Deep Eutectic Solvents: Are They Safe?
Abstract
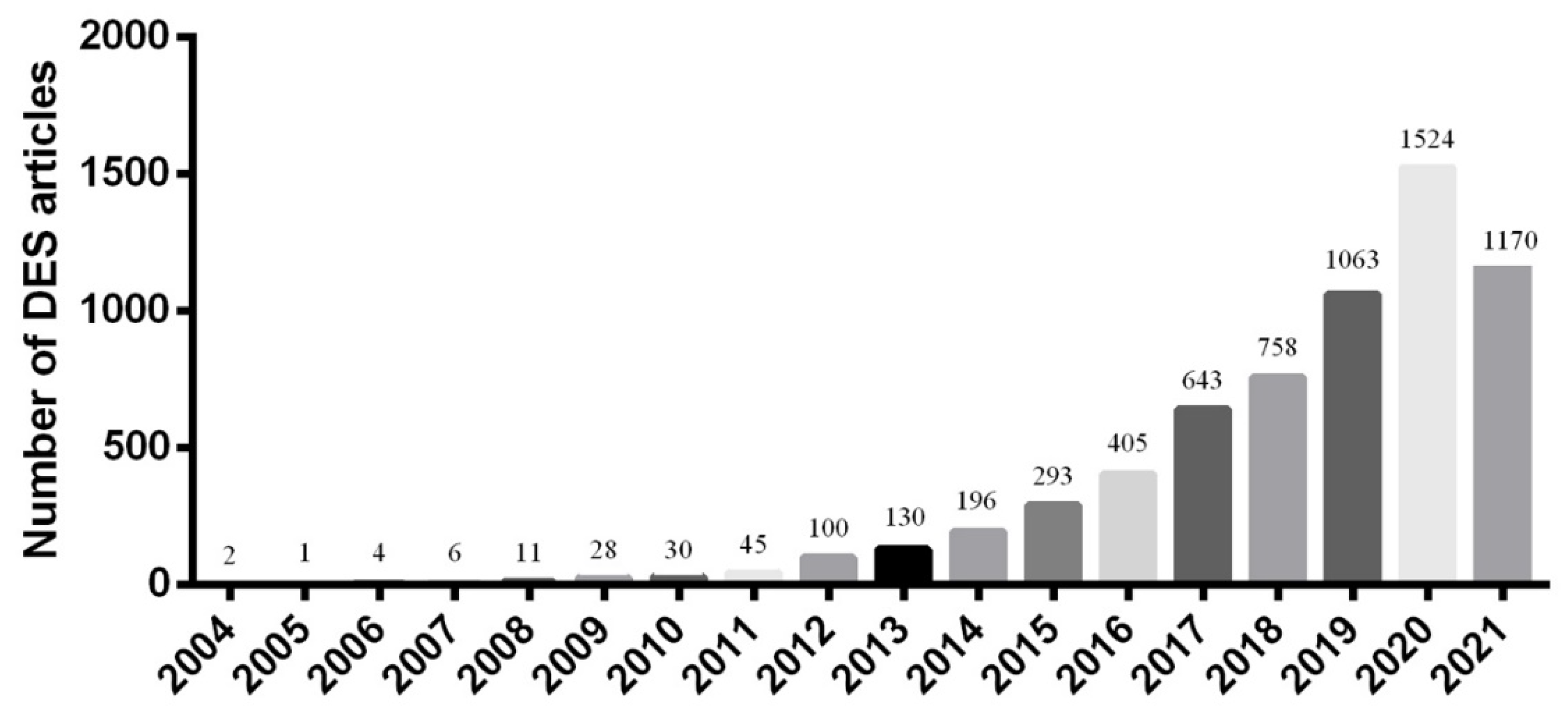
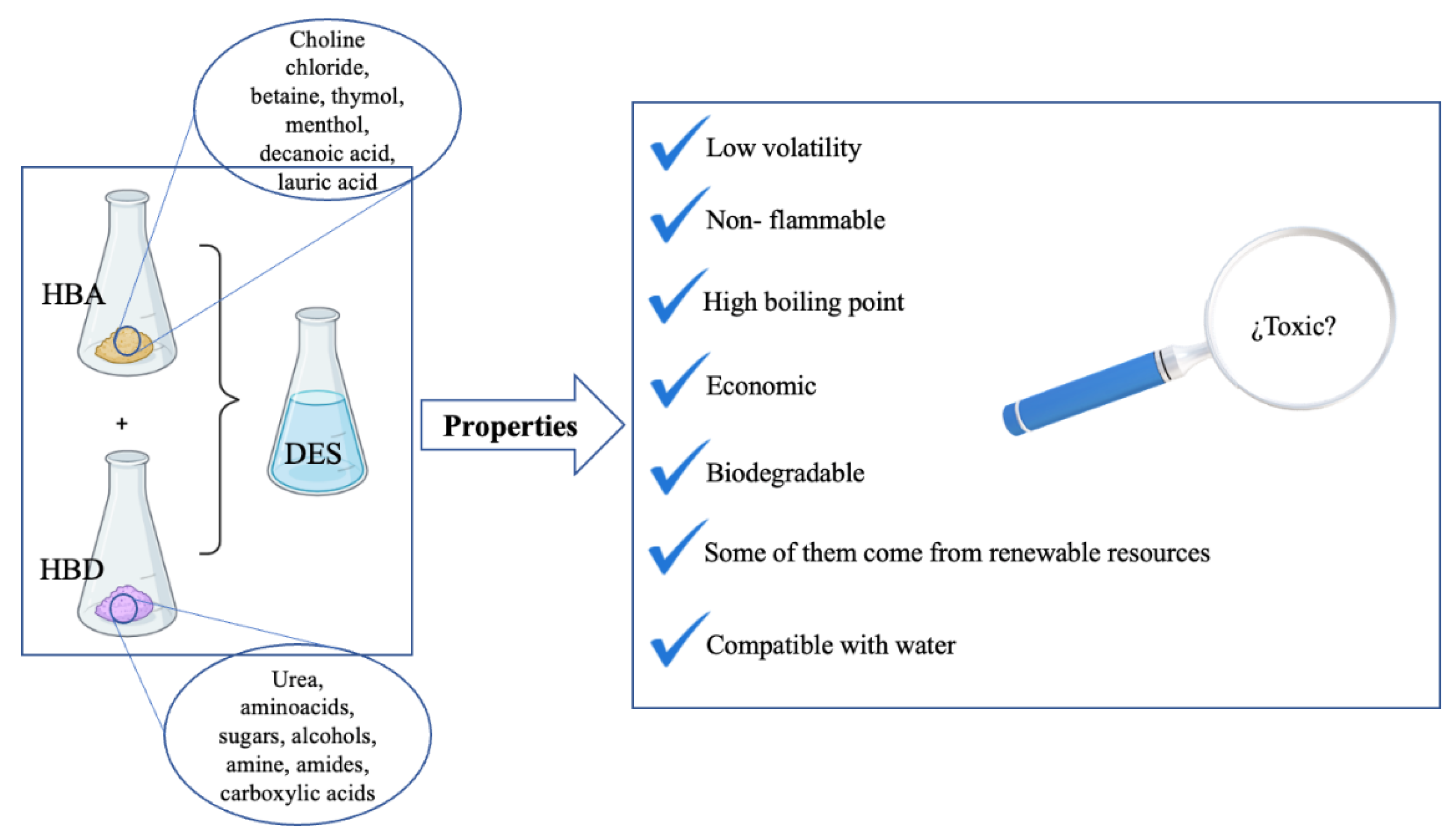
1. Deep Eutectic Solvents (DESs)
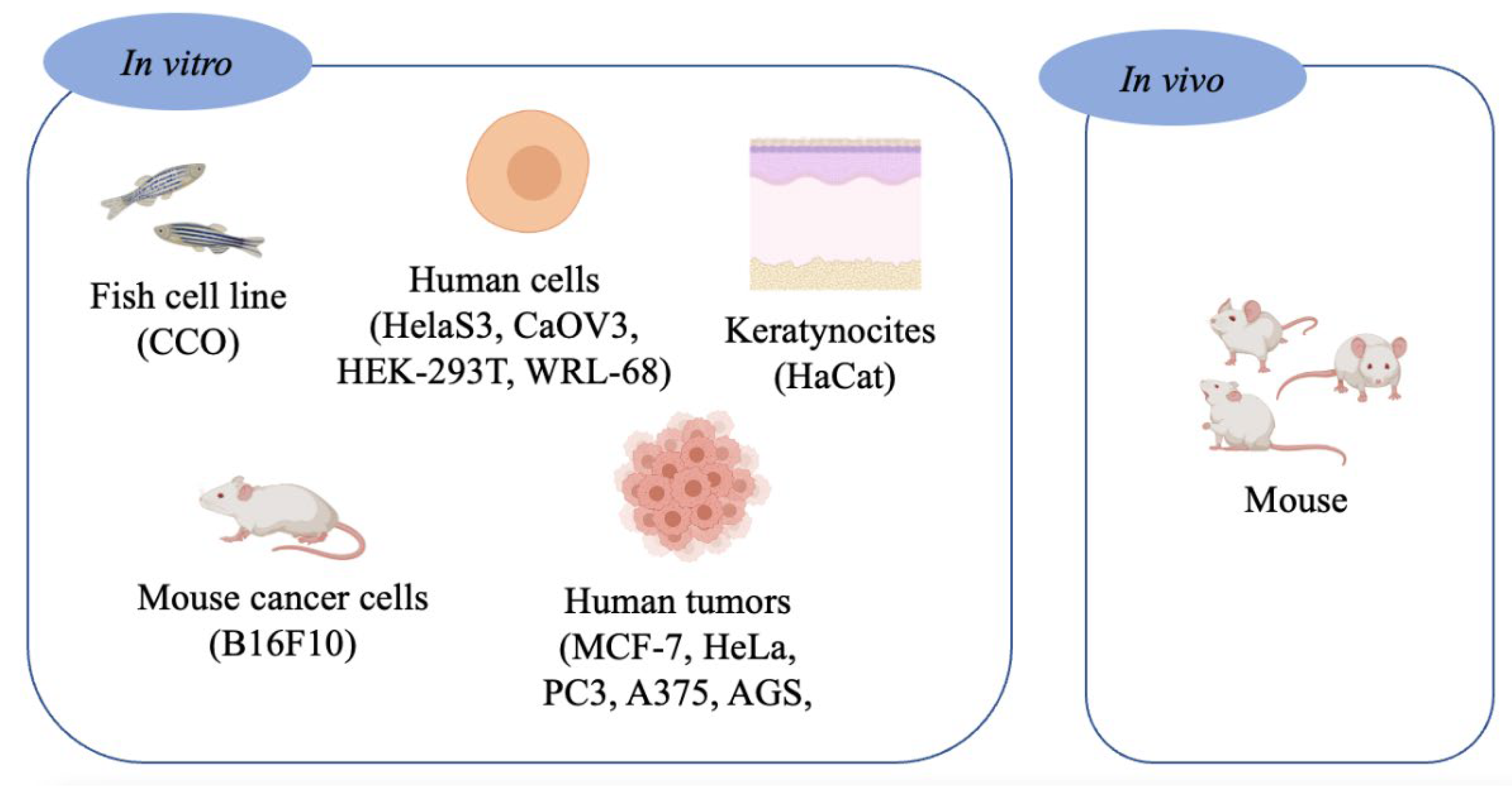
2. Cytotoxicity
2.1. In Vitro Assays
2.1.1. Choline Chloride (HBA)-HBD
2.1.2. Presence of Organic Acids
2.1.3. Influence of pH
2.1.4. Molar Ratio
2.1.5. Synergistic Effects
2.1.6. Natural Cellular Intermediaries
2.1.7. Viscosity
2.2. In Vivo Assays
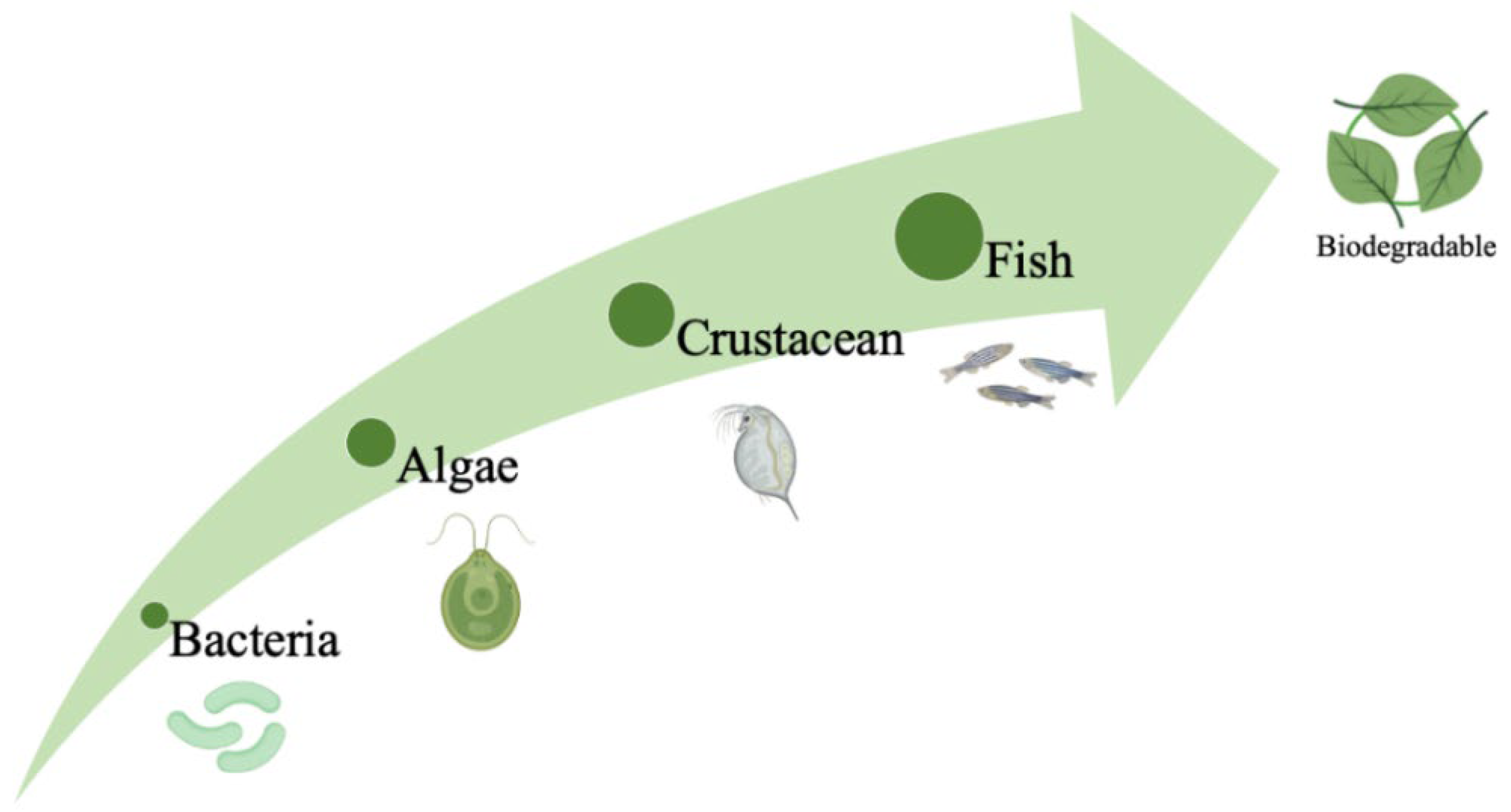
3. Ecotoxicity
3.1. Aquatic Biomodels
3.1.1. Bacteria Biomodel
3.1.2. Crustacean
3.1.3. Algae
3.1.4. Fish
3.2. Terrestrial Biomodels
3.3. Biodegradability
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. fischeri | Aliivibrio fischeri |
| A375 | Human malignant melanoma cell line |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| B16F10 | Murine melanoma cell line |
| CaOV3 | Human adenocarcinoma ovary cell line |
| CCO | Channel catfish ovary |
| ChCl | Choline chloride |
| D. magna | Daphnia magna |
| DESs | Deep eutectic solvents |
| EC50 | Effective concentration at 50% |
| IC50 | Inhibitory concentration at 50% |
| EG | Ethylene glycol |
| Etha | Ethaline |
| EthaW | Ethaline and water |
| Gly | Glycerine |
| GlyW | Glycerine and water |
| GlyW | Glycerine and water |
| H413 | Human squamous-cell carcinoma, buccal mucosa |
| HaCat | Human keratinocyte cell line |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| HEK-293 | Human embryonic kidney |
| HelaS3 | Human cervical cancer cell line |
| ILs | Ionic liquids |
| LC50 | Lethal concentration at 50% |
| MCF-7 | Human breast cancer cell line |
| MNT-1 | Human malignant melanoma cell line |
| MTPB | Methyltriphenylphosphonium bromide |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| N1111 | Tetramethylammonium chloride |
| N4444 | Tetrabutylammonium chloride |
| NADESs | Natural deep eutectic solvents |
| PC3 | Human prostate cancer cell line |
| R. subcapitata | Raphidocelis subcapitata |
| Rel | Reline |
| RelW | Reline and water |
| scCO2 | Supercritical carbon dioxide |
| scH2O | Supercritical water |
| scNH3 | Supercritical ammonia |
| TEG | Triethylene glycol |
| U | Urea |
| WRL-68 | Human cervical cancer cell line |
| WST-1 | Water-soluble tetrazolium salt |
References
- Bart, H.-J. Extraction of Natural Products from Plants—An Introduction; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.K.; Naraparaju, S.; Soujanya, C.; Gupta, S.D. Green Chemistry and Green Solvents: An Overview. Curr. Green Chem. 2020, 7, 314–325. [Google Scholar] [CrossRef]
- Abdussalam-Mohammed, W.; Qasem Ali, A.; O Errayes, A. Green Chemistry: Principles, Applications, and Disadvantages. Chem. Methodol. 2020, 4, 408–423. [Google Scholar] [CrossRef]
- Khalil, R.; Chaabene, N.; Azar, M.; Malham, I.B.; Turmine, M. Effect of the chain lengthening on transport properties of imidazolium-based ionic liquids. Fluid Phase Equilib. 2020, 503, 112316. [Google Scholar] [CrossRef]
- Shoja, S.M.R.; Abdouss, M.; Beigi, A.A.M. Synthesis and characterization of physicochemical properties of imidazolium-based ionic liquids and their application for simultaneous determination of sulfur compounds. J. Mol. Struct. 2021, 1230. [Google Scholar] [CrossRef]
- Bounsiar, R.; Gascón, I.; Amireche, F.; Lafuente, C. Volumetric properties of three pyridinium-based ionic liquids with a common cation or anion. Fluid Phase Equilib. 2020, 521, 112732. [Google Scholar] [CrossRef]
- Fang, J.; Wu, J.; Li, C.; Li, H.; Yang, Q. Molecular design and comprehensive evaluation of solvents capable of simultaneously extracting multiple active substances. Sep. Purif. Technol. 2020, 245, 116882. [Google Scholar] [CrossRef]
