Applications of Plant Polymer-Based Solid Foams: Current Trends in the Food Industry
Abstract
:1. Introduction
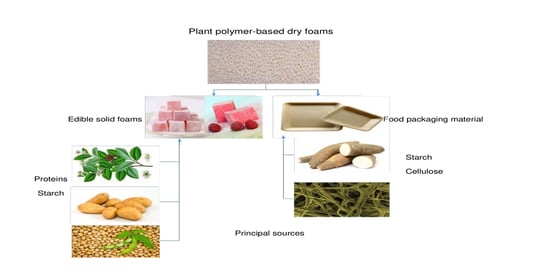
2. Food Industry Applications
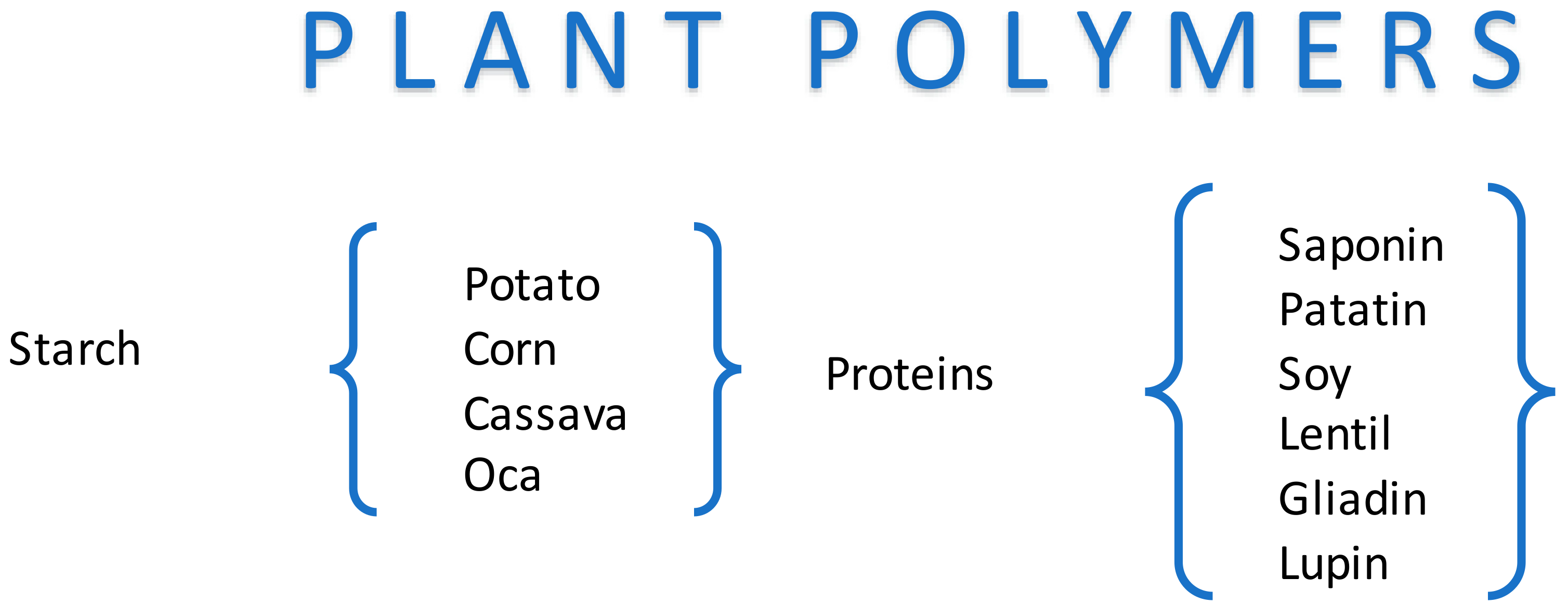
2.1. Plant Polymer-Based Foams as Edible Materials
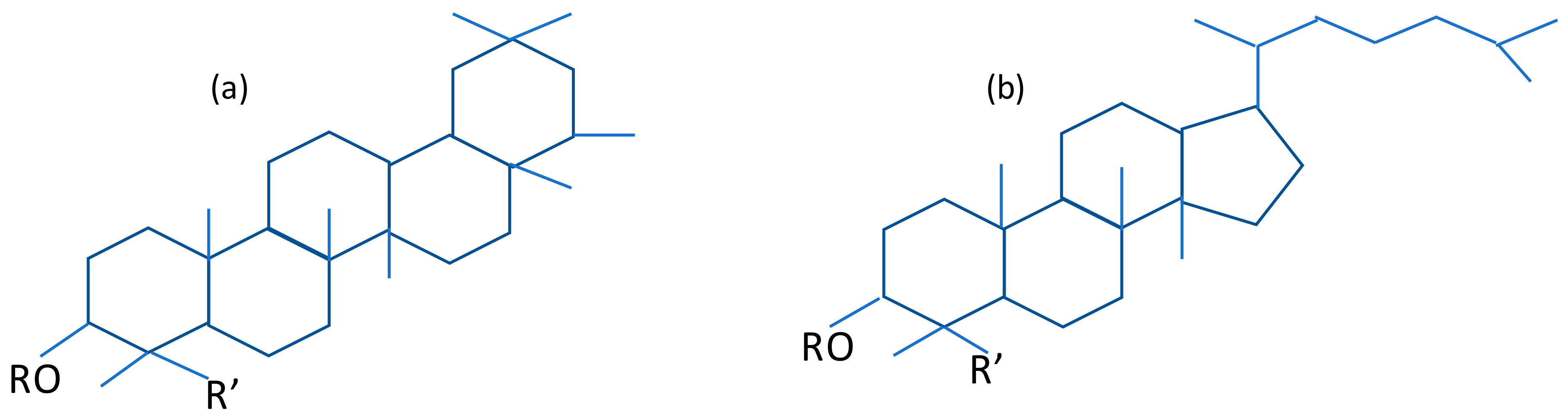
2.1.1. Saponins
2.1.2. Potato Protein
2.1.3. Soy Protein
2.1.4. Gliadin and Lupin
2.1.5. Starch
| Plant Polymer | Study | Type of Foam | Foam Characteristics | Polymer Characteristics |
|---|---|---|---|---|
| Proteins | ||||
| Saponin/Soapwort extract |
| Edible | Good stability and resistance to heating process | They are composed by an aglycone unit called sapogenin linked to one or more carbohydrate chains. The sapogenin unit consists of either a sterol or a triterpene unit, which is the more common. The carbohydrate side-chain is habitually attached to the 3 carbon of the sapogenin. The carbohydrate portion is water-soluble, whereas the sapogenin is fat-soluble; thus, saponins have surface-active properties [36] |
| Edible | Good stability and resistance to heating process. Similar behavior to egg white in sponge cake elaboration | ||
| Patatin |
| Edible | Foam structure is open and resembles a glassy membrane structure around the pores | Patatin belongs to a family of 40–42 kDa glycoproteins with isoelectric point values between 4.5 and 5.2. It shows a secondary structure composed of 35% alpha-helixes, 45% beta-strands and 15% aperiodic. It has a denaturation temperature of 60 °C at pH 7.0 and a relatively low stability as a function of pH showing loss of structure at pH ≤ 4.5 [38] |
| Soy protein isolate |
| Edible and packaging material | Foams have a tunable structure, e.g., size and density tailoring. They also showed uniform and smaller pores with an open-cell structure and pore sizes of about <50 μm, as well as improved mechanical properties | Soy protein is a globulin protein. Its polypeptide chains have a three-dimensional structure linked by disulfide and hydrogen bonds with a molecular weight ranging from 300,000 to 600,000 KDa. Proportion of two major protein polymers in soy protein are 35% conglycinin (7S) and 52% glycinin (11S), giving about 80% of the total soy protein [39,40] |
| Edible | Foams with dense porous structure that renders a crispier texture | ||
| Edible | Good foam expansion. There is no link given by the authors for foaming properties and structure | ||
| Edible | There is no link given by the authors for foaming properties and structure and there is no description of the latter | ||
| Edible | SPI by itself cannot guarantee a suitable foam structure to form the cake. SPI–MDG foams produce batters with correct specific density and appropriate nanostructure, though fewer and larger porosities are observed | ||
| Lentil protein concentrate |
| Edible | Lentil protein produced a foam that retains air bubbles due to strong networks around the air cells. The mean area of air cells is low, while the number of air cells per unit area is high | Lentil proteins are mainly comprised of albumins, (16%) and globulins (70%). Albumins have a molecular weight of about 20. Globulins contain both legumin- and vicilin-like proteins. The first group consists of six polypeptide pairs that interact noncovalently and have a molecular weight (Mw) of 320–380 kDa. Vicilins are trimers of glycosylated subunits with a Mw of 50–60 kDa [43] |
