species [2] and Zingiber officinale Roscoe. is the most commonly cultivated and used species. There are many other important species of Zingiber which are widely used as spices, food supplements and as crude drug in traditional medicines such as Zingiber montanum (J. Koenig) Link ex A. Dietr.
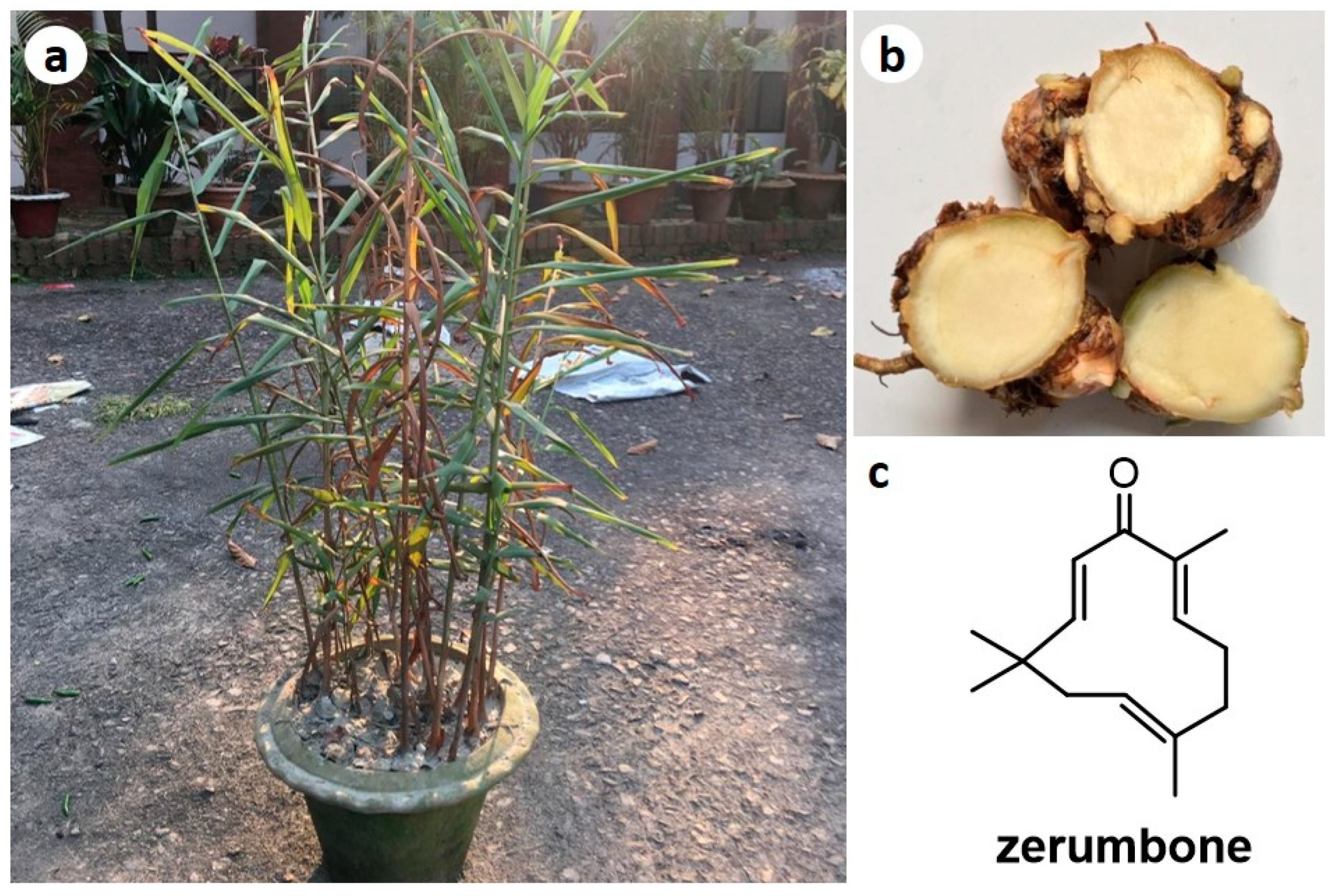
Zingiber montanum (Figure 1, Syns: Amomum cassumunar (Roxb.) Donn, Amomum montanum J. König, Amomum xanthorhiza Roxb. ex Steud., Cassumunar roxburghii Colla, Jaegera montana (J. König) Giseke, Zingiber anthorrhiza Horan., Zingiber cassumunar Roxb., Zingiber cassumunar var. palamauense Haines, Zingiber cassumunar var. subglabrum Thwaites, Zingiber cliffordiae Andrews, Zingiber luridum Salisb., Zingiber montanum (J. König ex Retz.) Theilade, Zingiber purpureum Roscoe, Zingiber purpureum var. palamauense (Haines) K.K. Khanna, Zingiber xantorrhizon Steud.) [3] is commonly known as “Banada” in Bangladesh, “Phlai” in Thailand, “Jangliadrak” in India, and “Bangle” in Malaysia. It is reported to be native to Southeast Asia and has been extensively planted in Thailand, Malaysia, and Indonesia [4]. The rhizomes of this plant are used in traditional medicines for the treatment of constipation, dyspepsia, gastritis, stomach bloating and stomach-ache. Various parts of Z. montanum are used in Thailand as daily diet [5], while the rhizome is used in the as vermifuge in Malaysia, and applied for abscesses, colic, diarrhea, fever and intestinal disorders. In Northeast India, the rhizome paste was reported to be used in the treatment of dyspepsia and stomach bloating [6,7].
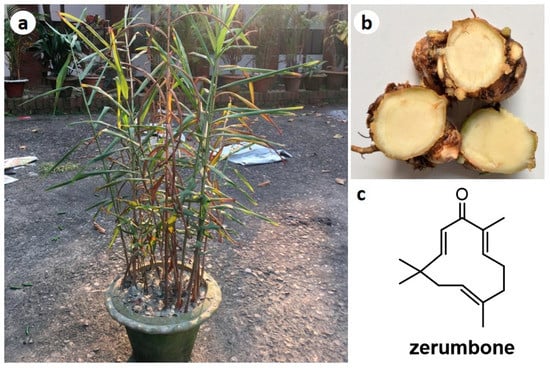
Figure 1.
Photographs of Zingiber montanum plant (a) and rhizomes (b), and chemical structure of zerumbone (ZER) (c).
Zerumbone (ZER) (Figure 1c), a sesquiterpenoid, is one of the major compounds in the essential oils and rhizomes of Z. montanum [4]. In recent years, it has received much attention among researchers as a potent antitumor and anti-inflammatory compound [8,9,10,11,12,13,14,15]. Z. montanum being extensively used in traditional medicine but very few investigations were found for their bioactive constituents and mechanism based pharmacological actions. Thus, the main aim of this review is to scientifically analyze the available scientific information about the chemical constituents and pharmacological activities of extracts and compounds isolated form Zingiber montanum along with the various activities of ZER.
2. Traditional Uses of Zingiber montanum
Zingiber montanum rhizomes are traditionally used for the treatment of asthma, cough, colic, constipation, dyspepsia, diarrhea, inflammation, sprains, stomach bloating and wounds [4,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. It is also used as a tonic and appetizer. It is given along with black pepper in the treatment of cholera and also used as a vermifuge [32]. The rhizome is also used to prepare cleansing solution for skin diseases [33]. The rhizome oil is applied in the treatment of swelling [25]. Rhizomes are also used as anti-inflammatory, antifungal, and antibacterial agent [19,34].
3. Bioactive Compounds
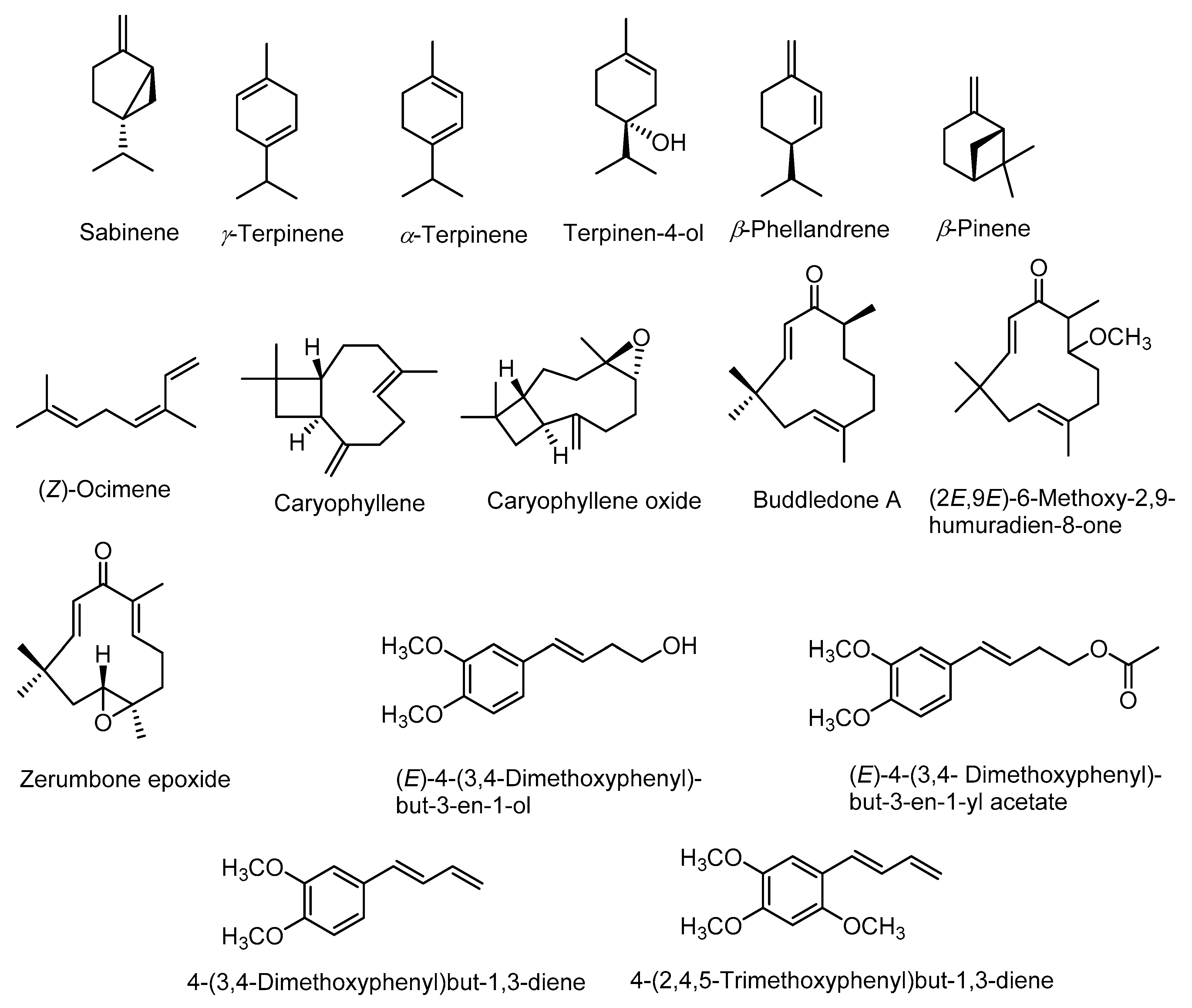
Phytochemical investigation of rhizomes of Z. montanum revealed the presence of numerous bioactive chemical constituents such as alkaloids, saponins, tannins, flavonoids, terpenoids, phenolic compounds, phlobatannins, steroids, and glycosides [35,36]. The gas chromatography-mass spectrometry (GC-MS) analysis of essential-oil constituents of fresh rhizomes of Z. montanum reported the presence of various compounds such as α-thujene, α-pinene, β-mycrene, α-terpinene, p-cymene, β-phellandrene, γ-terpinene, sabinene, sabinene hydrate, terpinolene, terpinen-4-ol, terpinyl acetate, β-sesquiphellandrene, and 4-(3,4-dimethoxyphenyl)but-1,3-diene (DMPBD) which were identified on the basis of retention time and comparison with standard compounds [37]. In another study, GC-MS analysis of essential oils of Z. montanum revealed the presence of sixty four constituents in leaf oil and thirty two constituents in the rhizome oil [38]. The major active chemical constituents of the rhizome oil were sabinene (27–34%), γ-terpinene (6–8%), α-terpinene (4–5%), terpinen-4-ol (30–5%), DMPBD (12–19%), triquinacene 1,4-bis (methoxy) (26.5%), (Z)-ocimene (22.0%), and β-phellandrene (1.0–4.4%) [35,37,38,39]. Whereas, the major constituents in leaf oil were sabinene (15.0%), β-pinene (14.3%), caryophyllene oxide (13.9%) and caryophyllene (9.5%) [38]. Kantoyos and Paisooksantivatana analyzed the chemical constituents in the essential oils obtained from ten Zingiber species in Thailand including Z. montanum. Among the studied plant species, the oil obtained from Z. montanum rhizomes had highest yield (0.89 ± 0.14%, v/w) and also showed highest total curcuminoid content (2.633% w/w) and terpinen-4-ol content (14.5 ± 2.59%) [37]. Structures of some of the main compounds in essential oils are given in Figure 2.
