Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals
Abstract
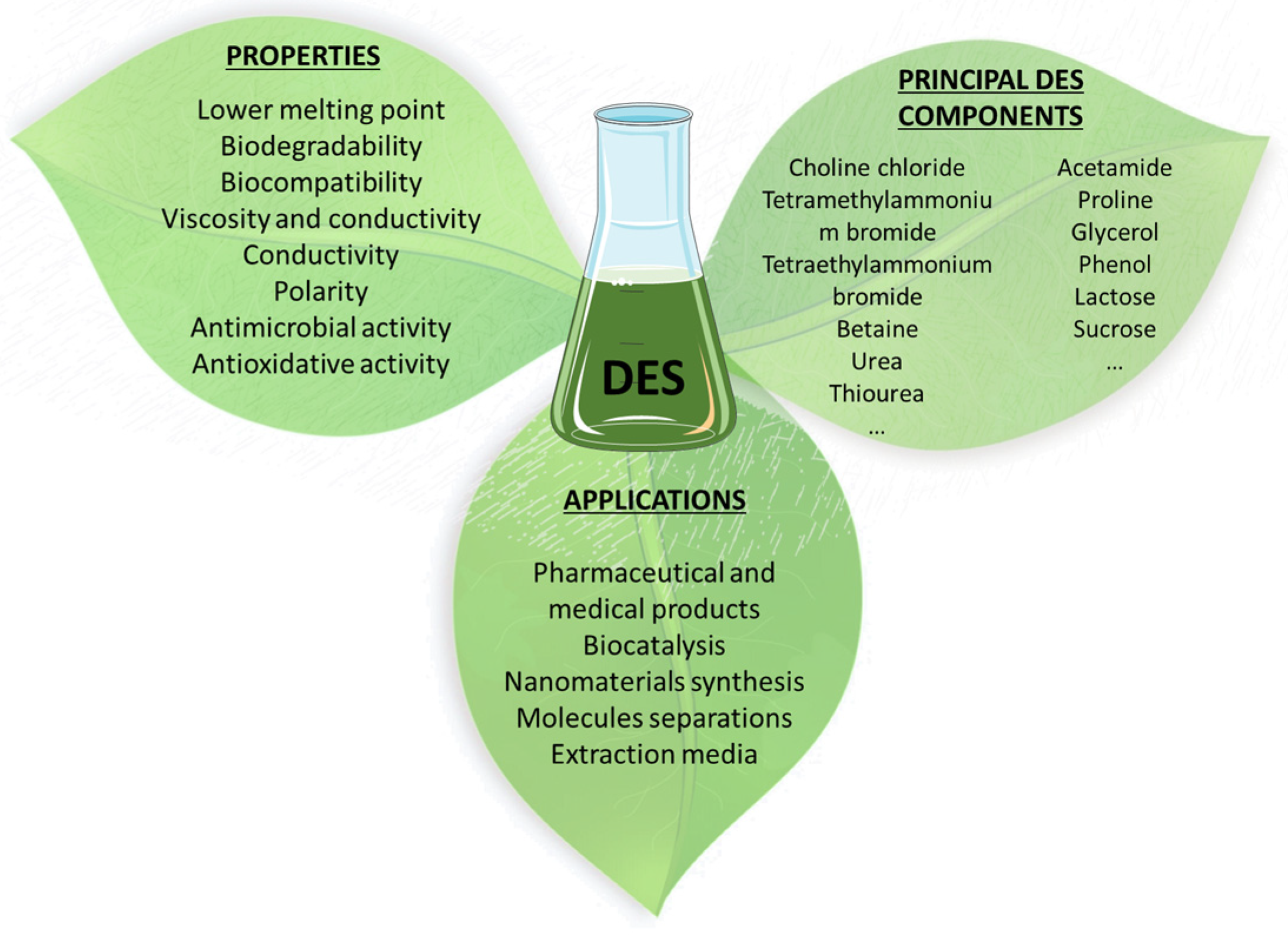
:1. Introduction
2. Applications Related to Peculiar Solubility Properties of DESs
Pharmaceutical Applications: Therapeutical Deep Eutectic Solvent-THEDES
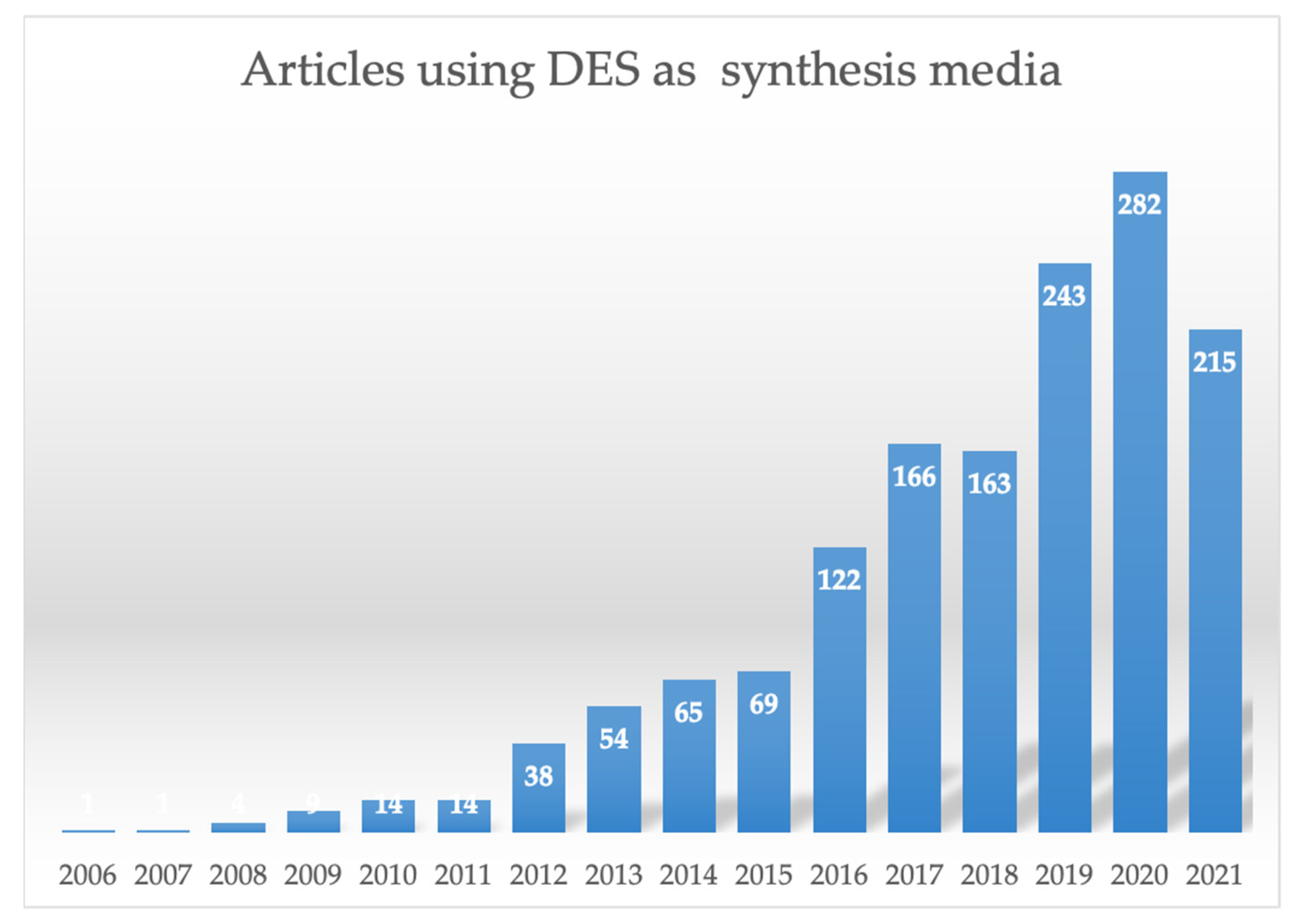
3. DES as Synthesis Media of Natural Based Chemicals
4. DES in Extraction Processes of Bioactive Products
5. Other Interesting Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry; Oxford University Press: Oxford, UK, 2000; Volume 4, pp. 437–438. [Google Scholar]
- Nelson, W.M. Are ionic liquids green solvents? In Ionic Liquids: Industrial Applications for Green Chemistry; Rogers, R.D., Seddon, K.R., Eds.; American Chemical Society: Washington, DC, USA, 2002; Volume 818, pp. 30–41. [Google Scholar]
- Capello, C.; Fischer, U.; Hungerbuhler, K. What is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Ahluwalia, V.K.; Varma, R.S. Green Solvents for Organic Synthesis; Alpha Science: Oxford, UK, 2009. [Google Scholar]
- Romero, A.; Santos, A.; Tojo, J.; Rodríguez, A. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008, 151, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Deetlefs, M.; Seddon, K.R. Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem. 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Castro, V.I.B.; Craveiro, R.; Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Natural deep eutectic systems as alternative nontoxic cryoprotective agents. Cryobiology 2018, 83, 15–26. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. Adv. Biochem. Eng. Biotechnol. 2019, 168, 31–59. [Google Scholar]
- Arriaga, S.; Aizpuru, A. Advances and Applications of Partitioning Bioreactors. In Advances in Chemical Engineering (54); Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Warrag, S.E.E.; Peters, C.J.; Kroon, M.C. Deep eutectic solvents for highly efficient separations in oil and gas industries. Curr. Opin. Green Sustain. Chem. 2017, 5, 55–60. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Doert, T.; Wang, H.; Zhang, S.; Ruck, M. Inorganic Synthesis Based on Reactions of Ionic Liquids and Deep Eutectic Solvents. Angew. Chem. Int. Ed. Engl. 2021, 60, 22148–22165. [Google Scholar] [CrossRef]
- Devi, T.J.; Singh, T.; Singh, R.R.; Sharma, K.; Singh, O. Synthesis of Tri-indolylmethane Derivatives Using a Deep Eutectic Solvent. Russ. J. Org. Chem. 2021, 57, 255–264. [Google Scholar] [CrossRef]
- Devi, T.J.; Singh, T.P.; Singh, O.M. The one-pot four-component eco-friendly synthesis of spirooxindoles in deep eutectic solvent. J. Chem. Sci. 2020, 132, 28. [Google Scholar] [CrossRef]
- Zhao, R.T.; Pei, D.; Yu, P.L.; Wei, J.T.; Wang, N.L.; Di, D.L.; Liu, Y.W. Aqueous two-phase systems based on deep eutectic solvents and their application in green separation processes. J. Sep. Sci. 2020, 43, 348–359. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, Z.; Yang, L.; Liu, H. Development and applications of deep eutectic solvent derived functional materials in chromatographic separation. J. Sep. Sci. 2021, 44, 1098–1121. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Nakhle, L.; Kfoury, M.; Mallard, I.; Landy, D.; Greige-Gerges, H. Microextraction of bioactive compounds using deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3747–3759. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Y.; Zhao, Q.; Chen, A.; Jiao, B. Ultrasound-assisted dispersive liquid-phase microextraction by solidifying L-menthol-decanoic acid hydrophobic deep eutectic solvents for detection of five fungicides in fruit juices and tea drinks. J. Sep. Sci. 2021, 44, 3870–3882. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.A.; Alabdullkarem, E.A.; ALOthman, Z.A.; Yilmaz, E.; Soylak, M. Thiomalic acid/ferric chloride-based deep eutectic solvent for microextraction of chromium in natural water samples prior to FAAS analysis. Int. J. Environ. Anal. Chem. 2020, 1–9. [Google Scholar] [CrossRef]
