Abstract
In recent years, hemp oils have become ubiquitous in health products on the European market. As the trend continues to grow and more cannabinoids are researched for their therapeutic benefits, more academic and industrial interests are drawn to this direction. Cannabidiol, Δ9-tetrahydrocannabinol, and their acidic forms remain the most examined cannabinoids in hemp and cannabis oils, in the case of cannabidiol due to its proven health implications in numerous articles, and in the case of Δ9-tetrahydrocannabinol, due to the legislation in the European area. These oils sold on the internet contain a wide range of cannabinoids that could demonstrate their effects and benefits. As a result of these claims, we developed a robust and rapid method that can identify and quantify 10 of the most common cannabinoids found in hemp oils: cannabivarin, cannabidiolic acid, cannabigerolic acid, cannabigerol, cannabidiol, cannabinol, Δ9-tetrahydrocannabinol, Δ8-tetrahydrocannabinol, cannabichromene, and tetrahydrocannabinolic acid in less than 11 min, with reverse-phase–high-performance liquid chromatography–photodiode matrix system (RP–UHPLC–PDA) equipped with C18 column, eluting in a gradient using water and acetonitrile with formic acid as mobile phases. The quantification of 9 sample products presented in different matrixes was performed using a calibration curve obtained by analyzing standard solutions from a 10-cannabinoid-mix-certified reference standard. The developed method demonstrated the ability to identify and quantify the main cannabinoids in hemp oil and is a useful tool for pharmaceutical professionals.
1. Introduction
Cannabis sativa L. (hemp), is an annual herbaceous plant belonging to the Cannabaceae family and has been used for ages to produce hemp fiber (for clothing, rope, and paper), seeds and also as a medicinal plant [1,2]. Cannabis sativa L. has also been used for recreational and medical purposes [3,4]. Cannabis has a complex chemical composition, with approximately 540 metabolites reported, such as (phyto) cannabinoids, terpenoids, flavonoids, and secondary metabolites used to treat epilepsy, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, pain and nausea in cancer patients, diabetes, and eating disorders [5,6]. The most active of these are cannabinoids, a class represented by over 150 terpenophenolic compounds that accumulate mainly in the resin secreted from the trichomes of female plants [3,6,7]. The therapeutic properties of cannabis are attributed to cannabinoids [8]. According to its use, cannabis is divided into two distinct groups: marijuana (medicinal and recreational) and industrial hemp. Hemp serves more as an agricultural commodity, being appreciated for its fibers and seeds and, recently, for the properties of cannabidiol (CBD) [9,10]. The two major neuroactive components in Cannabis plants are the main psychoactive cannabinoid, Δ9-tetrahydrocannabinol (THC), and the non-psychoactive cannabinoid, CBD [11]. THC and CBD are neutral homologues of tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), respectively [12]. A classification of cannabinoids can be made due to their chemical structure by dividing them into 11 groups, including cannabigerol (CBG), THC, cannabichromene CBD, cannabichromene (CBC), cannabinol (CBN), Δ8-tetrahydrocannabinol (Δ8-THC), cannabicyclol (CBL), cannabinodiol (CBND), cannabielsoin (CBE), cannabitriol (CBT), and miscellaneous types [13]. CBD products have grown in popularity due to their low THC content, as well as due to the medical benefits attributed to CBD [14]. As a result, a multitude of products are marketed as supplements, improved formulas with CBD, the most marketed being CBD oils [14]. As CBD oil consumers have limited means to analyze their chemical composition, they may accidentally purchase products with undesirable properties given the different effects of cannabinoids [15]. As a result, it is important to implement quality control methods so that consumers are confident that CBD products have the desired effects [5,16,17]. The legal status of Cannabis compounds is different from one country to another. There are countries in which THC and CBD are classified in the same class of prohibited substances, while in other countries CBD products are legal [1,18]. For this reason, it is becoming increasingly important to have methods for quantifying the profile and cannabinoid content of CBD oils to ensure product uniformity and quality [19,20]. Cannabinoids may be detected by many and different analytical methods, including immunoassays (EMIT®, Elisa, fluorescent polarization, radioimmunotest). Are used techniques of flat chromatography; classic thin layer chromatography (TLC) [20], optimum performance laminar chromatography (OPLC) and multiple development automatization (AMD), gas chromatography–mass spectrometry (GC–MS) [21,22,23], high-performance liquid chromatography–mass spectrometry (HPLC–MS) [20,24,25,26,27]. TLC is useful in the laboratory for rapid screening of cannabinoid content in a sample [20]. Various analysis techniques are used in conjunction with HPLC to detect cannabinoids [20,25,27,28,29,30,31]. Methods include MS and ultraviolet (UV) [19,20,24,25,26,27,31]. UV detection is less expensive and simpler than detecting MS [31]; therefore, it is used for the quantification of major cannabinoids, and MS is used for the quantification of minor cannabinoids. In general LC–MS/MS techniques are considered more sensitive and selective analysis procedures than UHPLC analysis [20,27], but the UHPLC analysis is a more economical and accessible procedure for quantifying 10 cannabinoids from hemp oil [27,32]. Differences in cannabinoid concentrations justify the need to provide stronger regulation and control over the composition of hemp oil and CBD oil [20,28]. Individual doses throughout administration should be adjusted for CBD bioavailability [33]. This is of fundamental importance for the safety of consumers, as hemp oil preparations are also used for therapeutic purposes, regardless of whether they are registered as food supplements [20,28,34,35]. In addition to the studied methods, it is necessary to develop new procedures in the analysis of hemp oils for the identification and quantification of the 10 most relevant cannabinoids, which can be used in the pharmaceutical field. This study describes the development of the method and validation, the separation and detection of the main cannabinoids in hemp oil by reverse-phase ultra-high-performance liquid chromatography using the photodiode matrix detector (RP–UHPLC–PDA) with a single wavelength.