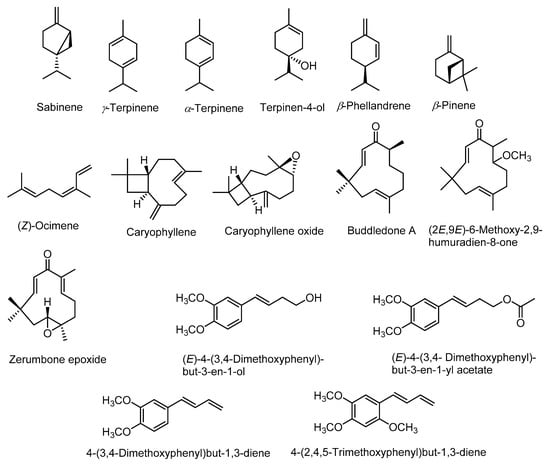
Figure 2.
Structures of major constituents of leaf and rhizome essential oils.
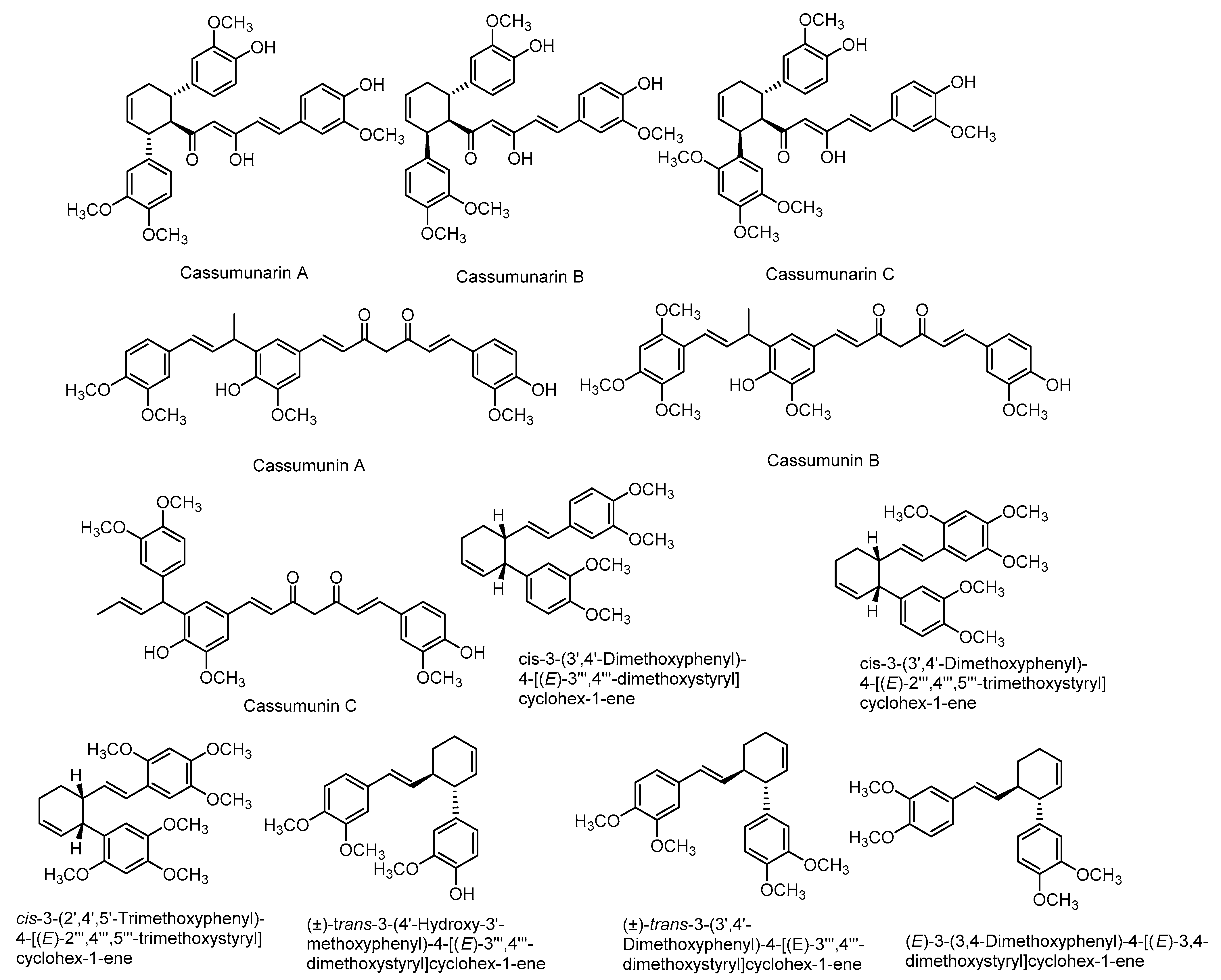
There are also various reports on the compounds isolated from the extracts including non-volatile compounds from the rhizomes. They include mainly phenylbutanoids (Figure 3), flavonoids (Figure 4), terpenes and many other compounds. A list of some of these compounds is provided in Table 1.
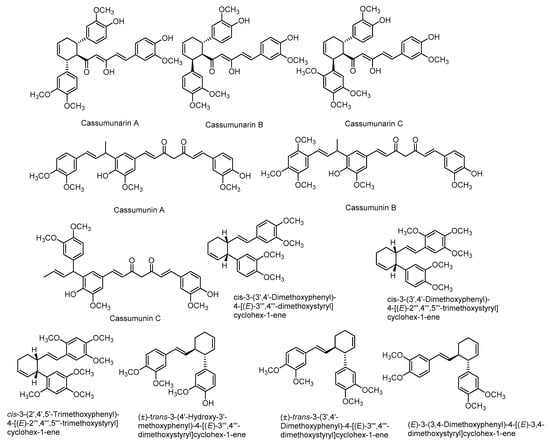
Figure 3.
Structures of some major compounds isolated from the extracts of rhizomes of Z. montanum.
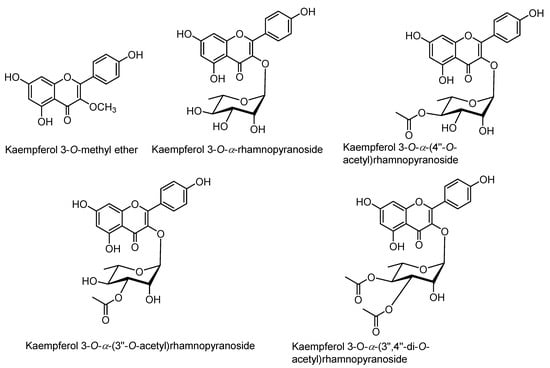
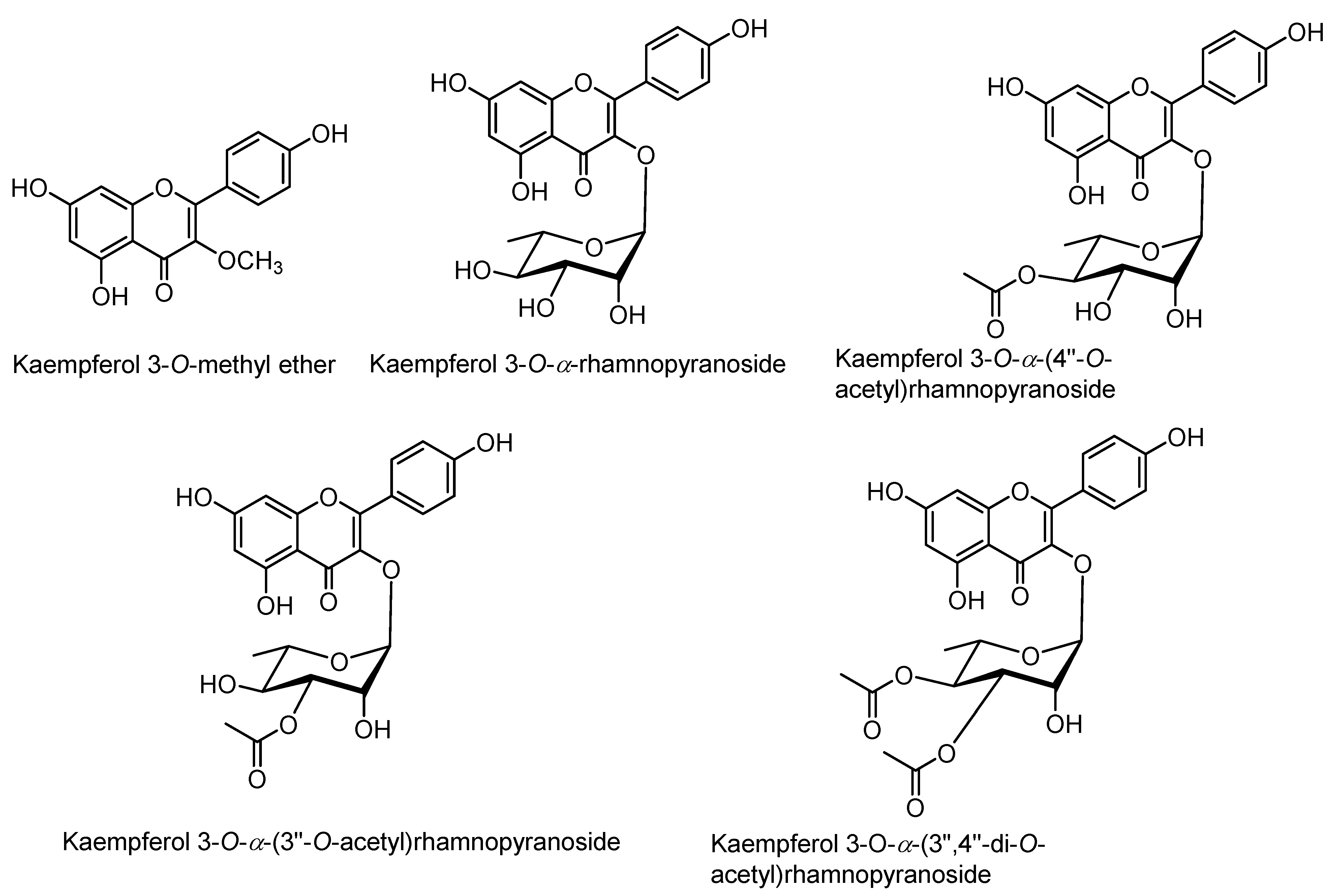
Figure 4.