- Nahar, Y.; Thickett, S.C. Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science. Polymers 2021, 13, 447. [Google Scholar] [CrossRef]
- Ghobadi, R.; Divsalar, A. Enzymatic behavior of bovine liver catalase in aqueous medium of sugar based deep eutectic solvents. J. Mol. Liq. 2020, 310, 113207. [Google Scholar] [CrossRef]
- Svigelj, R.; Dossi, N.; Grazioli, C.; Toniolo, R. Deep Eutectic Solvents (DESs) and Their Application in Biosensor Development. Sensors 2021, 21, 4263. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, M.; Garg, S.; Wu, Y.; Idros, M.N.; Hocking, R.; Duan, H.; Gao, S.; Yago, A.J.; Zhuang, L.; et al. Cobalt Electrochemical Recovery from Lithium Cobalt Oxides in Deep Eutectic Choline Chloride plus Urea Solvents. ChemSusChem 2021, 14, 2972–2983. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Álvarez, M.S.; Zhang, Y. Sketching neoteric solvents for boosting drugs bioavailability. J. Control. Release 2019, 311–312, 225–232. [Google Scholar] [CrossRef]
- Kim, J.; Shi, Y.; Kwon, C.J.; Gao, Y.; Mitragotri, S. A Deep Eutectic Solvent-Based Approach to Intravenous Formulation. Adv. Healthc. Mater. 2021, 10, 2100585. [Google Scholar] [CrossRef]
- Mustafa, N.R.; Spelbos, V.S.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations. Molecules 2021, 26, 2645. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: Glyceline, ethaline, and reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorovic, Z.B.; Dokic-Stojanovic, D.R.; Stamenkovic, O.S. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging Frontiers of Deep Eutectic Solvents in Drug Discovery and Drug Delivery Systems. J. Control. Release 2019, 316, 168–195. [Google Scholar] [CrossRef]
- Kumar, S.; Dilbaghi, N.; Rani, R.; Bhanjana, G.; Umar, A. Novel Approaches for Enhancement of Drug Bioavailability. Rev. Adv. Sci. Eng. 2013, 2, 133–154. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast Dissolving Curcumin Cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; Vega-Baudrit, J.R.; Guillén-Girón, T.; Navarro-Hoyos, M.; Cuffini, S.L. Drug Solubility Enhancement through the Preparation of Multicomponent Organic Materials: Eutectics of Lovastatin with Carboxylic Acids. Pharmaceutics 2019, 11, 112. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lee, P.I. Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives. Int. J. Pharm. 2016, 505, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, N.; Liu, Z.; Fei, Z.; Zheng, M.; Zhao, J.; Zhao, H. Solubility modelling and thermodynamic properties of allopurinol in aqueous solutions of four deep eutectic solvents. J. Chem. Thermodyn. 2019, 132, 363–372. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Yong, C.S.; Jung, S.H.; Rhee, J.D.; Choi, H.G.; Lee, B.J.; Kim, D.C.; Choi, Y.W.; Kim, C.K. Improved solubility and in vitro dissolution of Ibuprofen from poloxamer gel using eutectic mixture with menthol. Drug Deliv. 2003, 10, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Haghtalab, A. Experimental and thermodynamic modeling of cefixime trihydrate solubility in an aqueous deep eutectic system. J. Mol. Liq. 2020, 304, 112727. [Google Scholar] [CrossRef]
- Lu, C.; Cao, J.; Wang, N.; Su, E. Significantly improving the solubility of non-steroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration. Med. Chem. Commun. 2016, 7, 955–959. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert Opin. Drug Deliv. 2019, 16, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Olivares, B.; Martínez, F.; Rivas, L.; Calderón, C.; M Munita, J.; R Campodonico, P. A Natural Deep Eutectic Solvent Formulated to Stabilize β-Lactam Antibiotics. Sci. Rep. 2018, 8, 14900. [Google Scholar] [CrossRef] [Green Version]