2. Materials and Methods
2.1. Chemicals and Materials
Analytical and chromatographic grade chemicals and solvents that were used for validation and analysis include methanol, purchased from VWR Chemicals (Radnor, PA, USA), acetonitrile from Merk (Darmstadt, Germany), and formic acid, purchased from Fisher Chemicals (Pittsburgh, UK). A mix standard of 10 phytocannabinoids in acetonitrile was provided at a concentration of 250 µg/mL for each component consisting of CBG, Δ9-THC, CBD, CBC, CBN, Δ8-THC, CBL, CBND, CBE, CBT and was obtained from Cayman Chemical (Ann Arbor, MI, USA). Ultrapure water was supplied by a Mili-Q water purification system from Millipore (Bedford, MA, USA).
2.2. Instrumentation
PerkinElmer Flexar FX-15 UHPLC system was used, equipped with a photodiode array detector (PDA), quaternary pump, column oven, and autosampler. Chromatographic separation was performed using a PerkinElmer Brownlee™ SPP C18, 2.7 µm, 3.0 mm × 150 mm column (PerkinElmer, Shelton, CT, USA). All data analysis, peak purity, and processing were performed using the PerkinElmer Chromera® CDS software.
2.3. Chromatographic Condition
The analysis was performed using as mobile phases 100% ultrapure water + 0.1% formic acid (solvent A) and 100% acetonitrile + 0.1% formic acid (solvent B) in a gradient elution mode, starting from 33% A and 67% B, increasing to 95% B in 5.5 min and maintaining at 95% B for 2 min. Equilibration time was 4.5 min at 33% A and 67% B before each injection, 10 µL sample volume + 5 µL air volume was injected in partial-loop mode. The flow rate was set at 1 mL/min, the temperature in the samples’ compartment was 5 °C and 40 °C in the column oven. The acquisition was made at a wavelength of 228 nm.
2.4. Standard and Quality Control (QS) Solutions Preparation
A working stock solution of 50 µg/mL (also the first point on the calibration curve) was prepared by diluting 200 µL of the 10 phytocannabinoids standard mix with 800 µL acetonitrile. From this solution, serial dilutions were made at 25, 10, 5, and 2.5 µg/mL. The stock solution was also diluted to 16.7 µg/mL with acetonitrile, representing de QC solution.
2.5. Test Materials and Sample Preparation
Test materials consisting of 9 CBD (S1–S9) oils were obtained from reputable online retailers in order to validate the method on the most common matrixes used in the manufacturing process. Information about the composition and method employed in oil production was taken from the product label, and the resulting data were as follows: S1, S2, S5, and S6 were hemp extracts (HE) + hemp seed oil (HSO); S3 was produced by infusion under pressure in extra virgin hemp oil (INF + HO); S4 was produced by mixing HSO + CBD and terpenes (T); S7 by extracting with supercritical fluid CO2 (SCO2); S8 was CBD isolate + HSO and S9 is CBD isolate + medium-chain triglyceride oil (MCT). The subsequent sample preparation was carried out as follows: 1000 mg of vegetal oil was accurately weighted on a calibrated semi-micro balance in a 15 mL centrifuge tube. The extraction was conducted by adding 10 mL of methanol over the sample and vortexing at high speed for 3 min. A full 2 mL syringe was collected from the extract and filtered through a 0.45 µm nylon filter into a glass tube, and 1 mL was transferred in a new tube and diluted with 2 mL of methanol; 1 mL was then transferred to a 10 mL volumetric flask, which was brought to volume with methanol (300-fold dilution); the last step was repeated for 3000-fold dilution.
2.6. Method Validation Parameters
The method was validated for specificity, precision, accuracy, linearity, the limit of detection (LOD), and limit of quantification (LOQ), according to the International Conference on Harmonization (ICH) guidelines (ICH Q2A 1994; ICH Q2B 1996) to assure the reliability of the results.
