Anti-Inflammatory Activity of Cnidoscolus aconitifolius (Mill.) Ethyl Acetate Extract on Croton Oil-Induced Mouse Ear Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extract Preparation
2.3. Phytochemical Analysis
2.3.1. Thin-Layer Chromatography
2.3.2. Total Flavonoid Assay
2.4. Anti-Inflammatory Study
2.4.1. Animals
2.4.2. Croton Oil-Induced Assay
2.5. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Induced Mouse Ear Edema with Croton Oil Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nam, J.S.; Jagga, S.; Sharma, A.R.; Lee, J.H.; Park, J.B.; Jung, J.S.; Lee, S.S. Anti-inflammatory effects of traditional mixed extract of medicinal herbs (MEMH) on monosodium urate crystal-induced gouty arthritis. Chin. J. Nat. Med. 2017, 15, 561–575. [Google Scholar] [CrossRef]
- Bahta, T.; Karim, A.; Periasamy, G.; Gebremedhin, G.; Ur-Rehman, N.; Bitew, H.; Hagazi, K. Analgesic, Anti-inflammatory and In-vitro Hyaluronidase Inhibitory Properties of the Leaf Extract and Solvent Fractions of Otostegia Fruticosa (Forssk.) Schweinf. ex Penzig. Iran J. Pharm. Res. 2020, 19, 218–230. [Google Scholar]
- Alhawarri, M.B.; Dianita, R.; Razak, K.N.A.; Mohamad, S.; Nogawa, T.; Wahab, H.A. Antioxidant, Anti-Inflammatory, and Inhibition of Acetylcholinesterase Potentials of Cassia timoriensis DC. Flowers. Molecules 2021, 26, 2594. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Ross-Ibarra, J. Origen y domesticación de la chaya (Cnidoscolus aconitifolius Mill I. M. Johnst): La espinaca Maya. Mex. Stud. 2003, 19, 287–302. [Google Scholar] [CrossRef]
- Loarca-Pina, G.; Mendoza, S.; Ramos-Gomez, M.; Reynoso, R. Antioxidant, antimutagenic, and antidiabetic activities of edible leaves from Cnidoscolus chayamansa Mc. Vaugh. J. Food Sci. 2010, 75, H68–H72. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gonzalez, M.Z.; Gutierrez-Rebolledo, G.A.; Yepez-Mulia, L.; Rojas-Tome, I.S.; Luna-Herrera, J.; Jimenez-Arellanes, M.A. Antiprotozoal, antimycobacterial, and anti-inflammatory evaluation of Cnidoscolus chayamansa (Mc Vaugh) extract and the isolated compounds. Biomed. Pharmacother. 2017, 89, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Awoyinka, O.A.; Balogun, I.O.; Ogunnowo, A.A. Phytochemical screening andin vitro bioactivity of Cnidoscolus aconitifolius (Euphorbiaceae). J. Med. Plant Res. 2007, 1, 63–65. [Google Scholar]
- Johnston, M.I.M.; Nkeiruka, U.; Nsofor, A.; Anayo, L.; Ekwutosi, T.; Ugochi, J. An Evaluation of the Phytochemical and Nutritional Compositions of Fresh Leaves of Cnidoscolus aconitifolius. Int. J. Res. Stud. Biosci. 2016, 4, 21–28. [Google Scholar]
- Phumsuay, R.; Muangnoi, C.; Dasuni Wasana, P.W.; Hasriadi; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Molecular Insight into the Anti-Inflammatory Effects of the Curcumin Ester Prodrug Curcumin Diglutaric Acid In vitro and In vivo. Int. J. Mol. Sci. 2020, 21, 5700. [Google Scholar] [CrossRef] [PubMed]
- Guoyong, X.S.J.; Hongting, L.; Mingkun, A.; Feng, H.; Yiqun, D.; Wei, T.; Yan, Z.; Yucheng, Z.; Minjian, Q. Chemical constituents and antioxidative, anti-inflammatory and anti-proliferative activities of wild and cultivated Corydalis saxicola. Ind. Crop. Prod. 2021, 169, 113647. [Google Scholar]
- Adeniran, O.; Abimbade, S. Characterization of Compounds from Leaf Extracts of Tree Spinach (Cnidoscolus aconitifolius (Miller) I. M. Johnston. Int. J. Sci. Res. Chem. Eng. 2014, 1, 82–86. [Google Scholar]