- Firn, R.D.; Jones, C.G. Natural products—A simple model to explain chemical diversity. Nat. Prod. Rep. 2003, 20, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Ehrenström Reiz, G.M.; Reiz, S.L. EMLA—A eutectic mixture of local anaesthetics for topical anaesthesia. Acta Anaesthesiol. Scand. 1982, 26, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Gohel, M.C.; Nagori, S.A. Fabrication and design of transdermal fluconazole spray. Pharm. Dev. Technol. 2009, 14, 208–215. [Google Scholar] [CrossRef]
- Grønlien, K.G.; Pedersen, M.E.; Tønnesen, H.H. A natural deep eutectic solvent (NADES) as potential excipient in collagen-based products. Int. J. Biol. Macromol. 2020, 156, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gurau, G.; Shamshina, J.; Cojocaru, O.A.; Janikowski, J.; MacFarlane, D.R.; Davis, J.H., Jr.; Rogers, R.D. Simultaneous membrane transport of two active pharmaceutical ingredients by charge assisted hydrogen bond complex formation. Chem. Sci. 2014, 5, 3449–3456. [Google Scholar] [CrossRef]
- Ko, J.; Mandal, A.; Dhawan, S.; Shevachman, M.; Mitragotri, S.; Joshi, N. Clinical translation of choline and geranic acid deep eutectic solvent. Bioeng. Transl. Med. 2021, 6, e10191. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E.S. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Zeleznjak, J.; Bubalo, M.C.; Redovniković, I.R.; Slivac, I.; Srček, V.G. Comparative in vitro study of cholinium-based ionic liquids and deep eutectic solvents toward fish cell line. Ecotoxicol. Environ. Saf. 2016, 131, 30–36. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Chen, J.X.; Tang, Y.L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, M.; Zhang, L. The effect of deep eutectic solvent on the pharmacokinetics of salvianolic acid B in rats and its acute toxicity test. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1063, 60–66. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering-Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Mohd Ali, O. Toxicity profile of choline chloride-based Deep eutectic solvents for fungi and Cyprinus carpio fish. Environ. Sci. Pollut. Res. 2016, 23, 7648–7659. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, F.; Silva, J.M.; Matias, A.; Reis, R.L.; Duarte, A.R.C. Optimal Design of THEDES Based on Perillyl Alcohol and Ibuprofen. Pharmaceutics 2020, 12, 1121. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Santos, F.; Matias, A.; Paiva, A.; Duarte, A.R.C. Design and Processing of Drug Delivery Formulations of Therapeutic Deep Eutectic Systems for Tuberculosis. J. Supercrit. Fluids 2020, 161, 104826. [Google Scholar] [CrossRef]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the Anticancer Potential of Limomene Based Therapeutic Deep Eutectic Solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolfson, A.; Malcolm, R.; Campbell, K.; Jones, D.; Russell, J. Rheological, mechanical and membrane penetration properties of novel dual drug systems for percutaneous delivery. J. Control. Release 2000, 67, 395–408. [Google Scholar] [CrossRef]
- Nazzal, S.; Smalyukh, I.I.; Lavrentovich, O.D.; Khan, M.A. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: Mechanism and progress of emulsion formation. Int. J. Pharm. 2002, 235, 247–265. [Google Scholar] [CrossRef]
- Lodzki, M.; Godin, B.; Rakou, L.; Mechoulam, R.; Gallily, R.; Touitou, E. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release 2003, 93, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Ali, M.; Baboota, S.; Ahuja, A.; Kumar, A.; Ali, J. 2010. Solid dispersion as an approach for bioavailability enhancement of poorly water-soluble drug ritonavir. AAPS PharmSciTech 2010, 11, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Park, C.W.; Mansour, H.M.; Oh, T.O.; Kim, J.Y.; Ha, J.M.; Lee, B.J.; Chi, S.C.; Rhee, Y.S.; Park, E.S. Phase behavior of itraconazole-phenol mixtures and its pharmaceutical applications. Int. J. Pharm. 2012, 436, 652–658. [Google Scholar] [CrossRef]