2.6.1. Specificity
The specificity was determined by matching the acquired spectra and elution order of the compounds from the reference solution with those from the sample solution considering the available standard chromatogram and data from literature [36]; for this purpose, 10 µL from blank, reference, and sample solutions were injected into the system. In order to asset the matrix effect and interferences from other coeluting analytes, peak purity analysis was performed.
2.6.2. Precision
The system precision or the agreement between the area values for each analyte was evaluated as repeatability at the same concentration level following 6 successive injections from individual 2.5 µg/mL reference solutions. It was expressed as relative standard deviation (RSD%) with an acceptance criterion of ≤5% RSD.
2.6.3. Accuracy
The accuracy, as the measurement of the closeness of experimental value to the actual amount, was established by injecting 3 standard solutions of known concentration (16.7 µg/mL) and reporting the practical concentration determined by the system to the theoretical one. The accuracy was expressed as percent recovery, where 100 ± 10% was considered acceptable.
2.6.4. Linearity
Linearity, defined as the ability of the method to obtain test results that are directly proportional to the analyte concentration within a specific range [37], was assessed in the range of 2.5–50 µg/mL (0.75–15 mg/g from 3000-fold dilution, or 7.5–150 mg/g for 300-fold dilution, in sample units) from three injections for each point on the curve using the least-squares method and by calculating the coefficient of determination R2. An R2 coefficient higher than 0.99 is considered an acceptable criterion of linearity.
2.6.5. Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD, described as the smallest concentrations of an analyte that can be reliably distinguished from zero, and LOQ, as the lowest concentration of the analyte that can be determined with acceptable repeatability and trueness [38], were estimated using the standard deviations of y-intercepts of the regression line (σ) and calibration curve slope (S) based on the following formulas:
LOD = 3.3 σ/S
LOQ = 10 σ/S
3. Results
3.1. Specificity
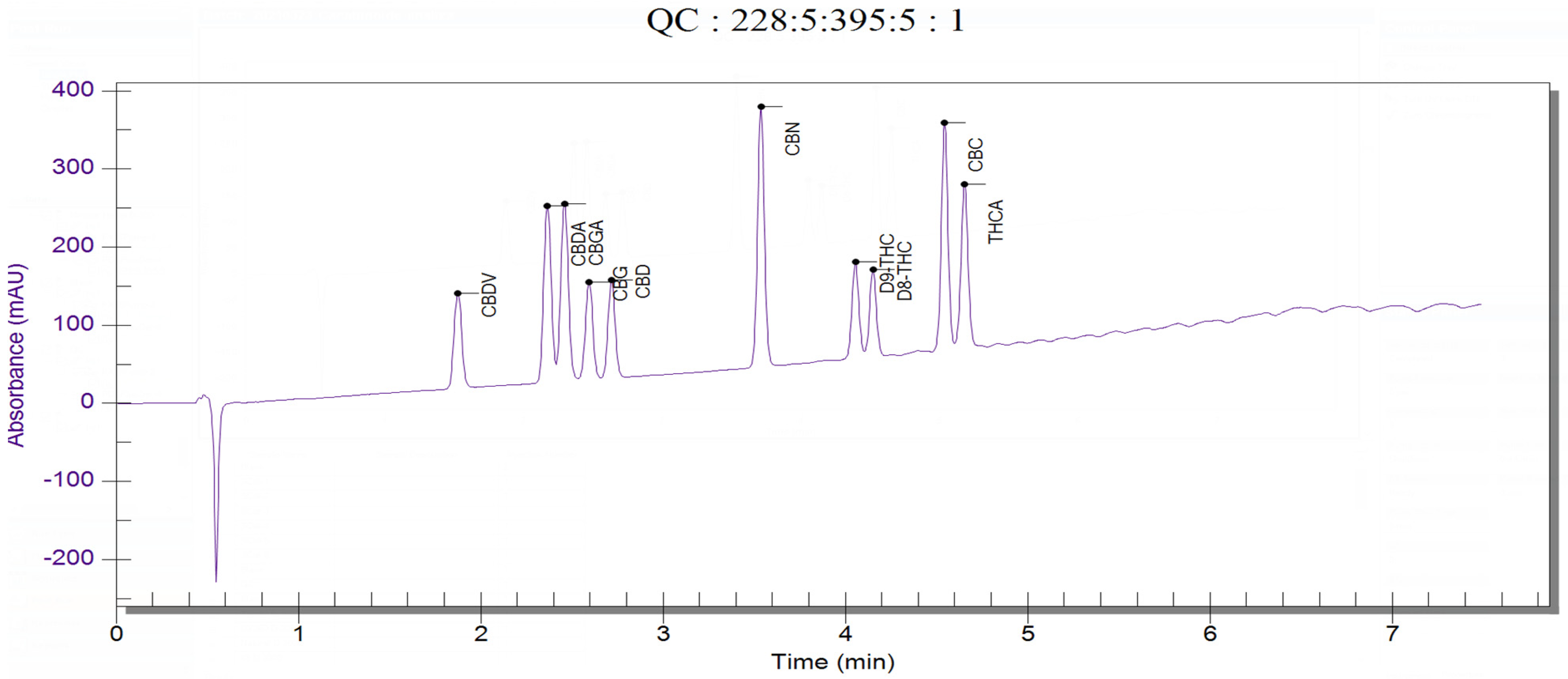
Spectra analysis and comparison confirmed the identity of each analyte in their eluting order on the reported method. As cannabis and hemp oil matrices contain a wide range of compounds that are extracted in the manufacturing process, good discrimination between matrix compounds and the target analytes was obtained with a suitable resolution that allows proper integration and quantification of each peak of interest, as shown in Figure 1. Retention times used for the identification of the compounds in the samples are displayed in Table 1.