- Hussain, A.; AlAjmi, M.F.; Hussain, I.; Ali, I. Future of Ionic Liquids for Chiral Separations in High-Performance Liquid Chromatography and Capillary Electrophoresis. Crit. Rev. Anal. Chem. 2019, 49, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jin, F.; Li, Z.; Song, H.; Chen, J. Research and Application of Supported Ionic Liquids. Prog. Chem. 2020, 32, 1274. [Google Scholar] [CrossRef]
- Cheng, K.; Wu, Q.; Jiang, L.; Liu, M.; Li, C. Protein stability analysis in ionic liquids by 19F NMR. Anal. Bioanal. Chem. 2019, 411, 4929–4935. [Google Scholar] [CrossRef] [PubMed]
- Javaherian, M.; Saghanezhad, S.J. Synthesis, Characterization and Applications of Dicationic Ionic Liquids in Organic Synthesis. Mini. Rev. Org. Chem. 2019, 17, 450–464. [Google Scholar] [CrossRef]
- Liu, R.; Shi, Y.; Zhang, R.; Dai, F. Progress in environmental behaviors and safety of ionic liquids. Chinese Sci. Bull. 2019, 64, 3158–3164. [Google Scholar] [CrossRef]
- Errazquin, D.; Mohamadou, A.; Dupont, L.; De Gaetano, Y.; García, C.B.; Lomba, L.; Giner, B. Ecotoxicity interspecies study of ionic liquids based on phosphonium and ammonium cations. Environ. Sci. Pollut. Res. 2021, 1–11. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Ayuso, M.; Villar-Chavero, M.M.; García, J.; Rodríguez, F. Ecotoxicity evaluation towards Vibrio fischeri of imidazolium- and pyridinium-based ionic liquids for their use in separation processes. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, S.; Shiksha; Gahlay, G.K.; Mithu, V.S. Cytotoxicity and Membrane Permeability of Double-Chained 1,3-Dialkylimidazolium Cations in Ionic Liquids. J. Phys. Chem. B 2021, 125, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Leitch, A.C.; Abdelghany, T.M.; Probert, P.M.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Cooke, M.P.; Blake, L.I.; Morse, K.; Rosenmai, A.K.; et al. The toxicity of the methylimidazolium ionic liquids, with a focus on M8OI and hepatic effects. Food Chem. Toxicol. 2020, 136, 111069. [Google Scholar] [CrossRef] [PubMed]
- Shirai, M.; Hiyoshi, N.; Rode, C.V. Stereoselective Aromatic Ring Hydrogenation over Supported Rhodium Catalysts in Supercritical Carbon Dioxide Solvent. Chem. Rec. 2019, 19, 1926–1934. [Google Scholar] [CrossRef]
- Huang, X.; Li, T.; Zhao, J.; Wang, C. Rigorous modeling of solubility of normal alkanes in supercritical CO2. Pet. Sci. Technol. 2019, 37, 1008–1015. [Google Scholar] [CrossRef]
- Ahmad Jelani, N.A.; Azlan, A.; Khoo, H.E.; Razman, M.R. Fatty acid profile and antioxidant properties of oils extracted from dabai pulp using supercritical carbon dioxide extraction. Int. Food Res. J. 2019, 26, 1587–1598. [Google Scholar]
- Park, S.I.S.H.J.M.S. Extraction of Active Compounds from Angelica gigas using Supercritical Carbon Dioxide and its Physiological Activity. J. Converg. Inf. Technol. 2021, 11, 206–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Chi, S.; Li, Z. Pulping of bamboo using supercritical ammonia with recovery and reuse of the ammonia. BioRes 2019, 14, 5586–5594. [Google Scholar]
- Zhou, F.; Hearne, Z.; Li, C.J. Water—the greenest solvent overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. [Google Scholar] [CrossRef]
- García, J.I.; Pires, E.; Aldea, L.; Lomba, L.; Perales, E.; Giner, B. Ecotoxicity studies of glycerol ethers in Vibrio fischeri: Checking the environmental impact of glycerol-derived solvents. Green Chem. 2015, 17, 4326–4333. [Google Scholar] [CrossRef]
- Lomba, L.; Muñiz, S.; Pino, M.R.; Navarro, E.; Giner, B. Ecotoxicity studies of the levulinate ester series. Ecotoxicology 2014, 23, 1484–1493. [Google Scholar] [CrossRef][Green Version]