- Benessam, S.; Khimeche, K.; Djellouli, F.; Benziane, M.; Dahmani, A. Phase diagram of ibuprofen with fatty acids. J. Therm. Anal. Calorim. 2013, 112, 317–320. [Google Scholar] [CrossRef]
- Abdelkader, H.; Abdallah, O.Y.; Salem, H.; Alani, A.W.G.; Alany, R.G. Eutectic, monotectic and immiscibility systems of nimesulide with water-soluble carriers: Phase equilibria, solid-state characterisation and in-vivo/pharmacodynamic evaluation. J. Pharm. Pharmacol. 2014, 66, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Tarate, B.; Bansal, A.K. Characterization of CoQ 10-lauric acid eutectic system. Thermochim. Acta 2015, 605, 100–106. [Google Scholar] [CrossRef]
- Gala, U.; Chuong, M.C.; Varanasi, R.; Chauhan, H. Characterization and Comparison of Lidocaine-Tetracaine and Lidocaine-Camphor Eutectic Mixtures Based on Their Crystallization and Hydrogen-Bonding Abilities. AAPS PharmSciTech 2015, 16, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Berton, P.; Di Bona, K.R.; Yancey, D.; Rizvi, S.A.A.; Gray, M.; Gurau, G.; Shamshina, J.L.; Rasco, J.F.; Rogers, R.D. Transdermal Bioavailability in Rats of Lidocaine in the Forms of Ionic Liquids, Salts, and Deep Eutectic. ACS Med. Chem. Lett. 2017, 8, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Cai, Y.; Liu, Y.; Zhao, Y.; Feng, J.; Liu, C. Microemulsions based on paeonol-menthol eutectic mixture for enhanced transdermal delivery: Formulation development and in vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Chadha, R.; Sharma, M.; Haneef, J. Multicomponent solid forms of felodipine: Preparation, characterisation, physicochemical and in-vivo studies. J. Pharm. Pharmacol. 2017, 69, 254–264. [Google Scholar] [CrossRef]
- Haneef, J.; Chadha, R. Drug-drug multicomponent solid forms: Cocrystal, coamorphous and eutectic of three poorly soluble antihypertensive drugs using mechanochemical approach. AAPS PharmSciTech 2017, 18, 2279–2290. [Google Scholar] [CrossRef]
- Khare, S.G.; Jena, S.K.; Sangamwar, A.T.; Khullar, S.; Mandal, S.K. Multicomponent pharmaceutical adducts of α-Eprosartan: Physicochemical properties and pharmacokinetic study. Cryst. Growth Des. 2017, 17, 1589–1599. [Google Scholar] [CrossRef]
- Haneef, J.; Chadha, R. Antioxidant-based eutectics of irbesartan: Viable multicomponent forms for the management of hypertension. AAPS PharmSciTech 2018, 19, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, R.A.; Essa, E.A.; El Maghraby, G.M. Eutexia for enhanced dissolution rate and anti-inflammatory activity of nonsteroidal anti-inflammatory agents: Caffeine as a melting point modulator. Int. J. Pharm. 2019, 563, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Jang, J.; Lee, S.; Kim, I.I.W. Binary Mixtures of Some Active Pharmaceutical. Ingredients with Fatty Alcohols—The Criteria of Successful Eutectic Formation and Dissolution Improvement. Pharmaceutics 2020, 12, 1098. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.D.; Raval, M.K.; Sheth, N.R. Formation of Diacerein—Fumaric acid eutectic as a multi-component system for the functionality enhancement. J. Drug Deliv. Sci. Technol. 2020, 58, 101562. [Google Scholar] [CrossRef]
- Patel, R.D.; Raval, M.K.; Pethani, T.M.; Sheth, N.R. Influence of eutectic mixture as a multi-component system in the improvement of physicomechanical and pharmacokinetic properties of diacerein. Adv. Powder Technol. 2020, 31, 1441–1456. [Google Scholar] [CrossRef]
- Marei, H.F.; Arafa, M.F.; Essa, E.A.; El Maghraby, G.M. Lidocaine as eutectic forming drug for enhanced transdermal delivery of nonsteroidal anti-inflammatory drugs. J. Drug Deliv. Sci. Technol. 2021, 61, 102338. [Google Scholar] [CrossRef]
- Knapik-Kowalczuk, J.; Kramarczyk, D.; Jurkiewicz, K.; Chmiel, K.; Paluch, M. Ternary eutectic ezetimibe−simvastatin−fenofibrate system and the physical stability of its amorphous form. Mol. Pharm. 2021, 18, 3588–3600. [Google Scholar] [CrossRef]