Figure 1.
Chromatogram of 10 cannabinoids standard mix (16.7 µg/mL).

Table 1.
Retention times (Rt) of cannabinoids identified in the standard mix.
At least 113 cannabinoids have been identified in cannabis and hemp plants. Due to the high probability of being coextracted and because of the close homology between the cannabinoid species, the risk of interferences and peak overlapping is increased. As shown in the table below, Table 2, the purity index provided for each sample shows that the method developed is capable of discriminating the target analytes from the sample matrix.

Table 2.
Peak purity indexes of each peak from sample solutions (300 fold).
High values for CBC in S 2, CBN in S 4, and CBDV in S7 indicate a high probability that matrix-specific interferences are present at those particular retention times.
3.2. Precision
Precision at the lowest point on the calibration curve (2.5 µg/mL) was evaluated based on reported concentration RSD%. The method was found to be reproducible, with an RSD value of 1.54% for CBDV; 0.45% for CBDA; 0.72% for CBGA; 1.88% for CBG; 1.63% for CBD%; 1.14% for CBN; 3.77% for Δ9-THC; 4.25% for Δ8-THC; 3.38% for CBC; 2.79% for THCA. These results indicate that the method is precise at the evaluated concentration.
3.3. Accuracy
To assess how close the values measured are from the true concentrations, accuracy at 16.7 µg/mL was calculated as percent recovery. The results indicated in Table 3 demonstrate a good accuracy of the proposed method at the evaluated concentration.

Table 3.
Results for accuracy.
3.4. Linearity
The calibration curves were linear within the concentration range of 2.5–50 µg/mL (0.75–15 mg/g from 3000-fold dilution, or 7.5–150 mg/g for 300-fold dilution, in sample units) for each analyzed compound. The calibration curve exhibited a good linear regression, and a value higher than 0.999 for R2 (coefficient of determination) was obtained for all cannabinoids, as shown in Table 4.

Table 4.
Results for linearity, regression equation, and R2 for each analyte.
Given the variable range of concentrations at which cannabinoids from hemp and cannabis oils are found at different manufacturers, a two-dilution system was implemented that allows all the compounds analyzed to be included in the calibration curve domain thus validated.
3.5. Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD and LOQ were estimated from the data acquired for linearity. It was found that the main cannabinoids of interest can be detected at a concentration of 0.22 mg/g for ∆9-THC (0.022%) and 0.19 mg/g for CBD (0.019%) and can easy be quantified at 0.68 mg/g for ∆9-THC (0.068%) and 0.58 mg/g (0.058%) for CBD. The proposed method provides sufficient sensibility for ∆9-THC, around 10 times lower than the legal limit imposed in some countries (0.2%). Higher limits for CBN are probably due to different absorption at 228 nm. Limits for other cannabinoids that can be analyzed with this method are displayed in Table 5.

Table 5.
Limit of detection (LOD) and limit of quantification (LOQ), determined by the calibration curves in standard units (µg/mL) and sample units (mg/g).
3.6. Cannabinoids in Hemp Oil
The method developed in this study was applied to the qualitative–quantitative analysis of the main cannabinoids from four samples of hemp oil. The amount of each cannabinoid was calculated using the equation obtained from a freshly prepared calibration curve. The following sequence was injected into the chromatographic system:
- 1× blank;
- 1× Calibration solution 2.5 µg/mL;
- 1× QC solution;
- 1× 3000-Fold samples;
- 1× 300-Fold samples;
- 1× QC every 10 samples;
- 1× QC after all samples;
- 1× Blank;
- 1× Wash.
As system suitability criteria, we chose to verify the accuracy of the 2.5 µg/mL calibration solution and QC solution; all the analyzed solutions passed the admissibility criteria (±10%).
Concentration in mg/g was calculated by following formula:
where C (µg/mL) is the concentration extrapolated on the calibration curve, d is dilution factor (300 or 3000), Swg is sample weight in grams, and 1000 represents the transformation factor from µg to mg.