Structures of kaempferol derivatives isolated from the extracts of rhizomes of Z. montanum.

Table 1.
List of compounds isolated from the rhizomes of Z. montanum.
4. Pharmacological Activities of Z. montanum Extracts and Compounds
Various pharmacological activities such as antimicrobial, anti-inflammatory, antioxidant, antihistaminic, smooth muscle relaxant, insecticidal activities are reported for the essential oils, extracts and some isolated compounds of Z. montanum. Some of these activities are discussed in detail in following sections.
4.1. Anti-Inflammatory Activity
The hexane extract of Z. montanum showed remarkable inhibitory effect on carrageenan-induced rat paw edema, acetic acid-inducing writhing reaction in mice and yeast-triggered hyperthermia in rats [56]. Moreover, phenylbutanoids have been reported as active constituents for anti-inflammatory activities [45]. Sabinene and terpinene-4-ol from essential oil of Z. montanum significantly reduced nuclear factor-kappa B (NF-κB) protein expression in human leukemic monocyte lymphoma cells and interleukin-6 (IL-6) secretion in lipopolysaccharide (LPS) stimulated mice macrophage (RAW264.7) [57]. Methyl t-butyl ether (MTBE) and methanol extracts of Zingiber were effective to inhibit LPS induced in vitro production of prostaglandin E2 (PGE2) and TNF-α in human promonocytic U937 cells [58]. The methanol extract and phenylbutanoids of Z. montanum rhizome showed inhibitory effects on the production of NO from LPS induced peritoneal macrophages from mouse [52]. Methanol extract and its fractions (petroleum ether, hexane and aqueous) of Z. montanum showed anti-inflammatory activity in carrageenan-induced edema in rats, and acetic acid-induced vascular permeability and writhing test in mice [45].
4.2. Antifungal Activity
Jantan et al. reported that the Z. montanum rhizome oil at a dose of 0.75 mg/disc showed significant fungicidal activity against five dermatophytes fungi (Trichophyton mentagrophytes, T. rubrum, Microsporum canis, M. nanum and Epidermophyton floccosum) and three filamentous fungi (Aspergillus niger, A. fumigatus and Mucor sp.) [59]. Another study reported that the essential oil of the rhizome showed antifungal activity against Thanetophorus cucumeris [60]. Z. montanum exhibited high activity against the yeasts namely Saccharomyces cerevisiae, Cryptococcus neoformans, Candida albicans, C. tropicalis, C. glabrata [59]. Tripathi et al. reported the essential oils of Z. montanum at 500 mg/L showed 100% growth inhibition of fruit fungus Botrytis cinerea [61].
4.3. Antioxidant Activity
Many studies have demonstrated the antioxidant properties of Z. montanum. Extract from Z. montanum exhibited potent antioxidant activity hydroxyl radical (OH) scavenging assay [62]. Anastasia et al. reported the antioxidant activities of different fractions of Z. montanum by using 1,1-diphenyl-2-picrylhydrazyl (DPPH), hydrogen peroxide (H2O2), β-carotene bleaching assays. Among different fractions, chloroform fraction showed highest antioxidant activities in DPPH radical scavenging assay, hexane fraction showed highest activity in H2O2 assays and ethyl acetate fraction in β-carotene bleaching assay [63]. Masuda et al. studied the antioxidant activity of cassumunins A, B and C isolated from Z. montanum rhizomes acetone extract using a thiocyanate method which demonstrated that all cassumunins at a dose of 2.7 μM inhibited accumulation of linoleic acid hyperoxide [64]. Bua-in and Paisooksantivatana reported the antioxidant activity of the extracts obtained from the rhizomes of Z. montanum collected from various localities in Thailand [65].
4.4. Antibacterial Activity
Z. montanum essential oil showed potent antibacterial activity against a number of Gram-positive and Gram-negative bacteria. Compared to methanolic extract, chloroform extract showed significant antimicrobial activity against a wide range of pathogens [66]. The rhizomes of Z. montanum are reported to be rich in essential oil effective against a range of pathogenic bacteria including Escherichia coli, Klebsiella pneumonia, Salmonella paratyphi, S. typhi and Shigella flexneri [27]. Z. montanum oil showed potent antimicrobial activity against seventy-four microbial strains with most potent activity against bacteria as such Bacillus subtilis, E. coli, and Salmonella typhi evaluated by disc-diffusion broth dilution method [19]. Boonyanugomol et al. reported significant antimicrobial activity of the essential oil of Z. montanum against Gram-negative Acinetobacter baumannii strains by agar disc-diffusion tests [67]. Sesquiterpenes, monoterpenes and diterpenes from Z. montanum showed various degrees of antimicrobial action against B. cereus, Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa [68].
4.5. Analgesic and Antipyretic Activity
Plai cream, a water in oil emulsion prepared from the essential oil of rhizomes of Z. montanum, was reported to reduce the delayed onset of muscle soreness in healthy volunteers [30,69]. Strong antipyretic action of Z. montanum hexane extract was observed in yeast induced hyperthermia rats and analgesic activity was observed on acetic acid-induced writhing response in mice [56]. In another study, strong analgesic activity was observed in hot plate method compared to the standard pentazocine in case of chloroform and dichloromethane extract of Z. montanum [70].
4.6. Antiulcer Activity
Al-Amin et al. evaluated the antiulcer activity of methanol extract of Z. montanum in mice and it showed 62.0% and 83.1% inhibition of stomach lesions induced by 1N hydrochloric acid (HCl) at doses of 200 mg/kg and 400 mg/kg, respectively. The major compound isolated from the extract i.e., zerumbone also showed potent antiulcer activity in ethanol and indomethacin induced gastric lesions in mice [16]. Another study reported that different concentration of rhizome extracts of Z. montanum showed significant antiulcer activity in comparison with control group in aspirin-induced rat model [71].
4.7. Anti-Allergic Activity
Ethanolic and aqueous extracts of Z. montanum exhibited the most potent anti-allergic activity in antigen induced beta hexosaminidase release in RBL-2H3 cell lines [72]. Capsules prepared from Z. montanum inhibited wheal and flare responses (Type 1 allergic reaction) induced by the mite skin prick test in allergic rhinitis patients [73].
4.8. Cytotoxicity Activity
Zulkhairi et al. reported the cytotoxicity activity of different extracts and compounds from rhizomes in human T-acute lymphoblastic leukemia cancer cells (CEMss) and human cervical cancer cells (HeLa) [74]. Crude methanolic extract of Z. montanum rhizomes showed significant cytotoxic activity in NIH 3T3 fibroblast cell line [65].
4.9. Other Activities
Dulpinijthmma et al. reported that Z. montanum capsule had remarkable role in the treatment of asthma by reducing the bronchial hyperresponsiveness [75]. Crude ethanolic Z. montanum extracts showed potent inhibitory effect on phorbol 12-myristate 13-acetate (PMA) induced mucous producing gene (MUC2, MUC5AC) as well as its protein expression in epithelial cell via inhibition of extracellular signal-regulated kinase pathway [76]. Dichloromethane extract from the rhizome of Z. montanum showed significant mosquito larvicidal activity [77]. Kato et al. reported the neutrophilic activity of phenylbutanoid constituents [17]. Okonogi et al. reported that essential oil showed moderate butyrylcholinesterase inhibitory [78].
5. Biological Activities of Zerumbone (ZER)
Zerumbone was initially isolated in 1960 from Z. zerumbet [79] and structurally characterized in 1965 [80]. Other than Z. zerumbet [81,82,83,84], it has been reported as one of the main constituents from Z. montanum [4,16,20,85]. It is also reported from many other species such as Z. aromaticum [84], Z. spectabile [86]. Zerumbone is widely studied for its various pharmacological activities such as such as anticancer, anti-inflammatory, antioxidant, antimicrobial, anti-ulcer, hepatoprotective activities among others [8,87,88,89,90]. These activities are explained in detail in following sections.
5.1. Anticancer Activity
Cancer is one of the leading causes of death worldwide [91]. Various studies have evaluated the anticancer potential of zerumbone. It was assessed against HeLa cell line and interestingly it showed a selective inhibition of HeLa cells proliferation (IC50 of 14.2 ± 0.5 μmol/L) via enhancement of cellular uptake compared to the normal cell line L929 [92]. Moreover, Rosa and co-workers revealed the anticancer mechanism of ZER on three cell lines including HeLa, B16F10 and undifferentiated Caco-2 cell lines. It was shown that ZER altered the total lipid and fatty acid profile in cancer cells, inducing marked changes in the phospholipid/cholesterol ratio [93]. In addition, the anticancer activity was assessed on Jurkat cells, human T cell leukemia, and it was found that ZER-pendant derivatives showed antiproliferative effects (IC50 values as low as 1–10 μM for most derivatives) [94]. A recent study reported that ZER inhibited cell migration of human esophageal squamous cancer by suppressing Rac1 expression, which is achieved through promoting Rac1 ubiquitination and degradation [95]. Wide number of studies had reported the in vivo, in vitro and in silico anticancer activities of ZER. Herein, an in vivo study showed that ZER significantly controls the growth of tumor and metastasis in BALB/c female mice injected with 4T1 (6-thioguanine resistant cell line) to spontaneously produce highly metastatic tumor [96]. Sithara et al. reported the anticancer activity of ZER against colorectal cancer cells, where they showed that ZER activates caspase 3, caspase 8, and caspase 9. ZER resulted in cell cycle arrest at the G2/M phase [97]. Similarly, other study reported induction of apoptosis in hepatoma HepG2 cells by ZER [98]. Eid and co-workers attempted to explore the underlying mechanism of ZER against breast cancer using in silico study. Since estrogen mediates several pathophysiological signaling pathways associated with cancer progression, the author had selected estrogen as a target for breast cancer and found that the promising molecular interaction, binding interaction, and stability of ZER and estrogen receptor-β (ERβ) suggests ZER as lead compound for breast cancer [99]. More details of these activities are given in Table 2.