- Boscariol, R.; Caetano, E.A.; Silva, E.C.; Oliveira, T.J.; Rosa-Castro, R.M.; Vila, M.M.D.C.; Balcao, V.M. Performance of choline geranate deep eutectic solvent as transdermal permeation enhancer: An in vitro skin histological study. Pharmaceutics 2021, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kong, Y.; Xu, H.; Li, J.P.; Dong, J.-X.; Lin, Z. Ionothermal synthesis of a three-dimensional zinc phosphate with DFT topology using unstable deep-eutectic solvent as template-delivery agent. Micropor. Mesopor. Mat. 2008, 115, 624–628. [Google Scholar] [CrossRef]
- Liu, L.; Wei, H.; Zhang, L.; Li, J.; Dong, J. Ionothermal synthesis of the MetalOrganic Framework compound Cu3(BTC)2. Stud. Surf. Sci. Catal. 2008, 174, 459–462. [Google Scholar]
- Drylie, E.A.; Wragg, D.S.; Parnham, E.R.; Wheatley, P.S.; Slawin, A.M.; Warren, J.E.; Morris, R.E. Ionothermal Synthesis of Unusual Choline-Templated Cobalt Aluminophosphates. Angew. Chem. Int. Ed. Engl. 2007, 46, 7839–7843. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.-N.; Trindade, T.; Rocha, J.; Paz, F.A.A. Hydro-Ionothermal Synthesis of Lanthanide-Organic Frameworks with 1,4-Phenylenebis(methylene)diphosphonate. Cryst. Growth Des. 2008, 8, 3917–3920. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Wragg, D.S.; Lightfoot, P.; Morris, R.E. A novel non-centrosymmetric metallophosphate-borate compound via ionothermal synthesis. Dalton Trans. 2009, 27, 5287–5289. [Google Scholar] [CrossRef]
- Morris, R. Ionothermal synthesis—Ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. (Camb. Engl.) 2009, 21, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Jiang, T.; Han, B.; Zhang, J.; Xie, Y.; Ma, X. Supported choline chloride/urea as a heterogeneous catalyst for chemical fixation of carbon dioxide to cyclic carbonates. Green Chem. 2007, 9, 169–172. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Cheng, W.; Ren, J. Hydroxyl-functionalized ionic liquid: A novel efficient catalyst for chemical fixation of CO2 to cyclic carbonate. Tetrahedron Lett. 2008, 49, 3588–3591. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ya-Qin, Y.; Li-Xin, C.; Ge, S.; Lan, Z.; Yong-Gang, W. Spindle Like BiVO4 Microtubes: Synthesis in Deep Eutectic Solvent and Photocatalytic Properties. Chin. J. Inorg. Chem. 2010, 26, 379–384. [Google Scholar]
- Liu, L.; Li, X.; Xu, H.; Li, J.; Lin, Z.; Dong, J. Template control in ionothermal synthesis of aluminophosphate microporous materials. Dalton Trans. 2009, 47, 10418–10421. [Google Scholar] [CrossRef]
- Chirea, M.; Freitas, A.; Vasile, B.S.; Ghitulica, C.; Pereira, C.M.; Silva, F. Gold nanowire networks: Synthesis, characterization, and catalytic activity. Langmuir 2011, 27, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Patiño, J.; Gutiérrez, M.C.; Carriazo, D.; Ania, C.O.; Parra, J.B.; Ferrer, M.L.; del Monte, F. Deep eutectic assisted synthesis of carbon adsorbents highly suitable for low-pressure separation of CO2–CH4 gas mixtures. Energy Environ. Sci. 2012, 5, 8699–8707. [Google Scholar] [CrossRef] [Green Version]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 4, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Baker, G.A. Ionic liquids and deep eutectic solvents for biodiesel synthesis: A review. J. Chem. Technol. Biot. 2013, 88, 3–12. [Google Scholar] [CrossRef]
- Gorke, J.T.; Kazlauskas, R.J.; Srienc, F.; Srienc, F. Enzymatic Processing in Deep Eutectic Solvents. U.S. Patent US8247198B2, 21 August 2012. [Google Scholar]
- Lindberg, D.; de la Fuente Revenga, M.; Widersten, M. Deep eutectic solvents (DESs) are viable cosolvents for enzyme-catalyzed epoxide hydrolysis. J. Biotechnol. 2010, 147, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Nagarajan, R. Deep Eutectic Solvent Mediated Synthesis of Quinazolinones and Dihydroquinazolinones: Synthesis of Natural Products and Drugs. RSC Adv. 2016, 6, 27378–27387. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Nagarajan, R. Total synthesis of penipanoid C, 2-(4-hydroxybenzyl)quinazolin-4(3H)-one and NU1025. Tetrahedron Lett. 2016, 57, 4277–4279. [Google Scholar] [CrossRef]
