Evaluation of Rice Straw Ash as a Pozzolanic Addition in Cementitious Mixtures
Abstract
1. Introduction
2. Materials and Method
2.1. Physical and Chemical Characterization of RSA
2.2. Analysis of RSA Reactivity
3. Results
3.1. Physical and Chemical Characterization of RSA
3.2. Analysis of RSA Reactivity
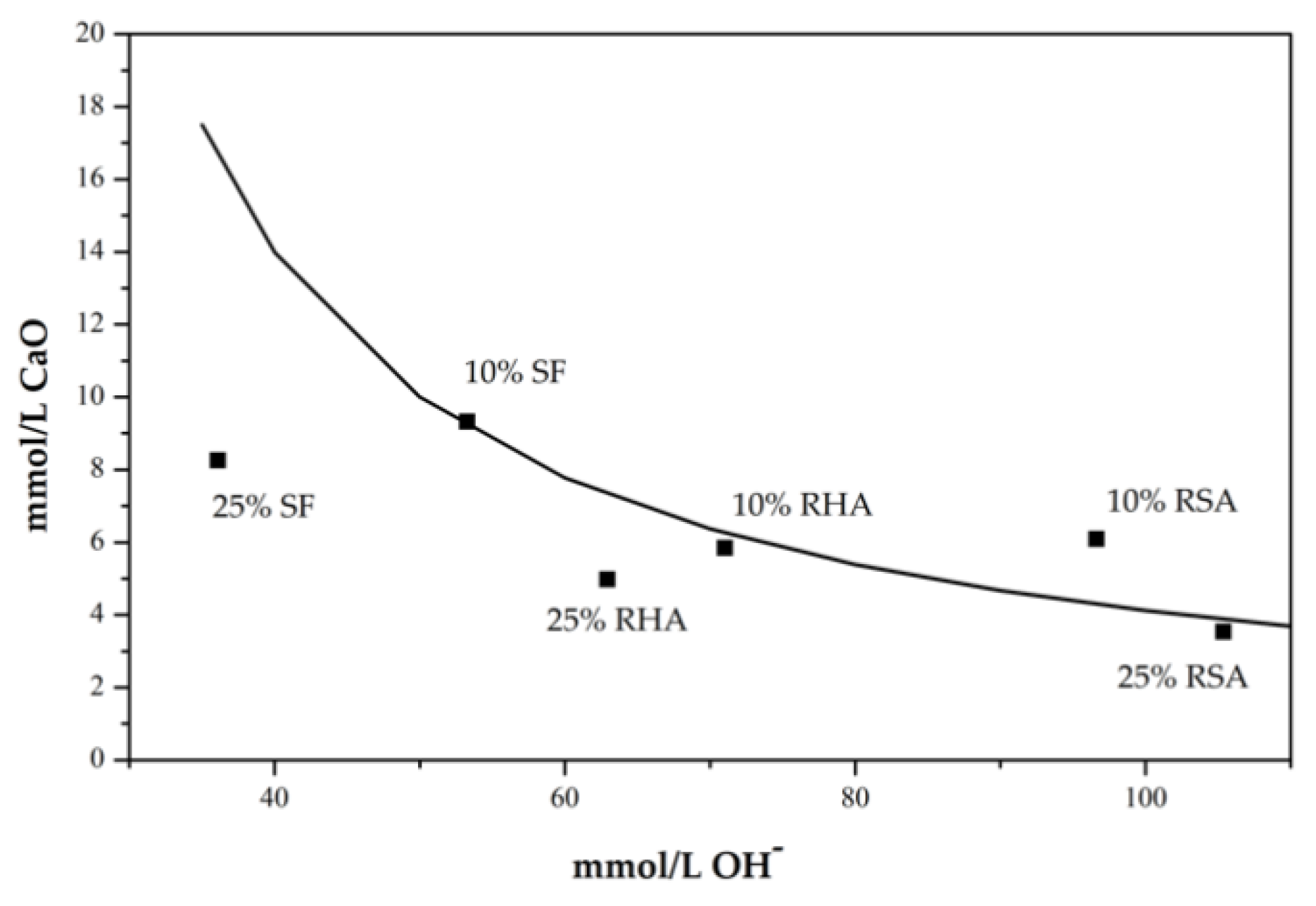
3.2.1. pH and Electrical Conductivity Studies
pOH = −log [OH−]
- [OH−]i: hydroxyl ion at a given time i