Table 2.
Anticancer activities of zerumbone (ZER).
5.2. Anti-Inflammatory Activity
The anti-inflammatory property of ZER is also reported by many studies in vitro and in vivo studies using different models. Various cellular mechanisms of anti-inflammatory activities are also reported. The details of these activities are given in Table 3.

Table 3.
Anti-inflammatory activity of ZER.
5.3. Antimicrobial Activity
Various studies have reported the potent antibacterial activity of zerumbone [87,149]. A recent study reported the inhibitory effect of ZER extract and its compounds against multi-drug resistant and methicillin resistant Staphylococcus aureus [20]. In addition, ZER has an anti-biofilm potential; where it is reported to significantly suppress the expression level of BmeB12 along with antibacterial activity against Bacteroides fragilis [150]. Moreover, a study reported the bactericidal action of ZER against the carcinogenic bacterium Streptococcus mutans (ATCC35668) [151]. Synthetic derivatives of zerumbone are also reported as potent antimicrobial compounds [152].
5.4. Other Pharmacological Activities
Various other pharmacological activities are also reported for ZER such as immunomodulatory activity, neuroprotective effect, antinociceptive, anti-platelet and anti-melanogenic activities (Table 4). Different studies reported the immunomodulatory properties of ZER. Keong et al. revealed ZER activates mice thymocytes, splenocytes and peripheral blood mononuclear cells (PBMC) at dose dependent pattern [153]. A similar study assessed a commercially obtained ZER on human peripheral blood, where it showed that ZER activates human lymphocytes and upregulates interleukin-12p70 cytokine [154]. For neuroprotective effect of ZER, Hamdi et al. reported that ZER oxide protects NG108-15 cells from H2O2 induced oxidative stress [155]. Apart from that, ZER has a gastroprotective effect, where ZER reduces submucosal edema and leukocyte infiltration. On the other hand, a recent in vivo study reported the antinociceptive activity of ZER on mouse, where ZER suppresses inflammatory mediators without any signs of sedation [156]. An in vivo assessment reported the anti-platelet action of ZER investigated from human blood [157]. For the anti-melanogenic activity, a recent study reported that ZER attenuates melanin accumulation in α-melanoma cells [158].

Table 4.
Other pharmacological activities of ZER.
Although zerumbone shows promising biological activities, its low water solubility and poor bioavailability are the limiting factors for wider applications of various formulations containing zerumbone. Few studies have been reported aimed at improving the solubility and bioavailability of zerumbone such as formulation inclusion complexes with cyclodextrin [8,175], nanostructured lipid careers [9], etc.
6. Conclusions and Future Prospects
This review highlighted the traditional food and medicinal uses, bioactive chemical constituents, and pharmacological activities of Z. montanum. Various bioactive compounds have been isolated and identified form the different plant parts. The most widely used and studied part was rhizome. Studies have reported both volatile and non-volatile compounds from the rhizomes. Sesquiterpene lactone, ZER was one of the main components in the rhizomes. ZER has been studied widely for its anticancer, anti-inflammatory, and other pharmacological activities. Future studies should focus on the exploration of various pharmacological activities of other compounds including flavonoids and phenylbutanoids. Bioassay guided isolation may result in the isolation of other active components from the extracts. Future studies should also focus on in vivo studies dealing with pharmacological and pharmacokinetic evaluations. Moreover, clinical studies should be conducted to validate the promising biological activities of ZER. Based on these data, Z. montanum can be a potential source for the development of functional and health beneficial food products.
Author Contributions
Conceptualization, H.P.D.; investigation, H.P.D., K.R.P., M.M.H.; writing—original draft preparation, H.P.D., K.R.P., M.M.H., A.A.-D., A.I.D., J.E., R.L., N.D.; writing—review and editing, H.P.D., K.R.P., N.K.J., S.K.S., P.M.H., Y.C., D.K.C., K.D. All authors have read and agreed to the published version of the manuscript.
Funding
Philip M. Hansbro (PMH) is funded by a Fellowship and grants from the National Health and Medical Research Council (NHMRC) of Australia (1175134) and by UTS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the genus Zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef]
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- World Flora Online (WFO) (2021): Zingiber montanum (J.Koenig) Link ex A.Dietr. Available online: http://www.worldfloraonline.org/taxon/wfo-0000617206 (accessed on 22 October 2021).
- Hassan, M.M.; Adhikari-Devkota, A.; Imai, T.; Devkota, H.P. Zerumbone and Kaempferol Derivatives from the Rhizomes of Zingiber montanum (J. Koenig) Link ex A. Dietr. from Bangladesh. Separations 2019, 6, 31. [Google Scholar] [CrossRef]
- Lim, T.K. Zingiber montanum. In Edible Medicinal and Non-Medicinal Plants; Springer: Cham, Switzerland, 2016; Volume 12. [Google Scholar]
- Wolff, X.Y.; Astuti, I.P.; Brink, M. Zingiber GR Boehmer. In Plant Resources of South-East Asia 13: Spices; Backhuys Publishers: Kerkwerve, The Netherlands, 1999; pp. 233–238. [Google Scholar]
- Anasamy, T.; Abdul, A.B.; Sukari, M.A.; Abdelwahab, S.I.; Mohan, S.; Kamalidehghan, B.; Azid, M.Z.; Muhammad Nadzri, N.; Andas, A.; Kuan Beng, N. A phenylbutenoid dimer, cis-3-(3′,4′-dimethoxyphenyl)-4-[(E)-3′′′,4′′′-dimethoxystyryl] cyclohex-1-ene, exhibits apoptogenic properties in T-acute lymphoblastic leukemia cells via induction of p53-independent mitochondrial signalling pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 939810. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Mohammed, A.F.A.; Elamin, K.M.; Devkota, H.P.; Ohno, Y.; Motoyama, K.; Higashi, T.; Imai, T. Improvement of pharmaceutical properties of zerumbone, a multifunctional compound, using cyclodextrin derivatives. Chem. Pharm. Bull. 2020, 68, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Foong, J.N.; Selvarajah, G.T.; Rasedee, A.; Rahman, H.S.; How, C.W.; Beh, C.Y.; Teo, G.Y.; Ku, C.L. Zerumbone-loaded nanostructured lipid carrier induces apoptosis of canine mammary adenocarcinoma cells. Biomed. Res. Int. 2018, 2018, 8691569. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, G.M.; Kim, J.-K. Zerumbone, sesquiterpene photochemical from ginger, inhibits angiogenesis. Korean J. Physiol. Pharmacol. 2015, 19, 335–340. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Yeap, S.K.; How, C.W.; Hafiza, W.A.G.W.N. Zerumbone-loaded nanostructured lipid carrier induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in a human lymphoblastic leukemia cell line. Int. J. Nanomed. 2014, 9, 527. [Google Scholar]
- Muhammad Nadzri, N.; Abdul, A.B.; Sukari, M.A.; Abdelwahab, S.I.; Eid, E.E.M.; Mohan, S.; Kamalidehghan, B.; Anasamy, T.; Ng, K.B.; Syam, S.; et al. Inclusion complex of zerumbone with hydroxypropyl-β-Cyclodextrin induces apoptosis in liver hepatocellular HepG2 Cells via caspase 8/BID cleavage switch and modulating Bcl2/Bax ratio. Evid. Based Complement. Altern. Med. 2013, 2013, 810632. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Tasrip, N.A.; Mokhtar, F.; Zakaria, Z.A.; Lajis, N.H.; Israf, D.A. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia 2010, 81, 855–858. [Google Scholar] [CrossRef]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of Zerumbone as an Anti-Cancer Agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Wang, Z.H.; Yan, H.Q.; Ren, M.Y.; Gao, S.Q.; Zhang, G.Q. Chemotherapeutic effect of Zerumbone on melanoma cells through mitochondria-mediated pathways. Clin. Exp. Dermatol. 2016, 41, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.; Sultana, G.N.N.; Hossain, C.F. Antiulcer principle from Zingiber montanum. J. Ethnopharmacol. 2012, 141, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Kubo, M.; Okamoto, Y.; Matsunaga, Y.; Kyo, H.; Suzuki, N.; Uebaba, K.; Fukuyama, Y. Safety Assessment of Bangle (Zingiber purpureum Rosc.) Rhizome Extract: Acute and Chronic Studies in Rats and Clinical Studies in Human. ACS Omega 2018, 3, 15879–15889. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Lily, M.K.; Dangwal, K. Evaluation and comparison of polyphenols and bioactivities of wild edible fruits of North-West Himalaya, India. Asian Pac. J. Trop. Dis. 2015, 5, 888–893. [Google Scholar] [CrossRef]
- Pithayanukul, P.; Tubprasert, J.; Wuthi-Udomlert, M. In vitro antimicrobial activity of Zingiber cassumunar (Plai) oil and a 5% Plai oil gel. Phyther. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 164–169. [Google Scholar]
- Siddique, H.; Pendry, B.; Rahman, M.M. Terpenes from Zingiber montanum and their screening against multi-drug resistant and methicillin resistant Staphylococcus aureus. Molecules 2019, 24, 385. [Google Scholar] [CrossRef]
- Kasahara, S.; Henmi, S. Medicine Herb Index in Indonesia. Eisai Indones. 1986, 92, 92. [Google Scholar]