C (mg/g) = C (µg/mL) × d/Swg × 1000
The main components CBD and CBDA were found in all samples, except two for CBDA in a concentration range of 1.42 to 166.32 mg/g for CBD and 1.62 to 18.80 mg/g for CBDA. Other cannabinoids also present in oils were CBDV (2.52 to 14.70 mg/g), CBGA, which was detected in two samples (1.19 to 2.64 mg/g) and as traces in the other two samples, CBG, which was quantified in only one sample (2.13 mg/g) and detected in other three, CBN (4.75 mg/g), which was found in one sample and identified in other three, Δ9-THC (1.06 to 1.35 mg/g), Δ8-THC, which was detected only in one sample in too low amount to be quantified, and CBC, which was present in six samples at a lower concentration, suggesting the need to decrease the dilution fold in order to be quantified and THCA (1.80–2.75 mg/g).
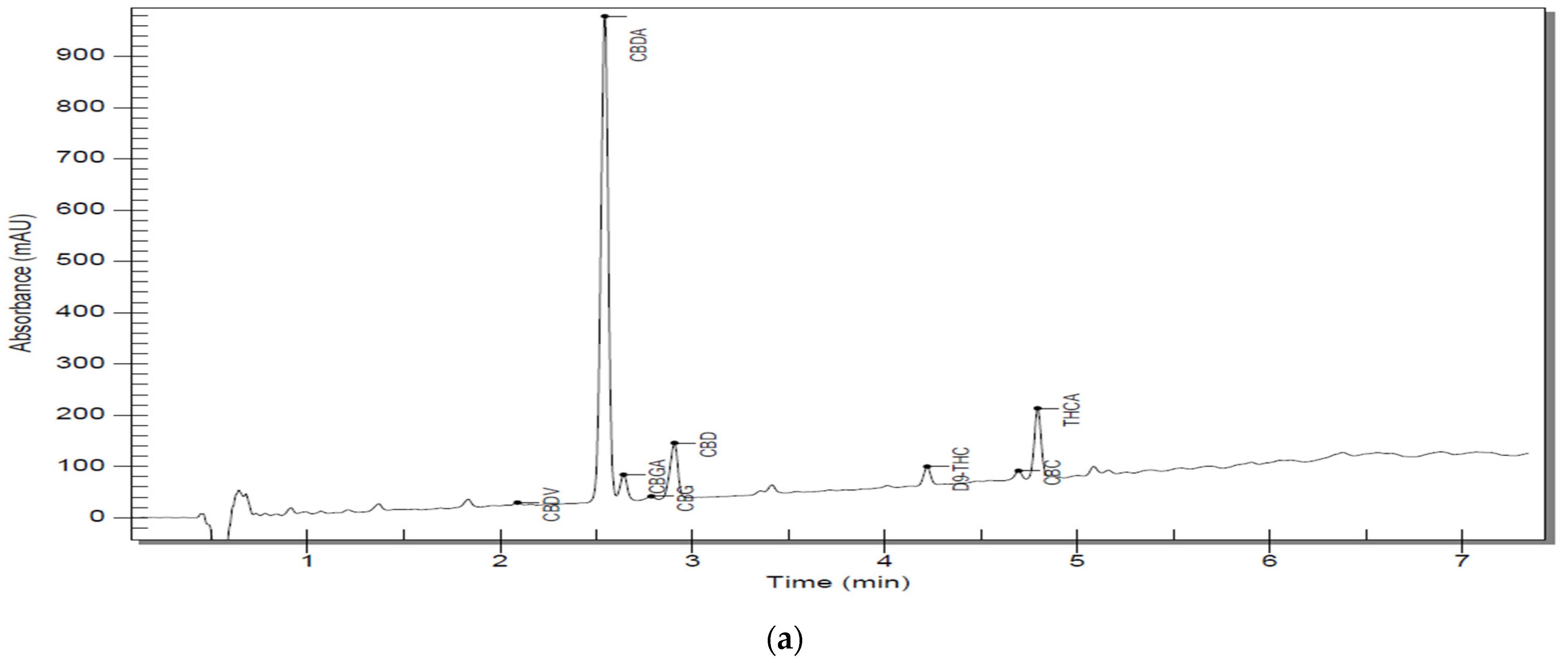
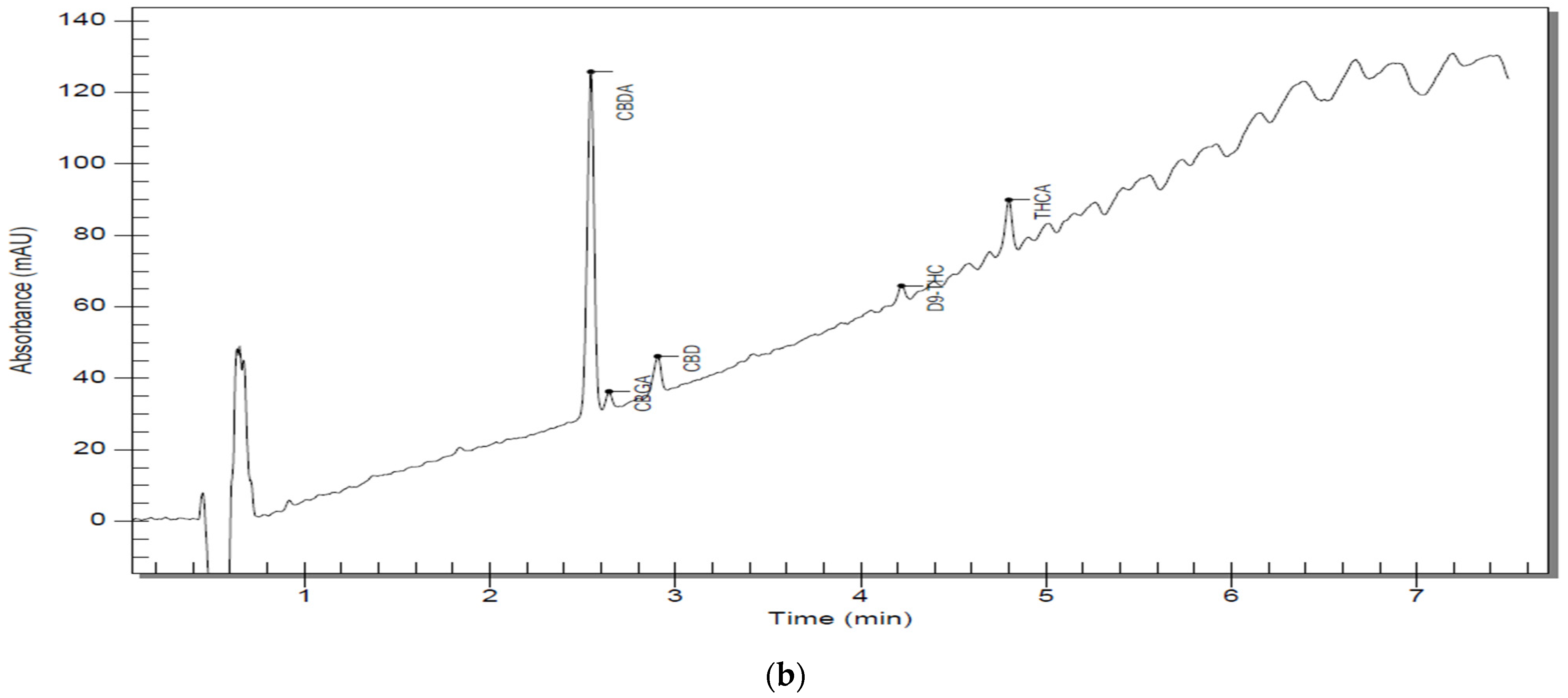
A more detailed view can be found in Table 6. Representative chromatograms of sample solutions S5 are shown in Figure 2a,b, and the rest of the chromatograms can be reviewed in the Supplementary Materials (Figures S1–S9).