- Pandey, A.; Dhingra, D.; Pandey, S. Hydrogen Bond Donor/Acceptor Cosolvent-Modified Choline Chloride Based Deep Eutectic Solvents. J. Phys. Chem. B. 2017, 121, 4202–4212. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 1–16. [Google Scholar] [CrossRef]
- Nakhle, L.; Kfoury, M.; Mallard, I.; Landy, D.; Greige-Gerges, H. Microextraction of bioactive compounds using deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3747–3759. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Y.; Zhao, Q.; Chen, A.; Jiao, B. Ultrasound-assisted dispersive liquid-phase microextraction by solidifying L-menthol-decanoic acid hydrophobic deep eutectic solvents for detection of five fungicides in fruit juices and tea drinks. J. Sep. Sci. 2021. [CrossRef]
- Habila, M.A.; Alabdullkarem, E.A.; Alothman, Z.A.; Yilmaz, E.; Soylak, M. Thiomalic acid/ferric chloride-based deep eutectic solvent for microextraction of chromium in natural water samples prior to FAAS analysis. Int. J. Environ. Anal. Chem. 2020, 1–9. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Pekel, A.G.; Toprakçı, İ.; Özçelik, G.; Bilgin, M.; Şahin, S. Hydrophobic carboxylic acid based deep eutectic solvent for the removal of diclofenac. Biomass Convers. Biorefinery 2020, 1–9. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Dokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Malolan, R.; Gopinath, K.P.; Vo, D.-V.N.; Jayaraman, R.S.; Adithya, S.; Ajay, P.S.; Arun, J. Green ionic liquids and deep eutectic solvents for desulphurization, denitrification, biomass, biodiesel, bioethanol and hydrogen fuels: A review. Environ. Chem. Lett. 2020, 19, 1001–1023. [Google Scholar] [CrossRef]
- Lima, F.; Branco, L.C.; Silvestre, A.J.D.; Marrucho, I.M. Deep desulfurization of fuels: Are deep eutectic solvents the alternative for ionic liquids? Fuel 2021, 293, 120297. [Google Scholar] [CrossRef]
- Zhang, T.; Doert, T.; Wang, H.; Zhang, S.; Ruck, M. Inorganic Synthesis Based on Reactions of Ionic Liquids and Deep Eutectic Solvents. Angew. Chemie Int. Ed. 2021, 60, 22148–22165. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Q.-X.; He, J.-R.; Liang, Z.-H.; Li, X.; Cheng, H.; Li, L.-L. L-proline-catalyzed Knoevenagel reaction promoted by choline chloride-based deep eutectic solvents. Biomass Convers. Biorefinery 2021, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Ghobadi, R.; Divsalar, A. Enzymatic behavior of bovine liver catalase in aqueous medium of sugar based deep eutectic solvents. J. Mol. Liq. 2020, 310. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; He, J.-R.; Yao, Q.-X.; Li, L.-L.; Liang, Z.-H.; Li, X. Enzymes-Catalyzed Knoevenagel Condensation Promoted by Ionic Liquid and Deep Eutectic Solvent. Catal. Lett. 2021, 1–9. [Google Scholar] [CrossRef]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. Adv. Biochem. Eng. Biotechnol. 2018, 168, 31–59. [Google Scholar] [CrossRef]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkumar, P.; Rajan, M.; Almoallim, H.S.; Alharbi, S.A. Targeted Delivery of Doxorubicin in HeLa Cells Using Self-Assembled Polymeric Nanocarriers Guided by Deep Eutectic Solvents. ChemistrySelect 2021, 6, 7232–7241. [Google Scholar] [CrossRef]
- Arriaga, S.; Aitor, A. Advances and Applications of Partitioning Bioreactors. In Advances in Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Álvarez, M.S.; Zhang, Y. Sketching neoteric solvents for boosting drugs bioavailability. J. Control. Release 2019, 311–312, 225–232. [Google Scholar] [CrossRef]
- Kim, J.; Shi, Y.; Kwon, C.J.; Gao, Y.; Mitragotri, S. A Deep Eutectic Solvent-Based Approach to Intravenous Formulation. Adv. Healthc. Mater. 2021, 10, 2100585. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Atilhan, M.; Aparicio, S. Theoretical Study on Deep Eutectic Solvents as Vehicles for the Delivery of Anesthetics. J. Phys. Chem. B 2020, 124, 1794–1805. [Google Scholar] [CrossRef]