| Polysaccharides | ||||
| Potato Starch |
| Packaging | TPS foam showed lower absorption with improved water resistance. The foam microstructure showed a sandwich-type structure, more or less dense outer layers, and a more compact cellular structure than pure TPS foam. Foams with more modified starch expanded more and became more porous | Potato starch granules are on average shorter than sweet potato starch granules, while bigger than rice starch granules. Amylose content is lower than wheat and corn starch and higher than tapioca and sweet potato starch. It also has the highest molecular weight and the lowest degree of branching. Amylopectin of potato is much less densely branched than other starches, it has much longer chains, and it carries mono-phosphate ester groups [45] |
| Packaging | Foams show the typical sandwich structure, with denser outer layers with small cells and an inner layer with larger and more expanded cells. Silylated starch foams have a more compact structure with thicker outer layers than traditional starch foams. They become mechanically more resistant and have less water absorption capacity | ||
| Packaging | Foams with essential oils had small cracks and holes. They displayed a more irregular but denser surface due to starch-lipid complexes forming during the thermal process. Starch and essential oils also formed strong interactions, resulting in starch–essential oil complexes in the foam layers. Thus, essential oil drops were trapped within the starch granules. Foams presented a sandwich structure with two well-defined layers and the presence of air cells. Essential oil addition and type also affected the layer thickness and the air cell size between the foams. | ||
| Packaging | The foam has a sandwich type structure with dense outer skins containing small cells comprising the surface of the foam. The interior of the foam has large cells with thin walls. Adding over 50% corn fiber, foamed trays contain few small cells in their outer skin. In the interior the cells are smaller, and the foam becomes denser. Trays containing only potato and PVA had thinner skins and larger cells with thicker walls. The outer skin of trays containing corn fiber show compressed and bounded fibers | ||
| Corn Starch |
| Edible | Starch foams were very amorphous. Spirulina–starch or hybrid foams showed a slightly more crystalline structure than the pure starch foam. Thus, hybrid foams showed more densely packed and well-connected porous structures, and foam texture is harder | Corn starch is, in general terms, similar to other cereal starches, and in specific properties has greatest similarity to its genetically closely related cousins, sorghum and the millets. Normal corn is composed of amylose and amylopectin. It is usually composed of 27% amylose and 73% amylopectin [49]. However, this amylose/amylopectin ratio varies slightly with different corn varieties, environmental and soil conditions. Waxy maize consists of amylopectin only, and high amylose corn contains amylose as high as 70% [50] |
| Packaging | Cross-linked starch foams had more expanded structures, and their cell walls were thinner than those of native foams. They showed areas of weak formation on the surface. The additives eliminated these zones. Addition of fiber, kaolin or beeswax increased the cell size in the center of the foams | |||