Table 6.
Results for hemp oils, analyzed with the proposed method and regression equations used for quantification.
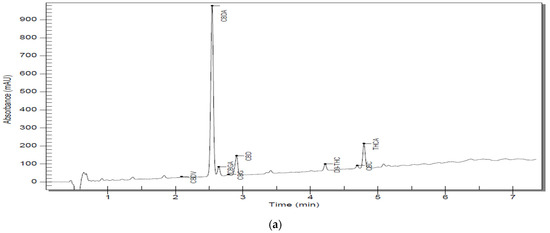
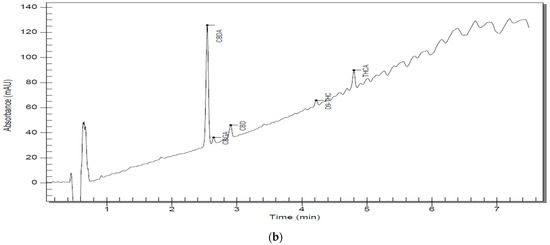
Figure 2.
(a) Chromatographic trace of S5 D300 analyzed by RP–HPLC–PDA; (b) chromatographic trace of an S5 D3000 analyzed by RP–HPLC–PD.
4. Conclusions
The developed assay was validated by evaluating specificity, linearity, LOD, LOQ, accuracy, and precision and it was successfully applied to the analysis of hemp oils. The described method focuses on the quantification of major cannabinoids present in oils but can also be applied to check THC and other cannabinoids at levels >0.075% in order to assess the compliance with each country’s own jurisdiction. Phytocannabinoids screening offers a unique view about the potency and health effects of hemp and cannabis oils that implies a synergic action of many compounds at various concentrations.