- Godínez-Santillán, R.I.; Chávez-Servín, J.L.; García-Gasca, T.; Guzmán-Maldonado, S.H. Phenolic characterization and antioxidant capacity of alcoholic extracts from raw and boiled leaves of Cnidoscolus aconitifolius (Euphorbiaceae). Acta Bot. Mex. 2019, 126, e1493. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Oyinloye, B.E.; Agboinghale, P.E.; Ojo, O.A. Cnidoscolus aconitifolius (Mill.) I. M. Johnst leaf extract prevents oxidative hepatic injury and improves muscle glucose uptake ex vivo. J. Food Biochem. 2019, 43, e13065. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, R.V.; Gutiérrez-Rebolledo, G.A.; Méndez-Bolaina, E.; Sánchez-Medina, A.; Maldonado-Saavedra, O.; Domínguez-Ortiz, M.Á.; Cruz-Sánchez, J.S. Cnidoscolus chayamansa Mc Vaugh, an important antioxidant, anti-inflammatory and cardioprotective plant used in Mexico. J. Ethnopharmacol. 2014, 151, 937–943. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Evaluation of Minerals, Phytochemical Compounds and Antioxidant Activity of Mexican, Central American, and African Green Leafy Vegetables. Plant Foods Hum. Nutr. 2015, 70, 357–364. [Google Scholar] [CrossRef]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J. Food Sci. Technol. 2018, 55, 2523–2532. [Google Scholar] [CrossRef]
- Rodríguez-De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef]
- The United States Pharmacopeia. Procedures of Thin Layer Chromatography; The United States Pharmacopeia: Rockville, MD, USA, 1979; pp. 7524–7526. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Pękal, A. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Gobierno de México. Norma Oficial Mexicana, Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio; Nom-062-Zoo; Diario Oficial de la Federación: Mexico City, Mexico, 1999; pp. 1–58. [Google Scholar]
- Santos, I.J.M.; Leite, G.O.; Costa, J.G.M.; Alves, R.R.N.; Campos, A.R.; Menezes, I.R.A.; Freita, F.R.V.; Nunes, M.J.H.; Almeida, W.O. Topical Anti-Inflammatory Activity of Oil from Tropidurus hispidus (Spix, 1825). Evid.