| Cassava Starch |
| Packaging | Foams showed a sandwich-type structure. The addition of cotton fibers, produced more dense structures, thicker cell walls, and lower area porosity | Cassava starch granules are round with a granule size between 5 and 35 μm. The starch has an A-type X-ray diffraction pattern, usually characteristic of cereals, and not the B type found in other root and tuber starches. The C-type spectrum, intermediate between A and B types, has also been reported. The nonglucosidic fraction of cassava starch is very low; the protein and lipid content are below 0.2%. There is thus no formation of an amylose complex with lipids in native starch. Amylose contents of 8–28% have been reported, but most values lie within the range of 16–18%. The starch gelatinizes at relatively low temperatures. Initial and final gelatinization occurs at 60 °C and 80 °C, respectively. The swelling power of the starch is also very high: 100 g of dry starch will absorb 120 g of water at 100 °C. At this temperature, over 50% of the starch is soluble [54] |
| Packaging | Foams exhibited a more compact, homogeneous, and dense microstructure. The cells were of moderate size, with fibers homogeneously spread throughout the whole material. Baked foams that included proteins were practically devoid of inner open cells | ||
| Packaging | Foams showed a sandwich-type structure with dense outer skins that enclose small cells. The interior of the foams had large air cells with thin walls. They have a good distribution of the malt bagasse throughout the polymeric matrix and showed good expansion with large air cells | ||
| Packaging | Foams exhibited sandwich-type structure with denser outer skins that enclose small cells whereas inner structure is less dense with large cells. They also showed good expansion | ||
| Packaging | Foams present dense and homogeneous external walls, with small, closed cell structure. The interior shows a structure with large open cells and a sandwich- type structure typical of thermoplastic starch-based materials obtained by thermal expansion | ||
| Packaging | Foams showed a good distribution of the pineapple shell fiber throughout the polymeric matrix and a semi-crystalline structure. They have a sandwich-type structure with dense outer skins and small cells comprising the surface of the foam and larger sized cells in the interior of the foam | ||
| Packaging | Foams have filler fibers well incorporated into the starch matrix and well distributed, making the material homogeneous | ||
| Packaging | Foams exhibited sandwich-type structure with denser outer skins that enclose small cells whereas the inner structure is less dense, with large cells. They also showed good expansion | |||
| Packaging | SEM micrographs of foams showed that the cells formed were open with connectivity between cells. They had a sandwich-type structure composed of two layers. The outer layers had a smaller cell size but a denser structure, whereas the interior had a larger cell size and a more expanded structure. NS foam showed a thinner cell wall with a broad distribution of cell sizes. CNS foam, revealed a smaller cell size and a denser structure. | ||