- Elliott, S.; Brimacombe, J. The medicinal plants of Gunung Leuser National Park, Indonesia. J. Ethnopharmacol. 1987, 19, 285–317. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Chen, Y.-P.; Jyl, S. Oriental Materia Medica: A Concise Guide; Oriental Healing Arts Institute: Los Angeles, CA, USA, 1986. [Google Scholar]
- Dymock, W. Pharmacographia Indica; Kegan Paul, Trench, Trubner & Co.: London, UK, 1983; Volume 3. [Google Scholar]
- Nayar, M.P.; Ramamurthy, K.; Agarwal, V.S. Economic Plants of India; Kailash Prakashan: Delhi, India, 1989. [Google Scholar]
- Tham, T.C.; Ng, M.X.; Gan, S.H.; Chua, L.S.; Aziz, R.; Abdullah, L.C.; Ong, S.P.; Chin, N.L.; Law, C.L. Impacts of different drying strategies on drying characteristics, the retention of bio-active ingredient and colour changes of dried Roselle. Chin. J. Chem. Eng. 2018, 26, 303–316. [Google Scholar] [CrossRef]
- Amatayakul, T.; Cannon, J.R.; Dampawan, P.; Dechatiwongse, T.; Giles, R.G.F.; Huntrakul, C.; Kusamran, K.; Mokkhasamit, M.; Rastton, C.L.; Reutrakul, V.; et al. Chemistry and crystal structures of some constituents of Zingiber cassumunar. Aust. J. Chem. 1979, 32, 71–88. [Google Scholar] [CrossRef]
- Cheechareoan, S.; Pathanawiriyasirikul, T.; Manmee, C.; Janpol, K. Efficacy of Plai cream in adult patients with muscle strain: A randomized, double-blind, placebo-controlled trial. J. Med. Assoc. Thai. 2016, 99, S147–S152. [Google Scholar]
- Chienthavorn, O.; Poonsukcharoen, T.; Pathrakorn, T. Pressurized Liquid and Superheated Water Extraction of Active Constituents from Zingiber Cassumunar Roxb. Rhizome; Kasetsart University: Bangkok, Thailand, 2011; Volume 46, ISBN 9742747431. [Google Scholar]
- Manimmanakorn, N.; Manimmanakorn, A.; Boobphachart, D.; Thuwakum, W.; Laupattarakasem, W.; Hamlin, M.J. Effects of Zingiber cassumunar (Plai cream) in the treatment of delayed onset muscle soreness. J. Integr. Med. 2016, 14, 114–120. [Google Scholar] [CrossRef]
- Srirochana, S. Efficacy of Plai Cream Compared with Diclofenac Gel in Osteoarthritis of Knee. Mahasarakham Hosp. J. 2010, 7, 53–60. [Google Scholar]
- Jain, S.K.; Tarafder, C.R. Medicinal plant-lore of the santals. Econ. Bot. 1970, 24, 241–278. [Google Scholar] [CrossRef]
- Oliveros, M.B. Preformulation studies on terpinen-4-ol from Zingiber purpureum Rosc. In Proceedings of the 2nd Symposium on the Family Zingiberaceae; Wu, T.L., Wu, Q.G., Chen, Z.Y., Eds.; Zhongshan University Press: Guangzhou, China, 1996; pp. 180–186. [Google Scholar]
- Chaiwongsa, R.; Ongchai, S.; Boonsing, P.; Kongtawelert, P.; Panthong, A.; Reutrakul, V. Active compound of Zingiber cassumunar Roxb. down-regulates the expression of genes involved in joint erosion in a human synovial fibroblast cell line. Afr. J. Tradit. Complement. Altern. Med. 2012, 10, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Joram, A.; Das, A.K.; Mahanta, D. Evaluation of antioxidant and phenolic contents of Zingiber montanum (J.Koenig) Link ex Dietr.: A potential ethomedicinal plant of Arunachal Pradesh, India. Pleione 2018, 12, 255. [Google Scholar] [CrossRef]
- Majaw, S.; Moirangthem, J. Qualitative and Quantitative Analysis of Clerodendron colebrookianum Walp. Leaves and Zingiber cassumunar Roxb. Rhizomes. Ethnobot. Leafl. 2009, 2009, 3. [Google Scholar]
- Kantayos, V.; Paisooksantivatana, Y. Antioxidant Activity and selected chemical components if 10 Zinger spp. In Thailand. J. Dev. Sustain. Argic 2012, 7, 89–96. [Google Scholar]
- Bhuiyan, M.N.I.; Chowdhury, J.U.; Begum, J. Volatile constituents of essential oils isolated from leaf and rhizome of Zingiber cassumunar Roxb. Bangladesh J. Pharmacol. 2008, 3, 69–73. [Google Scholar] [CrossRef]
- Verma, R.S.; Joshi, N.; Padalia, R.C.; Singh, V.R.; Goswami, P.; Verma, S.K.; Iqbal, H.; Chanda, D.; Verma, R.K.; Darokar, M.P.; et al. Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. J. Sci. Food Agric. 2018, 98, 321–327. [Google Scholar] [CrossRef]
- Taechowisan, T.; Suttichokthanakorn, S.; Phutdhawong, W.S. Antibacterial and cytotoxicity activities of phenylbutanoids from Zingiber cassumunar Roxb. J. Appl. Pharm. Sci. 2018, 8, 121–127. [Google Scholar]
- Tuntiwachwuttikul, P.; Pancharoen, O.; Jaipetch, T.; Reutrakul, V. Phenylbutanoids from Zingiber cassumunar. Phytochemistry 1981, 20, 1164–1165. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Fukushima, S.; Yoshihira, K.; Natori, S.; Dechatiwongse, T.; Mihashi, K.; Nishi, M.; Hara, S. Further Characterization of the Constituents of a Thai Medicinal Plant, Zingiber cassumunar ROXB. Chem. Pharm. Bull. 1980, 28, 2948–2959. [Google Scholar] [CrossRef]
- Dinter, H.; Hänsel, R.; Pelter, A. The Structures of Cassum unaquinones 1 and 2 from Zingiber cassumunar. Z. Für Naturforsch. C 1980, 35, 154–155. [Google Scholar] [CrossRef]
- Dinter, H.; Hänsel, R.; Pelter, A. Isolation of Two Phenylbutadiene Dimers and One Monomeric 4-Phenylbut-3-ene from Zingiber cassumunar (roxb.). Z. Für Naturforsch. C 1980, 35, 156–158. [Google Scholar] [CrossRef][Green Version]
- Ozaki, Y.; Kawahara, N.; Harada, M. Anti-inflammatory Effect of Zingiber cassumunar ROXB. and Its Active Principles. Chem. Pharm. Bull. 2011, 39, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Jitoe, A.; Nakatani, N. Structures of Cassumunin A, B, and C, New Potent Antioxidants from Zingiber cassumunar. Chem. Lett. 2006, 22, 189–192. [Google Scholar] [CrossRef]
- Jitoe, A.; Masuda, T.; Mabry, T.J. Novel Antioxidants, Cassumunarin A, B, and C, from Zingiber cassumunar. Tetrahedron Lett. 1994, 35, 981–984. [Google Scholar] [CrossRef]
- Jitoe, A.; Masuda, T.; Nakatani, N. Phenylbutenoid dimers from the rhizomes of Zingiber cassumunar. Phytochemistry 1993, 32, 357–363. [Google Scholar] [CrossRef]
- Masuda, T.; Jitoe, A. Phenylbutenoid monomers from the rhizomes of Zingiber cassumunar. Phytochemistry 1995, 39, 459–461. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Soontornsaratune, P.; Jarikasem, S.; Sematong, T.; Wasuwat, S.; Claeson, P. Topical antiinflammatory activity of the major lipophilic constituents of the rhizome of Zingiber cassumunar. Part I: The essential oil. Phytomedicine 1997, 3, 319–322. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, C.; Wang, Y.; Pan, Y. Preparative isolation and purification of two phenylbutenoids from the rhizomes of Zingiber cassumunar by upright counter-current chromatography. J. Chromatogr. A 2005, 1089, 258–262. [Google Scholar] [CrossRef]
- Nakamura, S.; Iwami, J.; Matsuda, H.; Wakayama, H.; Pongpiriyadacha, Y.; Yoshikawa, M. Structures of New Phenylbutanoids and Nitric Oxide Production Inhibitors from the Rhizomes of Zingiber cassumunar. Chem. Pharm. Bull. 2009, 57, 1267–1272. [Google Scholar] [CrossRef]
- Matsuda, H.; Nakamura, S.; Iwami, J.; Li, X.; Pongpiriyadacha, Y.; Nakai, M.; Kubo, M.; Fukuyama, Y.; Yoshikawa, M. Invasion Inhibitors of Human Fibrosarcoma HT 1080 Cells from the Rhizomes of Zingiber cassumunar: Structures of Phenylbutanoids, Cassumunols. Chem. Pharm. Bull. 2011, 59, 365–370. [Google Scholar] [CrossRef][Green Version]
- Han, A.R.; Min, H.Y.; Windono, T.; Jeohn, G.H.; Jang, D.S.; Lee, S.K.; Seo, E.K. A new cytotoxic phenylbutenoid dimer from the rhizomes of Zingiber cassumunar. Planta Med. 2004, 70, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Kim, M.S.; Jeong, Y.H.; Lee, S.K.; Seo, E.K. Cyclooxygenase-2 inhibitory phenylbutenoids from the rhizomes of Zingiber cassumunar. Chem. Pharm. Bull. 2005, 53, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Panthong, A.; Kanjanapothi, D.; Niwatananant, W.; Tuntiwachwuttikul, P.; Reutrakul, V. Anti-inflammatory activity of compound D {(E)-4-(3′,4′-dimethoxyphenyl)but-3-en-2-ol} isolated from Zingiber cassumunar Roxb. Phytomedicine 1997, 4, 207–212. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Leelapornpisid, P.; Phongpradist, R.; Viernstein, H.; Mueller, M. Development of microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS PharmSciTech 2017, 18, 1332–1342. [Google Scholar] [CrossRef]
- Jiang, H.; Xie, Z.; Koo, H.J.; McLaughlin, S.P.; Timmermann, B.N.; Gang, D.R. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc.). Phytochemistry 2006, 67, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.b.; Yassin, M.S.M.; Chin, C.B.; Chen, L.L.; Sim, N.L. Antifungal Activity of the Essential Oils of Nine Zingiberaceae Species. Pharm. Biol. 2003, 41, 392–397. [Google Scholar] [CrossRef]
- Crop, T.F.; Thammarat, N.S. Screening for Plant Extract, Antagonistic Microorganism and Fungicides to Control Ganoderma boninense Caused Stem Rot of Oil Palm in Vitro. Int. J. Agric. Technol. 2017, 13, 141–147. [Google Scholar]
- Tripathi, P.; Dubey, N.K.; Shukla, A.K. Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J. Microbiol. Biotechnol. 2008, 24, 39–46. [Google Scholar] [CrossRef]
- Sharma, G.J.; Chirangini, Z.A.; Mishra, K.P. Evaluation of antioxidant and cytotoxic properties of tropical ginger, Zingiber montanum (J. Konig) A Dietr. Gard. Bull. Singap. 2007, 59, 189–202. [Google Scholar]
- Indrianingsih, A.W.; Prihantini, A.I.; Indrianingsih, A.W.; Prihantini, A.I. In vitro antioxidant and α-glucosidase inhibitory assay of Zingiber cassumunar roxb. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2026, p. 20005. [Google Scholar]
- Masuda, T.; Jitoe, A. Antioxidative and Antiinflammatory Compounds from Tropical Gingers: Isolation, Structure Determination, and Activities of Cassumunins A, B, and C, New Complex Curcuminoids from Zingiber cassumunar. J. Agric. Food Chem. 1994, 42, 1850–1856. [Google Scholar] [CrossRef]