- Golgoun, S.; Mokhtarpour, M.; Shekaari, H. Solubility Enhancement of Betamethasone, Meloxicam and Piroxicam by Use of Choline-Based Deep Eutectic Solvents. Pharm. Sci. 2020, 27, 86–101. [Google Scholar] [CrossRef]
- Mustafa, N.R.; Spelbos, V.S.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Solubility and Stability of Some Pharmaceuticals in Natural Deep Eutectic Solvents-Based Formulations. Molecules 2021, 26, 2645. [Google Scholar] [CrossRef]
- Atilhan, M.; Costa, L.T.; Aparicio, S. On the behaviour of aqueous solutions of deep eutectic solvents at lipid biomembranes. J. Mol. Liq. 2017, 247, 116–125. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, I.I.I.; Bahamon, D.; Llovell, F.; Abu-Zahra, M.R.M.; Vega, L.F. Perspectives and guidelines on thermodynamic modelling of deep eutectic solvents. J. Mol. Liq. 2020, 298, 112183. [Google Scholar] [CrossRef]
- Giner, B.; Lafuente, C.; Lapeña, D.; Errazquin, D.; Lomba, L. QSAR study for predicting the ecotoxicity of NADES towards Aliivibrio fischeri. Exploring the use of mixing rules. Ecotoxicol. Environ. Saf. 2020, 191, 110004. [Google Scholar] [CrossRef] [PubMed]
- Sekharan, T.R.; Chandira, R.M.; Tamilvanan, S.; Rajesh, S.C.; Venkateswarlu, B.S. Deep Eutectic Solvents as an Alternate to Other Harmful Solvents. Review 2022, 12, 847–860. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents – Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. Springerplus 2016, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F.; Hashim, M.A. In Vitro and In Vivo Toxicity Profiling of Ammonium-Based Deep Eutectic Solvents. PLoS ONE 2015, 10, e0117934. [Google Scholar] [CrossRef]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Landeka Dragičević, T.; Redovniković, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2014, 112, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the cytotoxicity and metabolic pathways of binary natural deep eutectic solvent systems. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Hemmateenejad, B.; Safavi, A.; Shojaeifard, Z.; Mohabbati, M.; Firuzi, O. Assessment of cytotoxicity of choline chloride-based natural deep eutectic solvents against human HEK-293 cells: A QSAR analysis. Chemosphere 2018, 209, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Macário, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Mitar, A.; Panić, M.; Kardum, J.P.; Halambek, J.; Sander, A.; Kučan, K.Z.; Redovniković, I.R.; Radošević, K. Physicochemical Properties, Cytotoxicity, and Antioxidative Activity of Natural Deep Eutectic Solvents Containing Organic Acid. Chem. Biochem. Eng. Q. 2019, 33, 1–18. [Google Scholar] [CrossRef]
- Kareem, M.A.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Phosphonium-Based Ionic Liquids Analogues and Their Physical Properties. J. Chem. Eng. Data 2010, 55, 4632–4637. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The pH behavior of seventeen deep eutectic solvents. BioResources 2019, 13, 5042–5051. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; Alnashef, I.M.; Mirghani, M.E.S. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Spring, D.R.; Qian, X.; Cui, J.; Xu, Z. Quantitatively mapping cellular viscosity with detailed organelle information via a designed PET fluorescent probe. Sci. Rep. 2014, 4, 5418. [Google Scholar] [CrossRef] [PubMed]