-Based Complement. Altern. Med. 2015, 2015, 140247. [Google Scholar] [CrossRef][Green Version]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Us-Medina, U.; Millán-Linares, M.d.C.; Arana-Argaes, V.E.; Segura-Campos, M.R. Actividad antioxidante y antiinflamatoriain vitro de extractos de chaya (Cnidoscolus aconitifolius (Mill.) I.M. Johnst). Nutr. Hosp. 2020, 37, 46–55. [Google Scholar]
- Onasanwo, S.A.; Oyagbemi, A.A.; Saba, A.B. Anti-inflammatory and analgesic properties of the ethanolic extract of (Cnidoscolus aconitifolius in rats and mice. J. Basic Clin. Physiol. Pharmacol. 2011, 22, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.D.; Gunn, K.C.; Davidson, J.O.; Galinsky, R.; Graham, S.E.; Berry, M.J.; Bennet, L.; Gunn, A.J.; Dean, J.M. Anti-Inflammatory Therapies for Treatment of Inflammation-Related Preterm Brain Injury. Int. J. Mol. Sci. 2021, 22, 4008. [Google Scholar] [CrossRef]
- Castro, J.P.; Ocampo, Y.C.; Franco, L.A. In vivo andin vitroanti-inflammatory activity of Cryptostegia grandiflora Roxb. ex R. Br. leaves. Biol. Res. 2014, 47, 1–8. [Google Scholar] [CrossRef]
- Kelly, G.S. Quercetin Monograph. Alter. Med. Rev. 2011, 16, 172–194. [Google Scholar]
- Rajnarayana, K.; Reddy, M.S.; Chaluvadi, M.R.; Krishna, D.R. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef]
- Morikawa, K.; Nonaka, M.; Narahara, M.; Torii, I.; Kawaguchi, K.; Yoshikawa, T.; Kumazawa, Y.; Morikawa, S. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. 2003, 74, 709–721. [Google Scholar] [CrossRef] [PubMed]
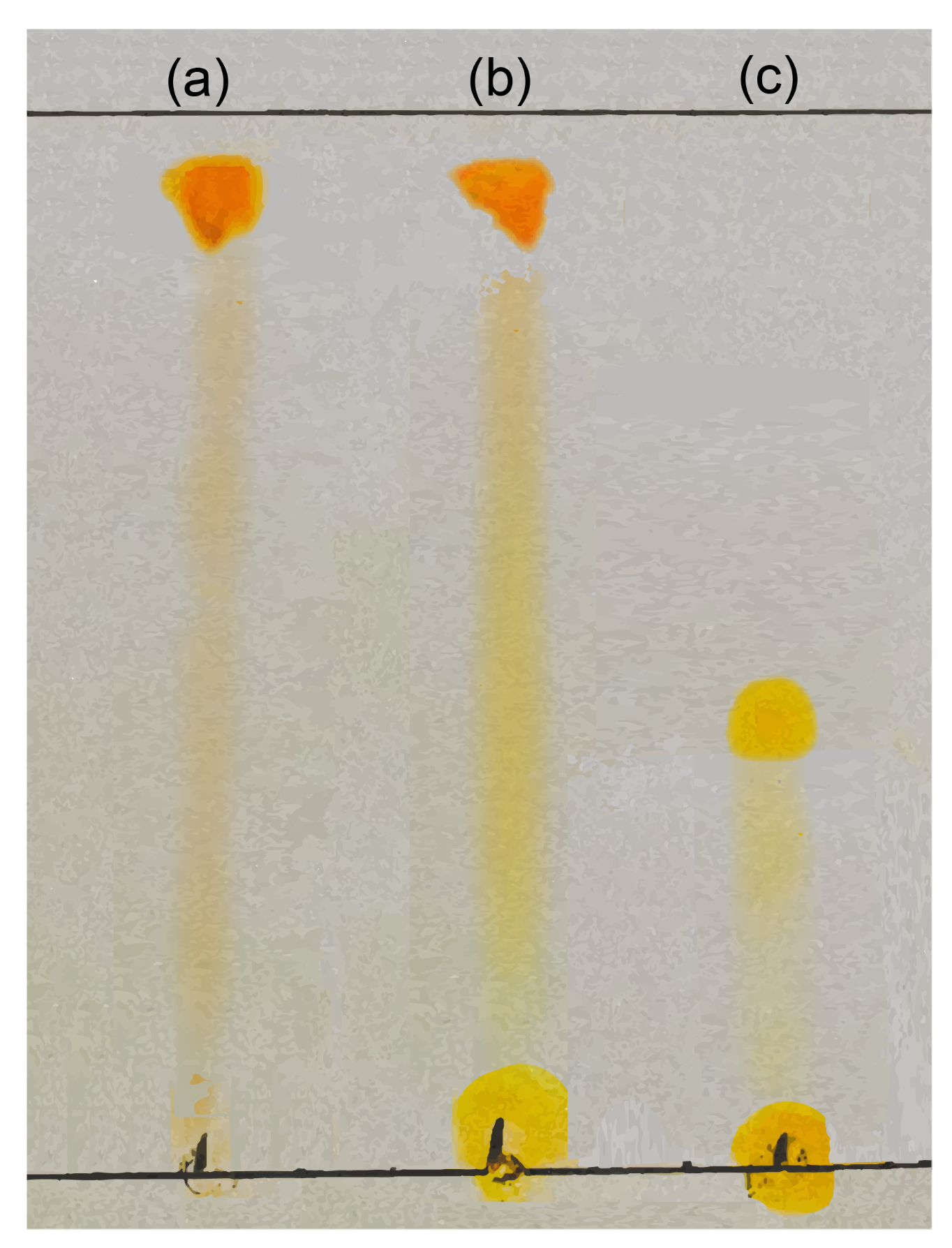
| Sample | Rf | Presence |
|---|---|---|
| C. aconitifolius | 0.79 | Q |
| 0.93 | Q | |
| Quercetin | 0.77 | Q |
| 0.92 | Q | |
| Rutin | 0.25 | R |
| 0.36 | R |
| Sample | Color | Presence |
|---|---|---|
| C. aconitifolius | Orange | Q |
| Quercetin | Orange | Q |
| Rutin | Yellow | R |
| Quercetin (mg/mL) | Mean (mg/mL) |
|---|---|