| Oca Starch |
| Packaging | Foams with addition of fibers showed a less compact structure and with distribution no homogenous of poreswhen compared to the control. The fiber distribution through the cellulose matrix was dissimilar for both SB and AP fiber. Trays with SB fiber had larger cells arranged in a thinner layer than those with AP fiber. Both exhibited the typical sandwich structure | Oca starch has a phosphorus content ∼60% lower than potato starch. Its amylose content is approximately 21% (lower than that of maize and potato starches). Amylopectin is similar to that of potato amylopectin, with some differences in the length of its internal chain and amount of fingerprint B-chains. Oca starch granules had a volume moment mean size of 34.5 μm and B-type polymorph [65] |
2.1.6. Plant Polymer-Based Egg Protein Replacers
2.2. Plant Polymer-Based Foams as Food Packaging Materials
2.2.1. Starch
2.2.2. Cellulose
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Svagan, A.J.; Samir, M.A.S.A.; Berglund, L.A. Biomimetic Foams of High Mechanical Performance Based on Nanostructured Cell Walls Reinforced by Native Cellulose Nanofibrils. Adv. Mater. 2008, 20, 1263–1269. [Google Scholar] [CrossRef]
- Wu, Q.; Lindh, V.H.; Johansson, E.; Olsson, R.T.; Hedenqvist, M.S. Freeze-dried wheat gluten biofoams; scaling up with water welding. Ind. Crop. Prod. 2017, 97, 184–190. [Google Scholar] [CrossRef]
- Cvrček, L.; Horáková, M. Chapter 14. Non-thermal plasma technology for polymeric materials. In Non-Thermal Plasma Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–407. [Google Scholar]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and characterization of starch-based composite films reinforced by cellulose nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2019, 3, 27–35. [Google Scholar] [CrossRef]
- Motloung, M.P.; Ojijo, V.; Bandyopadhyay, J.; Ray, S.S. Ray cellulose nanostructure-based biodegradable nanocomposite foams: A brief overview on the recent advancements and perspectives. Polymers 2019, 11, 1270. [Google Scholar] [CrossRef] [Green Version]
- Rydz, J.; Musioł, M.; Zawidlak-Węgrzyńska, B.; Sikorska, W. Present and future of biodegradable polymers for food packaging applications. Biopolym. Food Des. 2018, 431–467. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Basu, A.; Domb, A.J. Biodegradable polymers: Medical applications. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Jin, F.L.; Zhao, M.; Park, M.; Park, S.J. Recent trends of foaming in polymer processing: A review. Polymers 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, T.J. Polymers for food applications: News. In Polymers for Food Applications; Springer: Cham, Switzerland, 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Saiz-Arroyo, C.; Rodríguez-Pérez, M.; Velasco, J.I.; de Saja, J.A. Influence of foaming process on the structure–properties relationship of foamed LDPE/silica nanocomposites. Compos. Part B Eng. 2013, 48, 40–50. [Google Scholar] [CrossRef]
- Bergeron, V.; Walstra, P. Foams (chapter 7). In Fundamentals of Interface and Colloid Science, Volume V Soft Colloids; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; Available online: https://www.sciencedirect.com/bookseries/fundamentals-of-interface-and-colloid-science/vol/5/suppl/C (accessed on 28 September 2021).