- Bua-in, S.; Paisooksantivatana, Y. Essential Oil and Antioxidant Activity of Cassumunar Ginger (Zingiberaceae: Zingiber montanum (Koenig) Link ex Dietr.) Collected from Various Parts of Thailand. Kasetsart J. (Nat. Sci.) 2009, 43, 467–475. [Google Scholar]
- Jena, A.K.; Sahoo, R.K.; Subudhi, E.; Ghosh, G.; Subudhi, B.B.; Nayak, S. Studies on antioxidant, antimicrobial and phytochemical analysis of Zingiber capitatum roxb. Rhizome extracts. Int. J. Integr. Biol. 2011, 11, 127–133. [Google Scholar]
- Boonyanugomol, W.; Kraisriwattana, K.; Rukseree, K.; Boonsam, K.; Narachai, P. In vitro synergistic antibacterial activity of the essential oil from Zingiber cassumunar Roxb against extensively drug-resistant Acinetobacter baumannii strains. J. Infect. Public Health 2017, 10, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Lamlertthon, S.; Tiyaboonchai, W. Antimicrobial activity of essential oils against five strains of Propionibacterium acnes. Mahidol Univ. J. Pharm. Sci. 2007, 34, 60–64. [Google Scholar]
- Manimmanakorn, N.; Manimmanakorn, A.; Boobphachart, D.; Thuwakum, W.; Laupattarakasem, W.; Hamlin, M.J. Effects of Plai cream [Zingiber montanum (J.Koenig) Link ex A.Dietr. syn. Zingiber cassumunar Roxb.] combined with ultrasound on delayed muscle soreness. Indian J. Trait. Knowl. 2017, 16, 442–447. [Google Scholar]
- Koparde, A.A.; Magdum, C.S. Analgesic activity of extracts of Eulophia ochreata Lindl. and Zingiber cassumunar Roxb. in animal model. Int. J. Pharm. Sci. Res. 2018, 9, 1956–1962. [Google Scholar]
- Yuniarto, A.; Susilawati, E.; Rahman, T.A.; Setiawan, F.; Juanda, D. Gastric Ulcer Healing Effect of Bangle (Zingiber cassumunar (Roxb.)) Rhizome Extract in Aspirin-induced Rats Model. Indones. J. Pharm. Sci. Technol. 2017, 1, 29–34. [Google Scholar] [CrossRef][Green Version]
- Subhadhirasakul, S.; Tewtrakul, S. Anti-allergic activity of some selected plants in the Zingiberaceae family. J. Ethnopharmacol. 2007, 109, 535–538. [Google Scholar]
- Tanticharoenwiwat, P.; Kulalert, P.; Dechatiwongse Na Ayudhya, T.; Koontongkaew, S.; Jiratchariyakul, W.; Soawakontha, R.; Booncong, P.; Poachanukoon, O. Inhibitory effect of Phlai capsules on skin test responses among allergic rhinitis patients: A randomized, three-way crossover study. J. Integr. Med. 2017, 15, 462–468. [Google Scholar] [CrossRef]
- Zulkhairi, A.M.; Aspollah, S.M.; Lian, E.G.C.; Bustamam, A.A. Phytochemicals and cytotoxic studies of Zingiber cassumunar Roxb. J. Trop. Agric. Fd. Sc. 2017, 45, 187–197. [Google Scholar]
- Dulpinijthamma, J.; Poachanukoon, O.; Saiphoklang, N. The effect of Zingiber cassumunar (Phlai capsule) on bronchial hyperresponsiveness in asthmatic patients. J. Allergy Clin. Immunol. 2020, 145, AB20. [Google Scholar] [CrossRef]
- Limvuttegrijerat, T.; Poachanukoon, O.; Koontongkaew, S.; Ayudhya, T.D.N. Crude ethanolic extracts of Zingiber cassumunar ROXB. inhibit PMA-induced MUC2 and MUC5AC expression via ERK inhibition in human airway epithelial cells. Asian Pac. J. Allergy Immunol. 2014, 32, 328. [Google Scholar]
- Bandara, K.A.N.P.; Kumar, V.; Saxena, R.C.; Ramdas, P.K. Bruchid (Coleoptera: Bruchidae) ovicidal phenylbutanoid from Zingiber purpureum. J. Econ. Entomol. 2005, 98, 1163–1169. [Google Scholar] [CrossRef]
- Okonogi, S.; Chaiyana, W. Enhancement of anti-cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov. Ther. 2012, 6, 249–255. [Google Scholar] [CrossRef][Green Version]
- Dev, S. Studies in sesquiterpenes-XVI. Zerumbone, a monocyclic sesquiterpene ketone. Tetrahedron 1960, 8, 171–180. [Google Scholar] [CrossRef]
- Kitayama, T.; Okamoto, T.; Hill, R.K.; Kawai, Y.; Takahashi, S.; Yonemori, S.; Yamamoto, Y.; Ohe, K.; Uemura, S.; Sawada, S. Chemistry of Zerumbone. 1. Simplified isolation, conjugate addition reactions, and a unique ring contracting transannular reaction of its dibromide. J. Org. Chem. 1999, 64, 2667–2672. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Jitoe, A.; Kato, S.; Nakatani, N. Acetylated flavonol glycosides from Zingiber zerumbet. Phytochemistry 1991, 30, 2391–2392. [Google Scholar] [CrossRef]
- Chien, T.Y.; Chen, L.G.; Lee, C.J.; Lee, F.Y.; Wang, C.C. Anti-inflammatory constituents of Zingiber zerumbet. Food Chem. 2008, 110, 584–589. [Google Scholar] [CrossRef]
- Ruslay, S.; Abas, F.; Shaari, K.; Zainal, Z.; Maulidiani; Sirat, H.; Israf, D.A.; Lajis, N.H. Characterization of the components present in the active fractions of health gingers (Curcuma xanthorrhiza and Zingiber zerumbet) by HPLC-DAD-ESIMS. Food Chem. 2007, 104, 1183–1191. [Google Scholar] [CrossRef]
- Dai, J.R.; Cardellina, J.H.; McMahon, J.B.; Boyd, M.R. Zerumbone, an HIV-inhibitory and cytotoxic sesquiterpene of Zingiber aromaticum and Z. zerumbet. Nat. Prod. Lett. 1997, 10, 115–118. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Yeap, S.K.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Abdul, A.B.; How, C.W. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. BioMed Res. Int. 2014, 2014, 920742. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.K.; Khatun, A.; Ohtsuki, T.; Ishibashi, M. First isolation of sesquiterpenes and flavonoids from Zingiber spectabile and identification of zerumbone as the major cell growth inhibitory component. Nat. Prod. Res. 2007, 21, 1242–1247. [Google Scholar] [CrossRef]
- Kalantari, K.; Moniri, M.; Moghaddam, A.B.; Rahim, R.A.; Ariff, A.B.; Izadiyan, Z.; Mohamad, R. A Review of the biomedical applications of zerumbone and the techniques for its extraction from ginger rhizomes. Molecules 2017, 22, 1645. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sakao, K.; Singh, S.V. Notch2 activation is protective against anticancer effects of zerumbone in human breast cancer cells. Breast Cancer Res. Treat. 2014, 146, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Ohnishi, K.; Murakami, A.; Lee, J.S.; Kundu, J.K.; Na, H.K.; Ohigashi, H.; Surh, Y.J. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev. Res. 2011, 4, 860–870. [Google Scholar] [CrossRef]
- Saranya, J.; Dhanya, B.P.; Greeshma, G.; Radhakrishnan, K.V.; Priya, S. Effects of a new synthetic zerumbone pendant derivative (ZPD) on apoptosis induction and anti-migratory effects in human cervical cancer cells. Chem. Biol. Interact. 2017, 278, 32–39. [Google Scholar] [CrossRef]
- Agide, F.D.; Sadeghi, R.; Garmaroudi, G.; Tigabu, B.M. A systematic review of health promotion interventions to increase breast cancer screening uptake: From the last 12 years. Eur. J. Public Health 2018, 28, 1149–1155. [Google Scholar] [CrossRef]
- Ashraf, S.M.; Sebastian, J.; Rathinasamy, K. Zerumbone, a cyclic sesquiterpene, exerts antimitotic activity in HeLa cells through tubulin binding and exhibits synergistic activity with vinblastine and paclitaxel. Cell Prolif. 2019, 52, e12558. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Caprioglio, D.; Isola, R.; Nieddu, M.; Appendino, G.; Falchi, A.M. Dietary zerumbone from shampoo ginger: New insights into its antioxidant and anticancer activity. Food Funct. 2019, 10, 1629–1642. [Google Scholar] [CrossRef]
- Utaka, Y.; Kashiwazaki, G.; Tajima, S.; Fujiwara, Y.; Sumi, K.; Itoh, T.; Kitayama, T. Antiproliferative effects of zerumbone-pendant derivatives on human T-cell lymphoid cell line Jurkat cells. Tetrahedron 2019, 75, 1343–1350. [Google Scholar] [CrossRef]
- Wang, M.; Niu, J.; Gao, L.; Gao, Y.; Gao, S. Zerumbone inhibits migration in ESCC via promoting Rac1 ubiquitination. Biomed. Pharmacother. 2019, 109, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.E.; Abu, N.; Rahman, H.S.; Ky, H.; Ho, W.Y.; Lim, K.L.; How, C.W.; Rasedee, A.; Alitheen, N.B.; Yeap, S.K. Nanostructured lipid carrier improved in vivo anti-tumor and immunomodulatory effect of Zerumbone in 4T1 challenged mice. RSC Adv. 2015, 5, 22066–22074. [Google Scholar] [CrossRef]
- Sithara, T.; Dhanya, B.P.; Arun, K.B.; Sini, S.; Dan, M.; Kokkuvayil Vasu, R.; Nisha, P. Zerumbone, a Cyclic Sesquiterpene from Zingiber zerumbet Induces Apoptosis, Cell Cycle Arrest, and Antimigratory Effects in SW480 Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 602–612. [Google Scholar] [CrossRef]
- Tan, J.W.; Israf, D.A.; Tham, C.L. Major bioactive compounds in essential oils extracted from the rhizomes of Zingiber zerumbet (L.) Smith: A mini-review on the anti-allergic and immunomodulatory properties. Front. Pharmacol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.E.M.; Azam, F.; Hassan, M.; Taban, I.M.; Halim, M.A. Zerumbone binding to estrogen receptors: An in-silico investigation. J. Recept. Signal. Transduct. 2018, 38, 342–351. [Google Scholar] [CrossRef]
- Abdel Wahab, S.I.; Abdul, A.B.; Alzubairi, A.S.; Mohamed Elhassan, M.; Mohan, S. In Vitro Ultramorphological Assessment of Apoptosis Induced by Zerumbone on (HeLa). J. Biomed. Biotechnol. 2009, 2009, 769568. [Google Scholar] [CrossRef]
- Kang, C.G.; Lee, H.-J.; Kim, S.-H.; Lee, E.-O. Zerumbone Suppresses Osteopontin-Induced Cell Invasion Through Inhibiting the FAK/AKT/ROCK Pathway in Human Non-Small Cell Lung Cancer A549 Cells. J. Nat. Prod. 2016, 79, 156–160. [Google Scholar] [CrossRef]