| 154.58 | |
| 150.71 | 154.23 ± 3.35 |
| 157.39 |
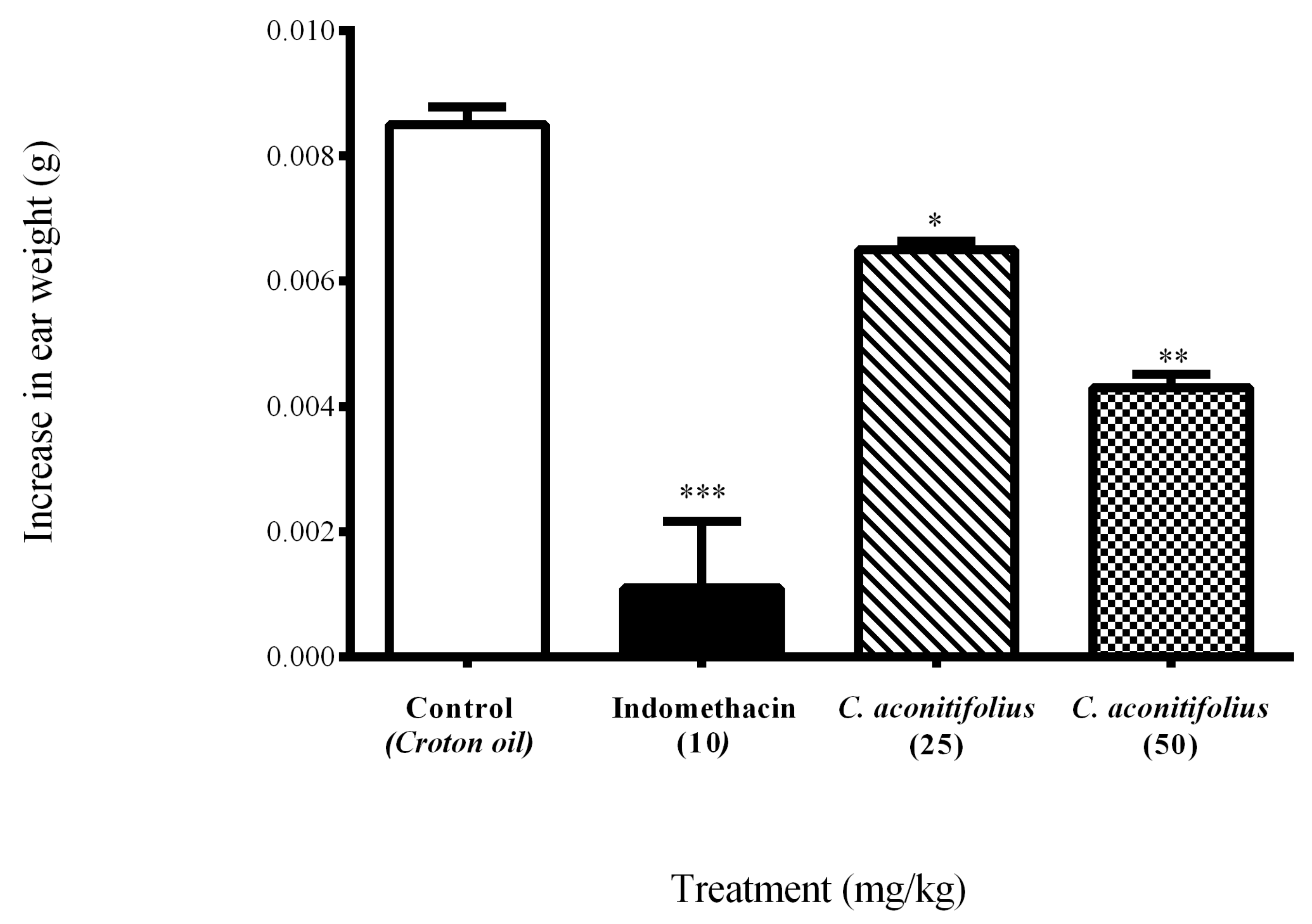
| Treatment | Dose (mg/kg) | Change in Ear Weight (g) | Inhibition (%) |
|---|---|---|---|
| Control | - | 0.0085 ± 0.00028 | 0.00 |
| Indomethacin | 10 | 0.0011 ± 0.00106 *** | 87.05 |
| C. aconitifolius | 25 | 0.0065 ± 0.00014 * | 23.52 |
| C. aconitifolius | 50 | 0.0043 ± 0.00021 ** | 49.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Camberos, E.; Torres-Gonzalez, O.R.; Sanchez-Hernandez, I.M.; Diaz-Martinez, N.E.; Hernandez-Perez, O.R.; Flores-Fernandez, J.M. Anti-Inflammatory Activity of Cnidoscolus aconitifolius (Mill.) Ethyl Acetate Extract on Croton Oil-Induced Mouse Ear Edema. Appl. Sci. 2021, 11, 9697. https://doi.org/10.3390/app11209697
Padilla-Camberos E, Torres-Gonzalez OR, Sanchez-Hernandez IM, Diaz-Martinez NE, Hernandez-Perez OR, Flores-Fernandez JM. Anti-Inflammatory Activity of Cnidoscolus aconitifolius (Mill.) Ethyl Acetate Extract on Croton Oil-Induced Mouse Ear Edema. Applied Sciences. 2021; 11(20):9697. https://doi.org/10.3390/app11209697
Chicago/Turabian StylePadilla-Camberos, Eduardo, Omar Ricardo Torres-Gonzalez, Ivan Moises Sanchez-Hernandez, Nestor Emmanuel Diaz-Martinez, Oscar Rene Hernandez-Perez, and Jose Miguel Flores-Fernandez. 2021. "Anti-Inflammatory Activity of Cnidoscolus aconitifolius (Mill.) Ethyl Acetate Extract on Croton Oil-Induced Mouse Ear Edema" Applied Sciences 11, no. 20: 9697. https://doi.org/10.3390/app11209697
APA StylePadilla-Camberos, E., Torres-Gonzalez, O. R., Sanchez-Hernandez, I. M., Diaz-Martinez, N. E., Hernandez-Perez, O. R., & Flores-Fernandez, J. M. (2021). Anti-Inflammatory Activity of Cnidoscolus aconitifolius (Mill.) Ethyl Acetate Extract on Croton Oil-Induced Mouse Ear Edema. Applied Sciences, 11(20), 9697. https://doi.org/10.3390/app11209697