- Kumar, S.; Singh, P.; Gupta, S.K.; Ali, J.; Baboota, S. Biodegradable and recyclable packaging materials: A step towards a greener future. In Reference Module in Materials Science and Materials Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 328–337. [Google Scholar]
- Nesic, A.; Castillo, C.; Castaño, P.; Cabrera-Barjas, G.; Serrano, J. Bio-based packaging materials. In Biobased Products and Industries; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–309. [Google Scholar]
- Dollet, B.; Raufaste, C. Comptes rendus physique rheology of aqueous foams rhéologie des mousses aqueuses. C. R. Phys. 2014, 15, 731–747. [Google Scholar] [CrossRef] [Green Version]
- Nastaj, M.; Sołowiej, B.G. The effect of various pH values on foaming properties of whey protein preparations. Int. J. Dairy Technol. 2020, 73, 683–694. [Google Scholar] [CrossRef]
- Alavi, F.; Tian, Z.; Chen, L.; Emam-Djomeh, Z. Effect of CaCl2 on the stability and rheological properties of foams and high-sugar aerated systems produced by preheated egg white protein. Food Hydrocoll. 2020, 106, 105887. [Google Scholar] [CrossRef]
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Góral, I.; Wojciechowski, K. Surface activity and foaming properties of saponin-rich plants extracts. Adv. Colloid Interface Sci. 2020, 279, 102145. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.J.; Sörensen, P.M. Characterization of saponin foam from Saponaria officinalis for food applications. Food Hydrocoll. 2019, 101, 105541. [Google Scholar] [CrossRef]
- Cam, I.B.; Topuz, A. Production of soapwort concentrate and soapwort powder and their use in Turkish delight and tahini halvah. J. Food Process Eng. 2018, 41, e12605. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Damgaard, H.; Greve-Poulsen, M.; Larsen, L.B.; Hammershøj, M. Foam and emulsion properties of potato protein isolate and purified fractions. Food Hydrocoll. 2018, 74, 367–378. [Google Scholar] [CrossRef]
- Ozcelik, M.; Ambros, S.; Morais, S.F.; Kulozik, U. Storage stability of dried raspberry foam as a snack product: Effect of foam structure and microwave-assisted freeze drying on the stability of plant bioactives and ascorbic acid. J. Food Eng. 2019, 270, 109779. [Google Scholar] [CrossRef]
- He, Z.; Li, W.; Guo, F.; Li, W.; Zeng, M.; Chen, J. Foaming characteristics of commercial soy protein isolate as influenced by heat-induced aggregation. Int. J. Food Prop. 2014, 18, 1817–1828. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhou, J.; Chen, J.; Li, B.; Li, Y.; Liu, S. Edible foam based on pickering effect of bacterial cellulose nanofibrils and soy protein isolates featuring interfacial network stabilization. Food Hydrocoll. 2019, 100, 105440. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Y.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Impact of pH on the interaction between soybean protein isolate and oxidized bacterial cellulose at oil-water interface: Dilatational rheological and emulsifying properties. Food Hydrocoll. 2021, 115, 106609. [Google Scholar] [CrossRef]
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Soponronnarit, S. Effects of foaming agents and foam density on drying characteristics and textural property of banana foams. LWT 2012, 47, 348–357. [Google Scholar] [CrossRef]
- Rajkumar, P.; Kailappan, R.; Viswanathan, R.; Raghavan, G.S.V.; Ratti, C. Foam mat drying of alphonso mango pulp. Dry. Technol. 2007, 25, 357–365. [Google Scholar] [CrossRef]
- Zheng, X.-Z.; Liu, C.-H.; Zhou, H. Optimization of Parameters for Microwave-Assisted Foam Mat Drying of Blackcurrant Pulp. Dry. Technol. 2011, 29, 230–238. [Google Scholar] [CrossRef]