- Annes, S.B.; Vigneshwar, K.; Nivedha, K.; Manojveer, S.; Ramesh, S. Deep Eutectic Solvent Mediated Alkyne-Carbonyl Metathesis (ACM) Reaction for the Synthesis of 2H-Chromene Derivatives. Chem. Select. 2019, 4, 6245–6249. [Google Scholar] [CrossRef]
- Aryan, R.; Beyzaei, H.; Nojavan, M.; Rezaei, M. Novel biocompatible glucose-based deep eutectic solvent as recyclable medium and promoter for expedient multicomponent green synthesis of diverse three and four substituted pyrazole-4-carbonitrile derivatives. Res. Chem. Intermed. 2017, 43, 4731–4744. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, Y.; Huang, Y.; Ding, X.; Chen, J.; Xu, K. Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 2014, 139, 2565–2573. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal. Chim. Acta 2015, 864, 9–20. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, S.; Zhang, H.; Row, K.H. Deep Eutectic Solvent-Based HS-SME Coupled with GC for the Analysis of Bioactive Terpenoids in Chamaecyparis obtusa Leaves. Chromatographia 2013, 77, 373–377. [Google Scholar] [CrossRef]
- Espino, M.; Fernández, M.A.; Gómez, F.J.V.; Boiteux, J.; Silva, M.F. Green analytical chemistry metrics: Towards a sustainable phenolics extraction from medicinal plants. Microchem. J. 2018, 141, 438–443. [Google Scholar] [CrossRef]
- Fu, M.; Lu, Z.; Ma, X. Enhanced extraction efficiency of natural D-borneol from Mei Pian tree leaves pretreated with deep eutectic solvents. Food Sci. Nutr. 2020, 8, 3806–3813. [Google Scholar] [CrossRef] [PubMed]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Fernández, M.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharm. Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, X.; Liu, W. Application of Deep Eutectic Solvents in Food Analysis: A Review. Molecules 2019, 24, 4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redha, A.A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Espino, M.; Solari, M.; Fernández, M.L.Á.; Boiteux, J.; Gómez, M.R.; Silva, M.F. NADES-mediated folk plant extracts as novel antifungal agents against Candida albicans. J. Pharm. Biomed. Anal. 2019, 167, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Grecco, C.F.; de Souza, I.D.; Queiroz, M.E.C. Novel materials as capillary coatings for in-tube solid-phase microextraction for bioanalysis. J. Sep. Sci. 2021, 44, 1662–1693. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. Evid.-Based Complement. Alternat. Med. 2013, 3, 820695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakeem, K.R.; Rehman, R.U.; Tahir, I. Plant Signaling: Understanding the Molecular Crosstalk; Springer: New Delhi, India, 2014. [Google Scholar]
- Alirezaei, M.; Khoshdel, Z.; Dezfoulian, O.; Rashidipour, M.; Taghadosi, V. Beneficial antioxidant properties of betaine against oxidative stress mediated by levodopa/benserazide in the brain of rats. J. Physiol. Sci. 2015, 65, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Grillo, G.; Gunjević, V.; Radošević, K.; Radojčić Redovniković, I.; Cravotto, G. Deep eutectic solvents and nonconventional technologies for blueberry-peel extraction: Kinetics, anthocyanin stability, and antiproliferative activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Li, Z.; Jiang, L.; Cao, X.; Gao, W.; Wang, J.; Luo, D.; Chen, F. Deep eutectic solvent-homogenate based microwave-assisted hydrodistillation of essential oil from Litsea cubeba (Lour.) Pers. fruits and its chemical composition and biological activity. J. Chromatogr. A 2021, 1646, 462089. [Google Scholar] [CrossRef]
- Goldsborough, A.S.; Bates, M.R. Sample Fixation and Stabilisation. U.S. Patent US9696247B2, 4 September 2014. [Google Scholar]