- Hu, Z.; Zeng, Q.; Zhang, B.; Liu, H.; Wang, W. Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. Biochimie 2014, 107, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jorvig, J.E.; Chakraborty, A. Zerumbone inhibits growth of hormone refractory prostate cancer cells by inhibiting JAK2/STAT3 pathway and increases paclitaxel sensitivity. Anticancer. Drugs 2015, 26, 160–166. [Google Scholar] [CrossRef]
- Jeon, M.; Han, J.; Nam, S.J.; Lee, J.E.; Kim, S. Elevated IL-1β expression induces invasiveness of triple negative breast cancer cells and is suppressed by zerumbone. Chem. Biol. Interact. 2016, 258, 126–133. [Google Scholar] [CrossRef]
- Hosseini, N.; Khoshnazar, A.; Saidijam, M.; Azizi Jalilian, F.; Najafi, R.; Mahdavinezhad, A.; Ezati, R.; Sotanian, A.; Amini, R. Zerumbone Suppresses Human Colorectal Cancer Invasion and Metastasis via Modulation of FAk/PI3k/NFκB-uPA Pathway. Nutr. Cancer 2019, 71, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.C.; Chien, T.Y.; Chen, L.G.; Wang, C.C. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005, 71, 219–224. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Liu, Y.; Qiao, H.; Liu, Y. Zerumbone, a Southeast Asian ginger sesquiterpene, induced apoptosis of pancreatic carcinoma cells through p53 signaling pathway. Evid.-Based Complement. Altern. Med. 2012, 2012, 936030. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.E.M.; Alanazi, A.S.; Koosha, S.; Alrasheedy, A.A.; Azam, F.; Taban, I.M.; Khalilullah, H.; Sadiq Al-Qubaisi, M.; Alshawsh, M.A. Zerumbone Induces Apoptosis in Breast Cancer Cells by Targeting αvβ3 Integrin upon Co-Administration with TP5-iRGD Peptide. Molecules 2019, 24, 2554. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Abdul, A.B.; Mohan, S.; Taha, M.M.E.; Syam, S.; Ibrahim, M.Y.; Mariod, A.A. Zerumbone induces apoptosis in T-acute lymphoblastic leukemia cells. Leuk. Res. 2011, 35, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-L.; Liang, J.-W.; Hsu, L.-C.; Chang, W.-L.; Lee, S.-S.; Guh, J.-H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2015, 388, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Dermani, F.K.; Amini, R.; Saidijam, M.; Pourjafar, M.; Saki, S.; Najafi, R. Zerumbone inhibits epithelial-mesenchymal transition and cancer stem cells properties by inhibiting the β-catenin pathway through miR-200c. J. Cell. Physiol. 2018, 233, 9538–9547. [Google Scholar] [CrossRef]
- Ma, S.; Lei, Y.; Zhang, L.; Wang, J. Effects of zerumbone on proliferation and apoptosis of esophageal cancer cells and on P53 and Bcl-2 expression levels. Oncol. Lett. 2018, 16, 4379–4383. [Google Scholar] [CrossRef]
- Murakami, A. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: The alpha, beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis 2002, 23, 795–802. [Google Scholar] [CrossRef]
- Kim, S.; Kil, W.H.; Lee, J.; Oh, S.-J.; Han, J.; Jeon, M.; Jung, T.; Lee, S.K.; Bae, S.Y.; Lee, H.C. Zerumbone suppresses EGF-induced CD44 expression through the inhibition of STAT3 in breast cancer cells. Oncol. Rep. 2014, 32, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.; Jeon, M.; Lee, J.E.; Nam, S.J. Zerumbone suppresses the motility and tumorigenecity of triple negative breast cancer cells via the inhibition of TGF-β1 signaling pathway. Oncotarget 2016, 7, 1544. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Zhang, W.; Han, X. Zerumbone suppresses the potential of growth and metastasis in hepatoma HepG2 cells via the MAPK signaling pathway. Oncol. Lett. 2018, 15, 7603–7610. [Google Scholar] [CrossRef] [PubMed]
- Jegannathan, S.D.; Arul, S.; Dayalan, H. Zerumbone, a Sesquiterpene, Controls Proliferation and Induces Cell Cycle Arrest in Human Laryngeal Carcinoma Cell Line Hep-2. Nutr. Cancer 2016, 68, 865–872. [Google Scholar] [CrossRef]
- Kim, M.; Miyamoto, S.; Yasui, Y.; Oyama, T.; Murakami, A.; Tanaka, T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int. J. Cancer 2009, 124, 264–271. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Abdul, A.B.; Devi, N.; Ehassan Taha, M.M.; Al-zubairi, A.S.; Mohan, S.; Mariod, A.A. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: Involvement of mitochondria-regulated apoptosis. Exp. Toxicol. Pathol. 2010, 62, 461–469. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Abdul, A.B.; Zain, Z.N.M.; Hadi, A.H.A. Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Int. Immunopharmacol. 2012, 12, 594–602. [Google Scholar] [CrossRef]
- Abdul, A.B.; Abdelwahab, S.I.; Jalinas, J.B.; Al-Zubairi, A.S.; Taha, M.M.E. Combination of zerumbone and cisplatin to treat cervical intraepithelial neoplasia in female BALB/c mice. Int. J. Gynecol. Cancer 2009, 19, 1004–1010. [Google Scholar] [CrossRef]
- Abdul, A.B.; Abdelwahab, S.I.; Al-Zubaira, A.S.; Elhassan, M.M.; Murali, S.M. Anticancer and antimicrobial activities of zerumbone from the rhizomes of Zingiber zerumbut. Int. J. Pharmacol. 2008, 4, 301–304. [Google Scholar] [CrossRef]
- Adbul, A.B.H.; Al-Zubairi, A.S.; Tailan, N.D.; Wahab, S.I.A.; Zain, Z.N.M.; Rusley, S.; Syam, M.M. Anticancer activity of natural compound (Zerumbone) extracted from Zingiber zerumbet in human HeLa cervical cancer cells. Int. J. Pharmacol. 2008, 4, 160–168. [Google Scholar]
- Sakinah, S.A.S.; Tri Handayani, S.; Hawariah, L.P.A. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007, 7, 4. [Google Scholar] [CrossRef]
- Al-Amin, M.; Eltayeb, N.M.; Khairuddean, M.; Salhimi, S.M. Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231. Nat. Prod. Res. 2019, 3166–3170. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.A.; Abdul, A.B.; Rahman, H.S.; Rasedee, A.; Ibrahim, T.A.T.; Keon, Y.S. Zerumbone suppresses angiogenesis in HepG2 cells through inhibition of matrix metalloproteinase-9, vascular endothelial growth factor, and vascular endothelial growth factor receptor expressions. Pharmacogn. Mag. 2017, 13, S731. [Google Scholar]
- Sehrawat, A.; Arlotti, J.A.; Murakami, A.; Singh, S.V. Zerumbone causes Bax- and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res. Treat. 2012, 136, 429–441. [Google Scholar] [CrossRef]
- Shamoto, T.; Matsuo, Y.; Shibata, T.; Tsuboi, K.; Nagasaki, T.; Takahashi, H.; Funahashi, H.; Okada, Y.; Takeyama, H. Zerumbone inhibits angiogenesis by blocking NF-κB activity in pancreatic cancer. Pancreas 2014, 43, 396–404. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Kim, C.; Sikka, S.; Siveen, K.S.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Abrogation of STAT3 signaling cascade by zerumbone inhibits proliferation and induces apoptosis in renal cell carcinoma xenograft mouse model. Mol. Carcinog. 2015, 54, 971–985. [Google Scholar] [CrossRef]
- Zainal, N.S.; Gan, C.P.; Lau, B.F.; Yee, P.S.; Tiong, K.H.; Abdul Rahman, Z.A.; Patel, V.; Cheong, S.C. Zerumbone targets the CXCR4-RhoA and PI3K-mTOR signaling axis to reduce motility and proliferation of oral cancer cells. Phytomedicine 2018, 39, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Shyanti, R.K.; Sehrawat, A.; Singh, S.V.; Mishra, J.P.N.; Singh, R.P. Zerumbone modulates CD1d expression and lipid antigen presentation pathway in breast cancer cells. Toxicol. Vitr. 2017, 44, 74–84. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Zeenathul, N.A.; Chartrand, M.S.; Yeap, S.K.; Abdul, A.B.; Tan, S.W.; Othman, H.H.; Ajdari, Z. Antileukemic effect of zerumbone-loaded nanostructured lipid carrier in WEHI-3B cell-induced murine leukemia model. Int. J. Nanomed. 2015, 10, 1649. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Cui, P.; Zhao, Q.; Tan, B.; Zhang, Z.; Liu, Y.; Jia, N. Zerumbone induces gastric cancer cells apoptosis: Involving cyclophilin A. Biomed. Pharmacother. 2016, 83, 740–745. [Google Scholar] [CrossRef]
- Wani, N.A.; Zhang, B.; Teng, K.; Barajas, J.M.; Motiwala, T.; Hu, P.; Yu, L.; Brüschweiler, R.; Ghoshal, K.; Jacob, S.T. Reprograming of glucose metabolism by zerumbone suppresses hepatocarcinogenesis. Mol. Cancer Res. 2018, 16, 256–268. [Google Scholar] [CrossRef]
- Weng, H.-Y.; Hsu, M.-J.; Wang, C.-C.; Chen, B.-C.; Hong, C.-Y.; Chen, M.-C.; Chiu, W.-T.; Lin, C.-H. Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells. J. Biomed. Sci. 2012, 19, 86. [Google Scholar] [CrossRef]
- Yan, H.; Ren, M.; Wang, Z.; Feng, S.; Li, S.; Cheng, Y.; Hu, C.; Gao, S.; Zhang, G. Zerumbone inhibits melanoma cell proliferation and migration by altering mitochondrial functions. Oncol. Lett. 2017, 13, 2397–2402. [Google Scholar] [CrossRef]
- Rajan, I.; Jayasree, P.R.; Kumar, P.R.M. Zerumbone induces mitochondria-mediated apoptosis via increased calcium, generation of reactive oxygen species and upregulation of soluble histone H2AX in K562 chronic myelogenous leukemia cells. Tumor Biol. 2015, 36, 8479–8489. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yun, J.M. Molecular mechanism of the protective effect of zerumbone on lipopolysaccharide-induced inflammation of THP-1 cell-derived macrophages. J. Med. Food 2019, 22, 62–73. [Google Scholar] [CrossRef]
- Ho, Y.C.; Lee, S.S.; Yang, M.L.; Huang-Liu, R.; Lee, C.Y.; Li, Y.C.; Kuan, Y.H. Zerumbone reduced the inflammatory response of acute lung injury in endotoxin-treated mice via Akt-NFκB pathway. Chem. Biol. Interact. 2017, 271, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Somchit, M.N.; Mak, J.H.; Bustamam, A.A.; Zuraini, A.; Arifah, A.K.; Adam, Y.; Zakaria, Z.A. Zerumbone isolated from Zingiber zerumbet inhibits inflammation and pain in rats. J. Med. Plants Res. 2012, 6, 177–180. [Google Scholar] [CrossRef]