- Ceresino, E.B.; Johansson, E.; Sato, H.H.; Plivelic, T.S.; Hall, S.A.; Kuktaite, R. Morphological and structural heterogeneity of solid gliadin food foams modified with transglutaminase and food grade dispersants. Food Hydrocoll. 2020, 108, 105995. [Google Scholar] [CrossRef]
- Ceresino, E.; Johansson, E.; Sato, H.; Plivelic, T.; Hall, S.; Bez, J.; Kuktaite, R. Lupin protein isolate structure diversity in frozen-cast foams: Effects of transglutaminases and edible fats. Molecules 2021, 26, 1717. [Google Scholar] [CrossRef] [PubMed]
- Philipp, C.; Oey, I.; Silcock, P.; Beck, S.M.; Buckow, R. Impact of protein content on physical and microstructural properties of extruded rice starch-pea protein snacks. J. Food Eng. 2017, 212, 165–173. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Larsson, E.; Filli, K.B.; Loupiac, C.; Assifaoui, A.; López-Rubio, A.; Lopez-Sanchez, P. Nano-/microstructure of extruded Spirulina/starch foams in relation to their textural properties. Food Hydrocoll. 2020, 103, 105697. [Google Scholar] [CrossRef]
- Mitrus, M.; Moscicki, L. Extrusion-cooking of starch protective loose-fill foams. Chem. Eng. Res. Des. 2014, 92, 778–783. [Google Scholar] [CrossRef]
- Savage, G.P. SAPONINS. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5095–5098. [Google Scholar]
- Çelik, I.; Yılmaz, Y.; Işık, F.; Üstün, O. Effect of soapwort extract on physical and sensory properties of sponge cakes and rheological properties of sponge cake batters. Food Chem. 2007, 101, 907–911. [Google Scholar] [CrossRef]
- Alting, A.C.; Pouvreau, L.; Giuseppin, M.L.F.; van Nieuwenhuijzen, N.H. Potato proteins. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 316–334. [Google Scholar]
- Silva, S.S.; Fernandes, E.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.; Reis, R. 2.11 Polymers of biological origin. Compr. Biomater. 2017, 2, 228–252. [Google Scholar]
- Sun, X.S. Thermal and mechanical properties of soy proteins. In Bio-Based Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2005; pp. 292–326. [Google Scholar]
- Lin, M.; Tay, S.H.; Yang, H.; Yang, B.; Li, H. Replacement of eggs with soybean protein isolates and polysaccharides to prepare yellow cakes suitable for vegetarians. Food Chem. 2017, 229, 663–673. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Wong, L.; Wismer, W.; Temelli, F.; Han, J.; Huang, W.; Eckhart, E.; Tian, Z.; Shi, K.; Sun, T.; et al. Quality characteristics of angel food cake and muffin using lentil protein as egg/milk replacer. Int. J. Food Sci. Technol. 2017, 52, 1604–1613. [Google Scholar] [CrossRef]
- Jarpa-Parra, M. Lentil protein: A review of functional properties and food application. An overview of lentil protein functionality. Int. J. Food Sci. Technol. 2018, 53, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Bergel, B.F.; Osorio, S.D.; da Luz, L.M.; Santana, R.M.C. Effects of hydrophobized starches on thermoplastic starch foams made from potato starch. Carbohydr. Polym. 2018, 200, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Semeijn, C.; Buwalda, P.L. Potato Starch; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bergel, B.F.; Araujo, L.L.; Silva, A.L.D.S.D.; Santana, R.M.C. Effects of silylated starch structure on hydrophobization and mechanical properties of thermoplastic starch foams made from potato starch. Carbohydr. Polym. 2020, 241, 116274. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Tirado, J.P.; Ferreira, R.S.B.; Lizárraga, E.; Tapia-Blacido, D.R.; Silva, N.C.C.; Angelats-Silva, L.; Siche, R. Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag. Shelf Life 2020, 23, 100457. [Google Scholar] [CrossRef]