- Ramezani, A.M.; Absalan, G. Employment of a natural deep eutectic solvent as a sustainable mobile phase additive for improving the isolation of four crucial cardiovascular drugs by micellar liquid chromatography. J. Pharm. Biomed. Anal. 2020, 186, 113259. [Google Scholar] [CrossRef]
- García-Argüelles, S.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; Yuste, L.; Rojo, F.; del Monte, F. Deep eutectic solvent-assisted synthesis of biodegradable polyesters with antibacterial properties. Langmuir 2013, 29, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Pradeepkumar, P.; Rajendran, N.K.; Shakila, H.; Houreld, N.N.; Farraj, D.A.; Elnahas, Y.M.; Elumalai, N.; Rajan, M. Natural deep eutectic solvent supported targeted solid–liquid polymer carrier for breast cancer therapy. RSC Adv. 2020, 10, 36989–37004. [Google Scholar] [CrossRef]
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F.; Hashim, M.A. In Vitro and In Vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS ONE 2015, 10, e0117934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Pereiro, A.B.; Rebelo, L.P.N.; Marrucho, I.M. Deep eutectic solvents as extraction media for azeotropic mixtures. Green Chem. 2013, 15, 1326–1330. [Google Scholar] [CrossRef]
- Zhang, Y.; de La Harpe, K.; Hariharan, M.; Kohler, B. Excited-state dynamics of mononucleotides and DNA strands in a deep eutectic solvent. Faraday Discuss. 2018, 207, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]

| Eutectic Mixture | Drug | Increased Solubility with Respect to Water Solution (T = 298.15 K) | Reference |
|---|---|---|---|
| Choline chloride and urea (1:2) | Benzoic acid | 80 times | [40] |
| AMG517 | 100 times | [40] | |
| Choline chloride and malonic acid (1:1) | Danazol | 320 times | [40] |
| AMG517 | 20,000 times | [40] | |
| Choline chloride and glycolic acid (1:2) | Itraconazole | 6700 times | [45] |
| Piroxicam | 430 times | [46] | |
| Lidocaine | 2. times | [46] | |
| Posaconazole | 6400 times | [46] | |
| Choline chloride, glycolic acid, and oxalic acid (1:1.7:0.3) | Itraconazol | 50,000 times | [45] |
| Piroxicam | 135 times | [46] | |
| Lidocain | 81 times | [46] | |
| Posaconazole | 7400 times | [46] | |
| Menthol | Ibuprofen | 2.5 times | [48] |
| Menthol:Poloxamer | 6 times | [48] | |
| Choline chloride and glycolic acid (1:2) | Cefixime trihydrate | 2418 times | [49] |
| THEDES System | Year | Experimental Study | Achievement | Administration Route | Reference |
|---|---|---|---|---|---|
| Ibuprofen:L-menthol Ibuprofen:thymol Ibuprofen:D-limonene Ibuprofene:cymene Ibuprofene:L-menthone | 1998 | Human epidermal membranes | Solubility enhancement | Transdermal | [54] |
| Methyl nicotinate:ibuprofen | 2000 | physicochemical studies | Enhance transdermal delivery | Transdermal | [72] |
| Menthol:coenzyme Q10 | 2002 | Physicochemical studies | Formulation of self-nanoemulsified drug delivery system (SNEDDS) to improve the solution of coenzyme Q10 | Oral * | [73] |
| Cannabidiol:phosphotidylcholine | 2003 | Mice | Enhance transdermal delivery and better accumulation (muscle and skin) | Transdermal | [74] |
| Fluconazole:camphor:menthol | 2009 | Rat skin (antifungicide activity, effective in vivo activity) | Improved drug transport | Transdermal | [56] |
| ritonavir:gelucire | 2010 | Albino Wistar rats | Increase of rate of absorption | Oral | [75] |
| Itraconazole:phenol | 2012 | Franz diffusion cells fitted with excised hairless mouse skins | Permeability enhancement | Topical | [76] |
| Ibuprofen:lauric acid Ibuprofen:palmitic acid | 2013 | Physicochemical studies | Information drug-excipient and its compatibility | Topical * | [77] |
| Nimesulide:PEG Nimesulide:urea | 2014 | rats | Increase of the analgesic effect | Oral | [78] |
| Coenzyme Q10:lauric acid | 2015 | Physicochemical studies | Solubility enhancement | Topical | [79] |