- Igarashi, Y.; Ohnishi, K.; Irie, K.; Murakami, A. Possible contribution of zerumbone-induced proteo-stress to its anti-inflammatory functions via the activation of heat shock factor 1. PLoS ONE 2016, 11, e0161282. [Google Scholar] [CrossRef]
- Hwang, S.; Jo, M.; Hong, E.J.; Park, O.C.; Lee, G.C.; Yun, M.; Rhee, K.-J. Zerumbone Suppresses Enterotoxigenic Bacteroides fragilis Infection-Induced Colonic Inflammation through Inhibition of NF-κΒ. Int. J. Mol. Sci. 2019, 20, 4560. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Jantan, I.; Harikrishnan, H. Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through MyD88-dependent NF-κB/MAPK/PI3K-Akt signaling pathways. Int. Immunopharmacol. 2018, 55, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Chen, S.-P.; Su, C.-H.; Ho, Y.-C.; Yang, M.-L.; Lee, S.-S.; Huang, L.R.; Yang, C.-P.; Chan, C.J.; Kuan, Y.-H. Zerumbone from Zingiber zerumbet ameliorates lipopolysaccharide-induced ICAM-1 and cytokines expression via p38 MAPK/JNK-IκB/NF-κB pathway in mouse model of acute lung injury. Chin. J. Physiol. 2018, 61, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.-S.; Yang, M.-L.; Lee, S.-S.; Kuo, C.-W.; Ho, Y.-C.; Huang-Liu, R.; Lin, H.-W.; Kuan, Y.-H. Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway. Int. Immunopharmacol. 2017, 46, 194–200. [Google Scholar] [CrossRef]
- Murakami, A.; Matsumoto, K.; Koshimizu, K.; Ohigashi, H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-γ-induced IκB degradation in RAW264.7 macrophages. Cancer Lett. 2003, 195, 17–25. [Google Scholar] [CrossRef]
- Murakami, A.; Shigemori, T.; Ohigashi, H. Zingiberaceous and citrus constituents, 1′-acetoxychavicol acetate, zerumbone, auraptene, and nobiletin, suppress lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264. 7 murine macrophages through different modes of action. J. Nutr. 2005, 135, 2987S–2992S. [Google Scholar] [CrossRef]
- Rücker, H.; Amslinger, S. Identification of heme oxygenase-1 stimulators by a convenient ELISA-based bilirubin quantification assay. Free Radic. Biol. Med. 2015, 78, 135–146. [Google Scholar] [CrossRef]
- Vishwanatha, H.N.; Niraguna Babu, P.; Gowrishankar, B.S.; Sridhar, S.B. Antimicrobial activity of zerumbone from Zingiber zerumbet against Staphylococcus epidermis and Aspergillus sp. Int. J. Appl. Biol. Pharm. Tech. 2012, 3, 40–43. [Google Scholar]
- Kim, H.R.; Rhee, K.J.; Eom, Y. Bin Anti-biofilm and antimicrobial effects of zerumbone against Bacteroides fragilis. Anaerobe 2019, 57, 99–106. [Google Scholar] [CrossRef]
- Moreira Da Silva, T.; Pinheiro, C.D.; Puccinelli Orlandi, P.; Pinheiro, C.C.; Soares Pontes, G. Zerumbone from Zingiber zerumbet (L.) smith: A potential prophylactic and therapeutic agent against the cariogenic bacterium Streptococcus mutans. BMC Complement. Altern. Med. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, T.; Yamamoto, K.; Utsumi, R.; Takatani, M.; Hill, R.K.; Kawai, Y.; Sawada, S.; Okamoto, T. Chemistry of zerumbone. 2. Regulation of ring bond cleavage and unique antibacterial activities of zerumbone derivatives. Biosci. Biotechnol. Biochem. 2001, 65, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Keong, Y.S.; Alitheen, N.B.; Mustafa, S.; Aziz, S.A.; Rahman, M.A.; Ali, A.M. Immunomodulatory effects of zerumbone isolated from roots of Zingiber zerumbet. Pak. J. Pharm. Sci. 2010, 23, 75–82. [Google Scholar] [PubMed]
- Dash, S.; Ray, M.; Mishra, A.; Shahbazi, S.; Gopinath Achary, A.; Nayak, S.; Singh, S. Zerumbone, a natural plant dietary compound induces expression of interleukin-12p70 cytokine in human peripheral blood mononuclear cells. Asian J. Pharm. Clin. Res. 2016, 9, 312–315. [Google Scholar] [CrossRef]
- Hamdi, O.A.A.; Ye, L.J.; Kamarudin, M.N.A.; Hazni, H.; Paydar, M.; Looi, C.Y.; Shilpi, J.A.; Kadir, H.A.; Awang, K. Neuroprotective and antioxidant constituents from Curcuma zedoaria Rhizomes. Rec. Nat. Prod. 2015, 9, 349–355. [Google Scholar]
- Gopalsamy, B.; Farouk, A.A.O.; Tengku Mohamad, T.A.S.; Sulaiman, M.R.; Perimal, E.K. Antiallodynic and antihyperalgesic activities of zerumbone via the suppression of IL-1β, IL-6, and TNF-α in a mouse model of neuropathic pain. J. Pain Res. 2017, 10, 2605–2619. [Google Scholar] [CrossRef]
- Jantan, I.; Raweh, S.M.; Sirat, H.M.; Jamil, S.; Mohd Yasin, Y.H.; Jalil, J.; Jamal, J.A. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine 2008, 15, 306–309. [Google Scholar] [CrossRef]
- Oh, T.I.; Jung, H.J.; Lee, Y.M.; Lee, S.; Kim, G.H.; Kan, S.Y.; Kang, H.; Oh, T.; Ko, H.M.; Kwak, K.C.; et al. Zerumbone, a tropical ginger sesquiterpene of Zingiber officinale Roscoe, attenuates α-MSH-induced melanogenesis in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 3149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Yang, D.; Jeong, H.; Park, I.S.; Lee, M.H.; Lim, C.W.; Kim, B. Dietary zerumbone, a sesquiterpene, ameliorates hepatotoxin-mediated acute and chronic liver injury in mice. Phyther. Res. 2019, 33, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Niu, J.; Ou, L.; Deng, B.; Wang, Y.; Li, S. Zerumbone protects against carbon tetrachloride (CCl4)-induced acute liver injury in mice via inhibiting oxidative stress and the inflammatory response: Involving the TLR4/NF-κB/COX-2 pathway. Molecules 2019, 24, 1964. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.; Lee, L.S.; Karim, S.R.; Jufri, N.F. Hepatoprotective effects of zerumbone against paracetamol-induced acute hepatotoxicity in rats. Malays. J. Med. Sci. MJMS 2018, 25, 64. [Google Scholar] [CrossRef] [PubMed]
- Ghazalee, N.S.; Jantan, I.; Arshad, L.; Haque, M.A. Immunosuppressive effects of the standardized extract of Zingiber zerumbet on innate immune responses in Wistar rats. Phyther. Res. 2019, 33, 929–938. [Google Scholar] [CrossRef]
- Akhtar, N.M.Y.; Jantan, I.; Arshad, L.; Haque, M.A. Standardized ethanol extract, essential oil and zerumbone of Zingiber zerumbet rhizome suppress phagocytic activity of human neutrophils. BMC Complement. Altern. Med. 2019, 19, 331. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.H.; Huang, H.M.; Wang, C.C.; Lee, C.C.; Fan, C.K.; Lee, Y.L. Zerumbone enhances the Th1 response and ameliorates ovalbumin-induced Th2 responses and airway inflammation in mice. Int. Immunopharmacol. 2015, 24, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Albaayit, S.F.A.; Maharjan, R.; Liao, J.W.; Rajendran, P.; Wu, J.J.; Hseu, Y.C. Immunomodulation of Zerumbone via Decreasing the Production of Reactive Oxygen Species from Immune Cells. Science 2018, 21, 475–479. [Google Scholar]
- Hemn, H.O. Influence of Zerumbone Supplementation a Natural Dietary Product from Zingiber zerumbet smith on Early-Developed Atherosclerotic Lesions in Cholesterol-Fed Rabbits. Open Conf. Proc. J. 2014, 4, 61–64. [Google Scholar] [CrossRef]
- Tzeng, T.F.; Lu, H.J.; Liou, S.S.; Chang, C.J.; Liu, I.M. Lipid-lowering effects of zerumbone, a natural cyclic sesquiterpene of Zingiber zerumbet Smith, in high-fat diet-induced hyperlipidemic hamsters. Food Chem. Toxicol. 2014, 69, 132–139. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Jung, C.H.; Choi, W.H.; Ha, T.Y. Zerumbone ameliorates high-fat diet-induced adiposity by restoring AMPK-regulated lipogenesis and microRNA-146b/SIRT1-mediated adipogenesis. Oncotarget 2017, 8, 36984. [Google Scholar] [CrossRef]
- Beattie, J.H.; Nicol, F.; Gordon, M.-J.; Reid, M.D.; Cantlay, L.; Horgan, G.W.; Kwun, I.-S.; Ahn, J.-Y.; Ha, T.-Y. Ginger phytochemicals mitigate the obesogenic effects of a high-fat diet in mice: A proteomic and biomarker network analysis. Mol. Nutr. Food Res. 2011, 55, S203–S213. [Google Scholar] [CrossRef]
- Shrikanth, C.B.; Chilkunda, N.D. Zerumbone Ameliorates High Glucose-Induced Reduction in AMP-Activated Protein Kinase Phosphorylation in Tubular Kidney Cells. J. Agric. Food Chem. 2017, 65, 9208–9216. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.-F.; Liou, S.-S.; Chang, C.J.; Liu, I.-M. Zerumbone, a tropical ginger sesquiterpene, ameliorates streptozotocin-induced diabetic nephropathy in rats by reducing the hyperglycemia-induced inflammatory response. Nutr. Metab. 2013, 10, 64. [Google Scholar] [CrossRef]
- Tzeng, T.-F.; Liou, S.-S.; Tzeng, Y.-C.; Liu, I.-M. Zerumbone, a Phytochemical of Subtropical Ginger, Protects against Hyperglycemia-Induced Retinal Damage in Experimental Diabetic Rats. Nutritiens 2016, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zou, S.; Cui, Z.; Guo, P.; Meng, Q.; Shi, X.; Gao, Y.; Yang, G.; Han, Z. Zerumbone protects INS-1 rat pancreatic beta cells from high glucose-induced apoptosis through generation of reactive oxygen species. Biochem. Biophys. Res. Commun. 2015, 460, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Tzeng, T.-F.; Liu, I.-M. Healing potential of zerumbone ointment on experimental full-thickness excision cutaneous wounds in rat. J. Tissue Viability 2017, 26, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.E.M.; Abdul, A.B.; Suliman, F.E.O.; Sukari, M.A.; Rasedee, A.; Fatah, S.S. Characterization of the inclusion complex of zerumbone with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2011, 83, 1707–1714. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).