- Barile, F.A. Clinical Toxicology: Principles and Mechanisms; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781420092264. [Google Scholar]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Q.; Liu, M.; Zhang, L. The effect of deep eutectic solvent on the pharmacokinetics of salvianolic acid B in rats and its acute toxicity test. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2017, 1063, 60–66. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Using Toxicity Tests in Ecological Risk Assessment. ECO Update; United States Environmental Protection Agency: Washington, DC, USA, 1994; Volume 2, number 1. [Google Scholar]
- Lagesson, A.; Fahlman, J.; Brodin, T.; Fick, J.; Jonsson, M.; Byström, P.; Klaminder, J. Bioaccumulation of five pharmaceuticals at multiple trophic levels in an aquatic food web - Insights from a field experiment. Sci. Total Environ. 2016, 568, 208–215. [Google Scholar] [CrossRef]
- de Morais, P.; Gonçalves, F.; Coutinho, J.A.P.; Ventura, S.P.M. Ecotoxicity of Cholinium-Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404. [Google Scholar] [CrossRef]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep eutectic solvents: An overview on their interactions with water and biochemical compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: Glyceline, ethaline, and reline. Environ. Sci. Pollut. Res. 2020, 28, 8812–8821. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Mohd Ali, O. Toxicity profile of choline chloride-based deep eutectic solvents for fungi and Cyprinus carpio fish. Environ. Sci. Pollut. Res. 2016, 23, 7648–7659. [Google Scholar] [CrossRef]
- Spasiano, D.; Siciliano, A.; Race, M.; Marotta, R.; Guida, M.; Andreozzi, R.; Pirozzi, F. Biodegradation, ecotoxicity and UV254/H2O2 treatment of imidazole, 1-methyl-imidazole and N,N’-alkyl-imidazolium chlorides in water. Water Res. 2016, 106, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.X.; Tang, Y.L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Jesus, F.; Pereira, J.L.; Ventura, S.P.M.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Unraveling the ecotoxicity of deep eutectic solvents using the mixture toxicity theory. Chemosphere 2018, 212, 890–897. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-Ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Asif Tahir, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Jafari, M.; Keshavarz, M.H.; Salek, H. A simple method for assessing chemical toxicity of ionic liquids on Vibrio fischeri through the structure of cations with specific anions. Ecotoxicol. Environ. Saf. 2019, 182, 109429. [Google Scholar] [CrossRef] [PubMed]
- Domínguez De María, P.; Maugeri, Z.; Flitsch, S.; Gotor, V. Ionic liquids in biotransformations: From proof-of-concept to emerging deep-eutectic-solvents This review comes from a themed issue on Biocatalysis and Biotransformation Edited Ionic liquids: Context and development. Curr. Opin. Chem. Biol. 2010, 15, 220–225. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Toxicity of Ionic Liquids: Eco(cyto)activity as Complicated, but Unavoidable Parameter for Task-Specific Optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Matzke, M.; Arning, J.; Ranke, J.; Jastorff, B.; Stolte, S. Design of Inherently Safer Ionic Liquids: Toxicology and Biodegradation. Handb. Green Chem. 2010, 6, 233–298. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. The antagonist and synergist potential of cholinium-based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2018, 165, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Navarro, A.; Romero González, M.C.; Rosas López, E.; Domínguez Aguilar, R.; Sachetin Marçal, W. Evaluation of Daphnia magna as an indicator of toxicity and treatment efficacy of textile wastewaters. Environ. Int. 1999, 25, 619–624. [Google Scholar] [CrossRef]