- Cinelli, P.; Chiellini, E.; Lawton, J.W.; Imam, S.H. Foamed articles based on potato starch, corn fibers and poly (vinyl alcohol). Polym. Degrad. Stab. 2006, 91, 1147–1155. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E.; Shen, X. Carbohydrates of the Kernel, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Loy, D.D.; Lundy, E.L. Nutritional Properties and Feeding Value of Corn and Its Coproducts, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Uslu, M.K.; Polat, S. Effects of glyoxal cross-linking on baked starch foam. Carbohydr. Polym. 2012, 87, 1994–1999. [Google Scholar] [CrossRef]
- Polat, S.; Uslu, M.K.; Aygün, A.; Certel, M. The effects of the addition of corn husk fibre, kaolin and beeswax on cross-linked corn starch foam. J. Food Eng. 2013, 116, 267–276. [Google Scholar] [CrossRef]
- Sanhawong, W.; Banhalee, P.; Boonsang, S.; Kaewpirom, S. Effect of concentrated natural rubber latex on the properties and degradation behavior of cotton-fiber-reinforced cassava starch biofoam. Ind. Crop. Prod. 2017, 108, 756–766. [Google Scholar] [CrossRef]
- Wheatley, C.C.; Chuzel, G.; Zakhia, N. CASSAVA|The Nature of the Tuber. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2003; pp. 964–969. [Google Scholar]
- Salgado, P.R.; Schmidt, V.C.; Ortiz, S.E.M.; Mauri, A.N.; Laurindo, J.B. Biodegradable foams based on cassava starch, sunflower proteins and cellulose fibers obtained by a baking process. J. Food Eng. 2008, 85, 435–443. [Google Scholar] [CrossRef]
- Mello, L.R.; Mali, S. Use of malt bagasse to produce biodegradable baked foams made from cassava starch. Ind. Crop. Prod. 2014, 55, 187–193. [Google Scholar] [CrossRef]
- Machado, C.M.; Benelli, P.; Tessaro, I.C. Sesame cake incorporation on cassava starch foams for packaging use. Ind. Crop. Prod. 2017, 102, 115–121. [Google Scholar] [CrossRef]
- Engel, J.B.; Ambrosi, A.; Tessaro, I.C. Development of biodegradable starch-based foams incorporated with grape stalks for food packaging. Carbohydr. Polym. 2019, 225, 115234. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, A.; Nuñez, J.; Tirado, L.J.P.C.; Vejarano, R.; Tapia-Blácido, D.R.; Arteaga, H.; Siche, R. Pineapple shell fiber as reinforcement in cassava starch foam trays. Polym. Polym. Compos. 2019, 27, 496–506. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Molina, G.; Pelissari, F. Biodegradable trays based on cassava starch blended with agroindustrial residues. Compos. Part B Eng. 2020, 183. [Google Scholar] [CrossRef]
- Matsuda, D.K.; Verceheze, A.E.; Carvalho, G.M.; Yamashita, F.; Mali, S. Baked foams of cassava starch and organically modified nanoclays. Ind. Crop. Prod. 2012, 44, 705–711. [Google Scholar] [CrossRef]
- Vercelheze, A.E.; Fakhouri, F.M.; Dall’Antônia, L.H.; Urbano, A.; Youssef, E.Y.; Yamashita, F.; Mali, S. Properties of baked foams based on cassava starch, sugarcane bagasse fibers and montmorillonite. Carbohydr. Polym. 2012, 87, 1302–1310. [Google Scholar] [CrossRef]
- Pornsuksomboon, K.; Holló, B.B.; Szécsényi, K.M.; Kaewtatip, K. Properties of baked foams from citric acid modified cassava starch and native cassava starch blends. Carbohydr. Polym. 2016, 136, 107–112. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Siche, R.; Cabanillas, A.; Díaz-Sánchez, L.; Vejarano, R.; Tapia-Blácido, D.R. Properties of baked foams from oca (Oxalis tuberosa) starch reinforced with sugarcane bagasse and asparagus peel fiber. Procedia Eng. 2017, 200, 178–185. [Google Scholar] [CrossRef]