High throughput of samples can be analyzed in a working day, leading to economies of scale in regard to standards, solvents, energy, and analyst’s time. Additionally, even if not all HPLC systems have the same performance, the proposed method can be operated on a wide variety of high-pressure liquids chromatographs due to the acceptable pressure generated by the system, solvents column, and temperature (around 5100 psi or 350 bar for our system). The two dilutions of 300-fold and 3000-fold allow a wide range of unknown samples to be analyzed within the method concentration range. If the proposed method is intended to be used for quality control of finished products, the sample dilution can also be optimized for the desired purpose; with regard to the various compositions of the extracts that can bring interferences, simple adjustments can be employed when validating the method for a specific product.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11209414/s1, Figure S1-a: Chromatogram of S1 D300 analyzed by RP-UHPLC-PDA; Figure S1-b: Chromatogram of S1 D3000 analyzed by RP-UHPLC-PDA; Figure S2-a: Chromatogram of S2 D300 analyzed by RP-UHPLC-PDA; Figure S2-b: Chromatogram of S2 D3000 analyzed by RP-UHPLC-PDA; Figure S3-a: Chromatogram of S3 D300 analyzed by RP-UHPLC-PDA; Figure S3-b: Chromatogram of S3 D3000 analyzed by RP-UHPLC-PDA; Figure S4-a Chromatogram of S4 D300 analyzed by RP-UHPLC-PDA; Figure S4-b: Chromatogram of S4 D3000 analyzed by RP-UHPLC-PDA; Figure S5-a: Chromatogram of S5 D300 analyzed by RP-UHPLC-PDA; Figure S5-b: Chromatogram of S5 D3000 analyzed by RP-UHPLC-PDA; Figure S6-a: Chromatogram of S6 D300 analyzed by RP-UHPLC-PDA; Figure S6-b: Chromatogram of S6 D3000 analyzed by RP-UHPLC-PDA; Figure S7-a: Chromatogram of S7 D3000 analyzed by RP-UHPLC-PDA; Figure S7-b; Chromatogram of S7 D300 analyzed by RP-UHPLC-PDA; Figure S8-a: Chromatogram of S8 D300 analyzed by RP-UHPLC-PDA; Figure S8-b: Chromatogram of S8 D3000 analyzed by RP-UHPLC-PDA; Figure S9-a: Chromatogram of S9 D300 analyzed by RP-HPLC-PDA; Figure S9-b: Chromatogram of S9 D3000 analyzed by RP-HPLC-PDA.
Author Contributions
Conceptualization, N.M.B. and D.R.; methodology, N.M.B.; software, T.C.; validation, D.R., T.C. and N.M.B.; formal analysis, D.R.; investigation, S.N.; resources, T.C.; data curation, D.R.; writing—original draft preparation, N.M.B.; writing—review and editing, N.M.B.; visualization, S.N.; supervision, T.C.; project administration, T.C.; funding acquisition, T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was performed in collaboration with the Research Center of Instrumental Analysis SCIENT, 1E Petre Ispirescu Street, 77167 Tancabesti, Ilfov, Romania.
Conflicts of Interest
All the authors declare that there is no conflict of interest with this study.
Abbreviations
| CBDV | Cannabidivarin |
| CBDA | Cannabidiolic acid |
| CBGA | Cannabigerolic acid |
| CBG | Cannabigerol |
| CBD | Cannabidiol |
| CBND | Cannabinodiol |
| CBE | Cannabielsoin |
| CBN | Cannabinol |
| ∆9-THC | Δ9-Tetrahydrocannabinol |
| Δ8-THC | Δ8-Tetrahydrocannabinol |
| CBC | Cannabichromene |
| THCA | Tetrahydrocannabinolic acid |
| CBL | Cannabicyclol |
| CBT | Cannabitriol |
References
- Lafaye, G.; Karila, L.; Blecha, L.; Benyamina, A. Cannabis, cannabinoids, and health. Dialogues Clin. Neurosci. 2017, 19, 309–316. [Google Scholar] [PubMed]
- Vlad, R.A.; Hancu, G.; Ciurba, A.; Antonoaea, P.; Rédai, E.M.; Todoran, N.; Muntean, D.L. Cannabidiol—Therapeutic and legal aspects. Pharmazie 2020, 75, 463–469. [Google Scholar] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 5, 300–315. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant. Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crop. Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Eržen, M.; Košir, I.J.; Ocvirk, M.; Kreft, S.; Čerenak, A. Metabolomic Analysis of Cannabinoid and Essential Oil Profiles in Different Hemp (Cannabis sativa L.) Phenotypes. Plants 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Hazekamp, A.; Choi, Y.H.; Verpoorte, R. Quantitative analysis of cannabinoids from Cannabis sativa using 1H-NMR. Chem. Pharm. Bull. 2004, 52, 718–721. [Google Scholar] [CrossRef] [Green Version]
- Sandler, L.N.; Beckerman, J.L.; Whitford, F.; Gibsonet, K.A. Cannabis as conundrum. Crop. Prot. 2019, 117, 37–44. [Google Scholar] [CrossRef]
- Blebea, N.M.; Bucur, L.A. Pharmacotherapeutic options in neoplastic diseases-part IV. Farmacist.ro 2021, 4, 15–18. [Google Scholar] [CrossRef]