- Lal, H.; Misra, V.; Viswanathan, P.N.; Murti, A.R.K. The Water Flea (Daphnia magna) as a Sensitive Indicator for the Assessment of Toxicity of Synthetic Detergents. Ecotoxicol. Environ. Saf. 1984, 8, 447–450. [Google Scholar] [CrossRef]
- Hartl, M.; Humpf, H.U. Toxicity assessment of fumonisins using the brine shrimp (Artemia salina) bioassay. Food Chem. Toxicol. 2000, 38, 1097–1102. [Google Scholar] [CrossRef]
- Erzinger, G.S.; Schmoeller, F.; Pinto, L.H.; Américo, L.; Hemmersbach, R.; Hauslage, J.; Häder, D.P. Bioluminescence systems in environmental biosensors. Bioassays Adv. Methods Appl. 2018, 241–262. [Google Scholar] [CrossRef]
- McCormick, P.V.; Cairns, J. Algae as indicators of environmental change. J. Appl. Phycol. 1994, 6, 509–526. [Google Scholar] [CrossRef]
- Passino, D.R.M.; Smith, S.B. Acute bioassays and hazard evaluation of representative contaminants detected in great lakes fish. Environ. Toxicol. Chem. 1987, 6, 901–907. [Google Scholar] [CrossRef]
- Fantke, P.; Bijster, M.; Guignard, C.; Hauschild, M.Z.; Huijbregts, M.A.J.; Jolliet, O.; Kounina, A.; Magaud, V.; Margni, M.; McKone, T.E.; et al. USEtox 2.0 Documentation (Version 1). USEtox International Center. 2017, p. 208. Available online: https://orbit.dtu.dk/en/publications/usetox-20-documentation-version-100 (accessed on 11 August 2021). [CrossRef]
- Clasen, B.; de Moura Lisbôa, R. Ecotoxicological Tests as a Tool to Assess the Quality of the Soil. Soil Contam. Altern. Sustain. Dev. 2019, 13–33. [Google Scholar] [CrossRef]
- Baretta, D.; JCP JCPS; Segat, J.C.; Geremia, E.V.; LCI OF; Alves, M.V. Edafic fauna and soil quality. In Topics in Soil Science; 2011; pp. 119–170. ISBN 1519-3934. [Google Scholar]
- Natal-Da-Luz, T.; Ojeda, G.; Pratas, J.; Van Gestel, C.A.M.; Sousa, J.P. Toxicity to Eisenia andrei and Folsomia candida of a metal mixture applied to soil directly or via an organic matrix. Ecotoxicol. Environ. Saf. 2011, 74, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Gainer, A.; Hogan, N.; Siciliano, S.D. Soil invertebrate avoidance behavior identifies petroleum hydrocarbon contaminated soils toxic to sensitive plant species. J. Hazard. Mater. 2019, 361, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Jiang, B.; Wei, D.; Ma, Y. Ecological criteria for zinc in Chinese soil as affected by soil properties. Ecotoxicol. Environ. Saf. 2020, 194. [Google Scholar] [CrossRef]
- Hou, X.-D.; Liu, Q.-P.; Smith, T.J.; Li, N.; Zong, M.-H. Evaluation of Toxicity and Biodegradability of Cholinium Amino Acids Ionic Liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef] [PubMed]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents. RSC Adv. 2015, 5, 83636–83647. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of water addition on choline chloride/glycol deep eutectic solvents: Characterization of their structural and physicochemical properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Hammershøj, R.; Birch, H.; Redman, A.D.; Mayer, P. Mixture Effects on Biodegradation Kinetics of Hydrocarbons in Surface Water: Increasing Concentrations Inhibited Degradation whereas Multiple Substrates Did Not. Environ. Sci. Technol. 2019, 53, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | DES | Result | Reference |
|---|---|---|---|
| Fibroblast-like cells (L929) | ChCl/glucose (1:1), ChCl/citric acid (1:1), ChCl/citric acid (2:1), ChCl/sucrose (4:1), ChCl/sucrose (1:1), ChCl/tartaric acid (2:1), ChCl/xylose (2:1), ChCl/xylose (3:1), Citric acid/sucrose (1:1), Citric acid/glucose (1:1), Glucose/tartaric acid (1:1) | HBD organic acids (tartaric and citric acids) are major enhancers of cytotoxicity | [58] |