- Zhu, F.; Cui, R. Comparison of molecular structure of oca (Oxalis tuberosa), potato, and maize starches. Food Chem. 2019, 296, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Ramos Diaz, J.M.; Kirjoranta, S.; Tenitz, S.; Penttilä, P.A.; Serimaa, R.; Lampi, A.M.; Jouppila, K. Use of amaranth, quinoa and kañiwa in extruded corn-based snacks. J. Cereal Sci. 2013, 58, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Anton, A.A.; Gary Fulcher, R.R.; Arntfield, S.D. Physical and nutritional impact of fortification of corn starch-based extruded snacks with common bean (Phaseolus vulgaris L.) flour: Effects of bean addition and extrusion cooking. Food Chem. 2009, 113, 989–996. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Zhang, B.; Drago, S.R.; Zhang, J. Relationships between the gelatinization of starches and the textural properties of extruded texturized soybean protein-starch systems. J. Food Eng. 2016, 174, 29–36. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.G.; Terpiłowski, K.; Mleko, S. Effect of erythritol on physicochemical properties of reformulated high protein meringues obtained from whey protein isolate. Int. Dairy J. 2020, 105, 104672. [Google Scholar] [CrossRef]
- Nastaj, M.; Mleko, S.; Terpiłowski, K.; Tomczyńska-Mleko, M. Effect of sucrose on physicochemical properties of high-protein meringues obtained from whey protein isolate. Appl. Sci. 2021, 11, 4764. [Google Scholar] [CrossRef]
- Emblem, A. Plastics properties for packaging materials. In Packaging Technology: Fundamentals, Materials and Processes; Elsevier: Amsterdam, The Netherlands, 2012; pp. 287–309. [Google Scholar]
- Ago, M.; Ferrer, A.; Rojas, O.J. Starch-based biofoams reinforced with lignocellulose nanofibrils from residual palm empty fruit bunches: Water sorption and mechanical strength. ACS Sustain. Chem. Eng. 2016, 4, 5546–5552. [Google Scholar] [CrossRef]
- Löbmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef]
- Cervin, N.T.; Andersson, L.; Ng, J.B.S.; Olin, P.; Bergström, L.; Wågberg, L. Lightweight and strong cellulose materials made from aqueous foams stabilized by nanofibrillated cellulose. Biomacromolecules 2013, 14, 503–511. [Google Scholar] [CrossRef]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Thermoplastic starch foamed composites reinforced with cellulose nanofibers: Thermal and mechanical properties. Carbohydr. Polym. 2018, 197, 305–311. [Google Scholar] [CrossRef]
- Hassan, M.; Tucker, N.; Le Guen, M. Thermal, mechanical and viscoelastic properties of citric acid-crosslinked starch/cellulose composite foams. Carbohydr. Polym. 2019, 230, 115675. [Google Scholar] [CrossRef]
- Luo, X.; Mohanty, A.; Misra, M. Lignin as a reactive reinforcing filler for water-blown rigid biofoam composites from soy oil-based polyurethane. Ind. Crop. Prod. 2013, 47, 13–19. [Google Scholar] [CrossRef]
- Silva, M.C.; Takahashi, J.A.; Chaussy, D.; Belgacem, M.N.; Silva, G.G. Composites of rigid polyurethane foam and cellulose fiber residue. J. Appl. Polym. Sci. 2010, 117, 3665–3672. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarpa-Parra, M.; Chen, L. Applications of Plant Polymer-Based Solid Foams: Current Trends in the Food Industry. Appl. Sci. 2021, 11, 9605. https://doi.org/10.3390/app11209605
Jarpa-Parra M, Chen L. Applications of Plant Polymer-Based Solid Foams: Current Trends in the Food Industry. Applied Sciences. 2021; 11(20):9605. https://doi.org/10.3390/app11209605
Chicago/Turabian StyleJarpa-Parra, Marcela, and Lingyun Chen. 2021. "Applications of Plant Polymer-Based Solid Foams: Current Trends in the Food Industry" Applied Sciences 11, no. 20: 9605. https://doi.org/10.3390/app11209605
APA StyleJarpa-Parra, M., & Chen, L. (2021). Applications of Plant Polymer-Based Solid Foams: Current Trends in the Food Industry. Applied Sciences, 11(20), 9605. https://doi.org/10.3390/app11209605