- Ibarra-Lecue, I.; Mollinedo-Gajate, I.; Meana, J.J.; Callado, L.F.; Diez-Alarcia, R.; Uriguen, L. Chronic cannabis promotes pro-hallucinogenic signaling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology 2018, 43, 2028–2035. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [Green Version]
- VanDolah, H.J.; Bauer, B.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [Green Version]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T. Isolation of Delta9-THCA-A from hemp and analytical aspects concerning the determination of Delta9-THC in cannabis products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef]
- Fischedick, J.; Kooy, F.V.D.; Verpoorte, R. Cannabinoid receptor 1 binding activity and quantitative analysis of Cannabis sativa L. smoke and vapor. Chem. Pharm. Bull. 2010, 58, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corroon, J.; Kight, R. Regulatory Status of Cannabidiol in the United States: A Perspective. Cannabis Cannabinoid Res. 2018, 3, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Omar, J.; Navarro, P.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Identification and quantification of cannabinoids in Cannabis sativa L. plants by high performance liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 7549–7560. [Google Scholar] [CrossRef] [PubMed]
- Blebea, N.M.; Negreș, S. Methods for quantification of the main cannabinoids in CBD oil. In Proceedings of the Geolinks International Conference on Environmental Sciences 2021, Online Conference on Environmental Sciences, Burgas, Bulgaria, 17–18 May 2021; pp. 57–64. [Google Scholar]
- Rodrigues, A.; Yegles, M.; Van Elsué, N.; Schneider, S. Determination of cannabinoids in hair of CBD rich extracts consumers using gas chromatography with tandem mass spectrometry (GC/MS-MS). Forensic Sci. Int. 2018, 292, 163–166. [Google Scholar] [CrossRef]
- Cardenia, V.; Gallina Toschi, T.; Scappini, S.; Rubino, R.C.; Rodriguez-Estrada, M.T. Development and validation of a Fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018, 26, 1283–1292. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Foss, F.W.; Schug, K.A. Determination of cannabinoids from a surrogate hops matrix using multiple reaction monitoring gas chromatography with triple quadrupole mass spectrometry. J. Sep. Sci. 2018, 41, 459–468. [Google Scholar] [CrossRef]
- Patel, B.; Wene, D.; Fan, Z.T. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Szijj, J.; Inglott, A.; Azzopardi, L.M. Analytical Techniques Used for Analysis of Cannabinoids. Cannabis Sci. Technol. 2021, 4, 34–46. [Google Scholar]
- Blebea, N.M.; Costache, T.; Negreș, S. The qualitative and quantitative analysis of CBD in hemp oils by UHPLC with PDA and applications. Sci. Pap. Ser. D Anim. Sci. 2019, 62, 138–142. [Google Scholar]
- Raharjo, T.J.; Verpoorte, R. Methods for the analysis of cannabinoids in biological materials: A review. Phytochem. Anal. 2004, 15, 79–94. [Google Scholar] [CrossRef]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm. Biomed. Anal. 2018, 147, 565–579. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. A review of methods for the chemical characterization of cannabis natural products. J. Sep. Sci. 2018, 41, 398–415. [Google Scholar] [CrossRef]
- Mandrioli, M.; Tura, M.; Scotti, S.; Toschi, T.G. Fast Detection of 10 Cannabinoids by RP-HPLC-UV Method in Cannabis sativa L. Molecules 2019, 4, 2113. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazekamp, A. The Trouble with CBD Oil. Med. Cannabis Cannabinoids 2018, 1, 65–72. [Google Scholar] [CrossRef]
- Winkler, P. The analysis of pesticides and cannabinoids in cannabis using LC-MS/MS. In Comprehensive Analytical Chemistry; Ferrer, I., Thurman, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 90, pp. 277–313. [Google Scholar]
- Hazekamp, A.; Peltenburg, A.; Verpoorte, R.; Giroud, C. Chromatographic and Spectroscopic Data of Cannabinoids from Cannabis sativa L. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2361–2382. [Google Scholar] [CrossRef]
- Le, T.H.H.; Phung, T.H.; Le, D.C. Development and Validation of an HPLC Method for Simultaneous Assay of Potassium Guaiacolsulfonate and Sodium Benzoate in Pediatric Oral Powder. J. Anal. Methods Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized Guidelines for single-laboratory validation of method of analyses (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).