| HelaS3, CaOV3, MCF-7 (human), B16F10 (murine cell line) | ChCl/malonic acid, ChCl/fructose/water, ChCl/glucose/water, ChCl/sucrose/water, ChCl/glycerol/water | HBD organic acids (e.g., malonic acid) increase toxicity. Glycerol (HBD) shows a lower toxicity profile. | [59] |
| MCF-7, A375, HT29, H413, PC3 (human cancer cells), HepG2 and OKF6 (human normal cells) | ChCl/glycerine, ChCl/glycerine/water ChCl/ethylene glycol, ChCl/ethylene glycol/water, ChCl/triethylene glycol, ChCl/triethylene glycol/water, ChCl/urea, ChCl/urea/water | DESs show greater toxicity than individual components. Molar ratio affects toxicity. | [60] |
| Fish cell lines (CCO), MCF-7 (human tumor cells) | (ChCl/glucose (1:2), ChCl/oxalic acid (1:1), ChCl/glycerol (2:1) | ClChl-based DESs show low or moderate (oxalic acid) toxicity. | [61] |
| Hela and MCF-7 (human) | ChCl/glucose, ChCl/fructose, ChCh/xylose, ChCl/malic acid | ClChl-based DESs show low toxicity (EC50 > 2000 mg/L) | [62] |
| HeLa, MCF-7 human tumor cells) and HEK-293T (human normal cells) | ChCl/oxalic acid (1:1), ChCl/urea (1:2), ChCl/xylitol (5:2), ChCl/sorbitol (2:3), Betaine/glucose (5:2), Betaine/malic acid:proline (1:1:1), Betaine/malic acid:glucose (1:1:1), Citric acid/proline (1:1), Citric acid:glucose:glycerol (1:1:1), Citric acid:fructose:glycerol (1:1:1). | Toxicity depends on cellular type and NADES nature. Tumor cells suffer great toxicity (higher energy demands). Oxalic acid ChCl-based NADES shows a lower EC50 in tumor cells. ChCl/urea increases pH and toxicity in MCF-7 | [63] |
| HelaS3, PC3, A375, AGS, MCF-7, and WRL-68 hepatic cell lines | ChCl/glucose (2:1) and ChCl/fructose (2:1) | In vitro (cell lines) NADESs are less toxic than DESs (less acidity). | [64] |
| HEK-293 cell line (human) | 28 selected ChCl-based NADESs | NADESs are more toxic than their individual components. HBA:HBD molar ratio and the number of HBD carbons have negative effects on toxicity, although dependent on the compounds. | [65] |
| Hacat and MNT-1 | Combinations of HBA: ChCl, tetramethylammonium chloride (N1111), and tetrabutylammonium chloride (N4444); and HBD: hexanoic and butanoic acids, ethylene glycol, 1-propanol, and urea | ChCl- and N111-based DESs show great biocompatibility, while N4444 shows toxicity. Some compounds increased cell viability. | [66] |
| Cell Line | DES | Result | Reference |
|---|---|---|---|
| Mice | ChCl/glycerine, ChCl/ethylene glycol, ChCl/triethylene glycol, ChCl/urea | DESs show greater toxicity than their individual components. Molar ratio affects toxicity. | [60] |
| Mice | ChCl/glucose (2:1) and ChCl/fructose (2:1) | In vivo (mice) NADESs are more toxic than DESs (more viscosity) | [64] |
| Male Wistar rats | Betaine:glycerol (1:2) | Short-term oral administration toxicity in rats. | [78] |
| Mice | ChCl-glycerol (1:2) | No toxicity in mice (LD50 = 7733 mg/kg) | [79] |
| DES (Molar Ratio) | EC50 (mg/L) 15 min | EC50 (mg/L) 30 min | Reference |
|---|---|---|---|
| ChCl:cetic acid (1:2) | 125 ± 15 | 130 ± 9 | [82] |
| ChCl:acetic acid | 200 ± 14 | 197 ± 17 | [82] |
| ChCl:acetic acid (2:1) | 343 ± 32 | 337 ± 34 | [82] |
| ChCl:lactic acid (1:2) | 34.2 ± 2.6 | 33.6 ± 0.8 | [82] |
| ChCl:lactic acid | 63.0 ± 6.0 | 61.8 ± 11.6 | [82] |
| ChCl:lactic acid (2:1) | 68.6 ± 12.3 | 67.0 ± 36.0 | [82] |
| ChCl:glycolic acid (1:2) | 30.4 ± 1.6 | 30.2 ± 0.8 | [82] |
| ChCl:glycolic acid | 33.1 ± 6.1 | 32.9 ± 5.6 | [82] |
| ChCl:glycolic acid (2:1) | 63.0 ± 12.2 | 62.2 ± 12.4 | [82] |
| ChCl:citric acid (1:2) | 15.6 ± 0.9 | 15.6 ± 0.6 | [82] |
| ChCl:citric acid | 22.5 ± 2.6 | 22.4 ± 2.4 | [82] |
| ChCl:citric acid (2:1) | 31.1 ± 2.6 | 31.9 ± 4.6 | [82] |
| [N1111]Cl:1-propanol (1:1) | 19,930 ± 2310 | 20,870 ± 3950 | [89] |