| Lidocaine:tertracaine Lidocaine:camphor | 2015 | physicochemical studies | Liquid formulation | Topical * | [80] |
| acetylsalicylic acid:ChCl acetylsalicylic acid:menthol | 2016 | Microbiology studies in E. coli, S. aureus, B. subtilis. | Enhanced transporters and delivery vehicles for bioactive molecules | Oral * | [9] |
| Ibuprofen:lidocaine | 2017 | Transdermal Bioavailability in rats | Enhancers of skin permeation | Transdermal | [81] |
| Resorcinol:ChCl acetylsalycilic acid:ChCl salicylic acid:ChCl Paracetamol:ChCl ranitidine:glycerol diphenine:glycerol phenformin:glycerol ticlopidine:glycerol tetracycline:glycerol ranitidine:urea. adiphenine:urea adiphenine:acetylsalycilic acid | 2017 | Physicochemical studies | Solubility enhancement | Oral * | [82] |
| Paeonol:menthol | 2017 | In vitro permeation and deposition study on mouse skin | Transdermal delivery | Transdermal | [83] |
| Ibuprofen:menthol | 2017 | Physicochemical studies | Solubility and permeability increases | Oral * | [84] |
| Felodipine:nicotinamide Felodipine:malonic acid | 2017 | Animal model | Improvement of AUC compared to free drug solution | Oral | [85] |
| Hydrochlorothiazide:atenolol | 2017 | Female Winstar rats | Decrease in systolic blood pressure was more pronounced when it was compared to physical mixtures | Oral | [86] |
| alpha-eprosartan:p-hydroxy benzoic acid | 2017 | Female Sprague-Dawley rats | Oral bioavailability and AUC increases | Oral | [87] |
| Ibersartan:nicotinic acid Ibersartan:ascorbic acid | 2018 | pharmacokinetic/pharmacodynamic studies and oxidative stress analysis in rats | Improvement of AUC | Oral | [88] |
| Caffeine:meloxicam Caffeine:aceclofenac Caffeine:flurbiprofen | 2019 | Male Winstar rats | Increase in solubility and effect | Oral | [89] |
| Ibuprofen:Limonene | 2019 | HT29 cell line | Enhancement of anti-inflammatory activity of ibuprofen | Oral * | [71] |
| Ibuprofen:1-tetradecanol Ibuprofen:1-octadecanol Ibuprofen:1-docosanol | 2020 | Physicochemical studies | In vitro release | Topical * | [90] |
| Diacerrein:fumaric acid | 2020 | Pharmacokinetic study in Sprague-Dawley rats | Improvement of Cmax and AUC | Oral | [91] |
| Diacerin:2,4-dihydroxybenzoic acid | 2020 | Pharmacokinetic study in Sprague-Dawley rats | Higher bioavailability and lower tmax compared to pure drug and | Oral | [92] |
| Citric acid:L-arginine:H2O | 2020 | L929 fibroblasts and system characterization | Design and development of drug formulation | Inhalatory * | [70] |
| Lidocaine:ketoprofen Lidocaine:flurbiprofen Lidocaine:aceclofenac Lidocaine:meloxicam Lidocaine:tenoxicam | 2021 | Albino male rabbit ear model. Skin permeation studies using vertical glass Franz diffusion cells | Use of lidocaine as eutectic co-former for enhanced skin delivery of NSAIDs. | Transdermal | [93] |
| Ezetimibe:simvastatin:fenofibrate | 2021 | Physicochemical studies | New way of selecting therapeutic concentrations | Oral * | [94] |
| Curcumin:choline geranate deep eutectic solvent | 2021 | Domestic pig ears | Increase the transdermal permeation of curcumin, insulin, and bacteriophage particles. It will be use as non-invasive delivery | Transdermal | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Appl. Sci. 2021, 11, 10156. https://doi.org/10.3390/app112110156
Lomba L, García CB, Ribate MP, Giner B, Zuriaga E. Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Applied Sciences. 2021; 11(21):10156. https://doi.org/10.3390/app112110156
Chicago/Turabian StyleLomba, Laura, Cristina B. García, Mª Pilar Ribate, Beatriz Giner, and Estefanía Zuriaga. 2021. "Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals" Applied Sciences 11, no. 21: 10156. https://doi.org/10.3390/app112110156
APA StyleLomba, L., García, C. B., Ribate, M. P., Giner, B., & Zuriaga, E. (2021). Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Applied Sciences, 11(21), 10156. https://doi.org/10.3390/app112110156