| [N1111]Cl:1-propanol (2:1) | 15,420 ± 3680 | 16,150 ± 4050 | [89] |
| [N1111]Cl:1-propanol (4:1) | 16,430 ± 1720 | 15,360 ± 1360 | [89] |
| [N2222]Cl:1-propanol (1:2) | 20,710 ± 3200 | 22,260 ± 3110 | [89] |
| [N2222]Cl:1-propanol (1:1) | 17,590 ± 5140 | 18,090 ± 4650 | [89] |
| [N2222]Cl:1-propanol (2:1) | 13,860 ± 2250 | 15,550 ± 2750 | [89] |
| [N2222]Cl:1-propanol (4:1) | 8571 ± 898 | 9500 ± 945 | [89] |
| [N3333]Cl:1-propanol (1:2) | 1467 ± 328 | 1555 ± 374 | [89] |
| [N3333]Cl:1-propanol (1:1) | 6106 ± 2350 | 4981 ± 2199 | [89] |
| [N3333]Cl:1-propanol (2:1) | 1700 ± 210 | 1845 ± 227 | [89] |
| [N3333]Cl:1-propanol (4:1) | 989.6 ± 184.6 | 1120 ± 214.5 | [89] |
| [N1111]Cl:EG (1:2) | 29,010 ± 2630 | 30,200 ± 9610 | [89] |
| [N1111]Cl:EG (1:1) | 52,110 ± 5320 | 53,990 ± 6110 | [89] |
| [N1111]Cl:EG (2:1) | 42,410 ± 3600 | 49,250 ± 2780 | [89] |
| [N1111]Cl:EG (4:1) | 54,520 ± 16,970 | 65,620 ± 18,260 | [89] |
| [N2222]Cl:EG (1:2) | 18,980 ± 3620 | 18,930 ± 3660 | [89] |
| [N2222]Cl:EG (1:1) | 24,500 ± 3310 | 23,940 ± 3420 | [89] |
| [N2222]Cl:EG (2:1) | 9069 ± 187 | 18,610 ± 4860 | [89] |
| [N2222]Cl:EG (4:1) | 37,180 ± 9530 | 36,390 ± 8600 | [89] |
| [N3333]Cl:EG (1:2) | 778.6 ± 68.6 | 971.3 ± 237.7 | [89] |
| [N3333]Cl:EG (1:1) | 3074 ± 125 | 3665 ± 224 | [89] |
| [N3333]Cl:EG (2:1) | 908.7 ± 104.5 | 945.3 ± 119.4 | [89] |
| [N3333]Cl:EG (4:1) | 1273 ± 110 | 1285 ± 143 | [89] |
| ChCl:glycerol (1:2) | 86,726 ± 8727 | [84] | |
| ChCl:glycerol:water (1:2:1) | 143,686 ± 8885 | [84] | |
| ChCl:urea (1:2) | 26,346 ± 24 | [84] | |
| ChCl:urea:water (1:2:1.7) | 98,409 ± 9598 | [84] | |
| ChCl:EG (1:2) | 108,526 ± 19,101 | [84] | |
| ChCl:EG:water (1:2:1) | 115,450 ± 17,943 | [84] |
| DES (Molar Ratio) | EC50 (mg/L) | Reference |
|---|---|---|
| ChCl:glycerol (1:2) | 2528 ± 151 | [84] |
| ChCl:glycerol:water (1:2:1) | 1762 ± 83 | [84] |
| ChCl:urea (1:2) | 1099 ±25 | [84] |
| ChCl:urea:water (1:2:1.7) | 1533 ± 37 | [84] |
| ChCl:EG (1:2) | 1868 ± 175 | [84] |
| ChCl:EG:water (1:2:1) | 2353 ± 46 | [84] |
| DES (Molar Ratio) | EC50 (mg/L) | Reference |
|---|---|---|
| ChCl:glycerol (1:2) | 7080 ± 65 | [84] |
| ChCl:glycerol:water (1:2:1) | 6617 ± 159 | [84] |
| ChCl:urea (1:2) | 8532 ± 157 | [84] |
| ChCl:urea:water (1:2:1.7) | 2896 ± 103 | [84] |
| ChCl:EG (1:2) | 9196 ± 795 | [84] |
| ChCl:EG:water (1:2:1) | 3536 ± 77 | [84] |
| DES (Molar Ratio) | LC50 (mg/L) | Reference |
|---|---|---|
| ChCl:ZnCl2 (1:2) | 4099 | [85] |
| ChCl:urea (1:2) | >100 | [85] |
| ChCl:glycerol (1:3) | >100 | [85] |
| ChCl:EG (1:3) | >100 | [85] |
| ChCl:diethylene glycol (1:2) | >100 | [85] |
| ChCl:TEG (1:3) | >100 | [85] |
| ChCl:fructose (2:1) | >100 | [85] |
| ChCl:glucose (2:1) | >100 | [85] |
| ChCl:malonic acid (1:1) | >100 | [85] |
| ChCl:p-toluene sulfonic acid (1:3) | >100 | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M. P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. https://doi.org/10.3390/app112110061
Lomba L, Ribate MP, Sangüesa E, Concha J, Garralaga MP, Errazquin D, García CB, Giner B. Deep Eutectic Solvents: Are They Safe? Applied Sciences. 2021; 11(21):10061. https://doi.org/10.3390/app112110061
Chicago/Turabian StyleLomba, Laura, Mª Pilar Ribate, Estela Sangüesa, Julia Concha, M ª Pilar Garralaga, Diego Errazquin, Cristina B. García, and Beatriz Giner. 2021. "Deep Eutectic Solvents: Are They Safe?" Applied Sciences 11, no. 21: 10061. https://doi.org/10.3390/app112110061
APA StyleLomba, L., Ribate, M. P., Sangüesa, E., Concha, J., Garralaga, M. P., Errazquin, D., García, C. B., & Giner, B. (2021). Deep Eutectic Solvents: Are They Safe? Applied Sciences, 11(21), 10061. https://doi.org/10.3390/app112110061