- [OH−]o: initial hydroxyl ion at time 0 immediately after homogenisation
- Ci is the corrected electrical conductivity value for a given time (t)
- Ct is the electrical conductivity in the CH/RSA aqueous suspension measured for t hours
- CRSA is the electrical conductivity of RSA in water for a given time (t)
- % RSA is the percentage of RSA in CH/RSA
3.2.2. Frattini Test
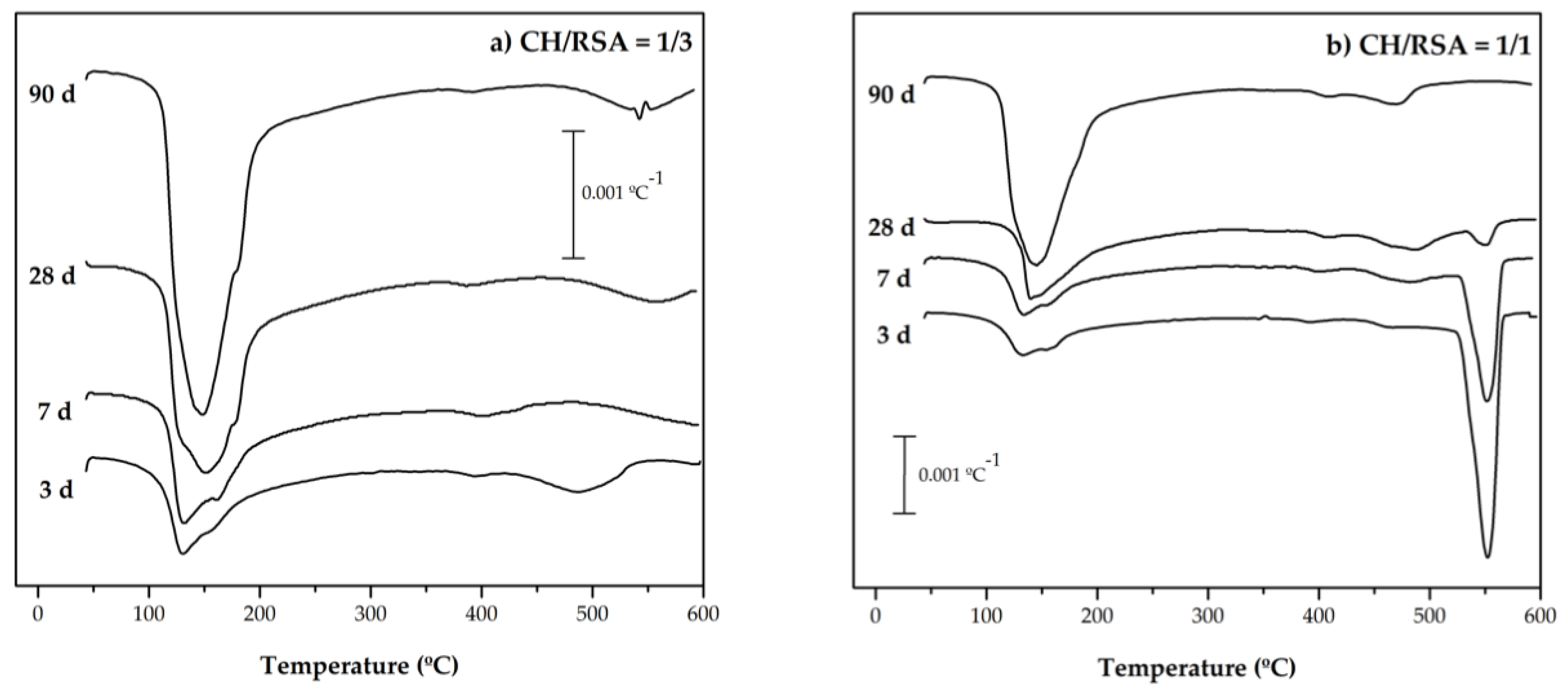
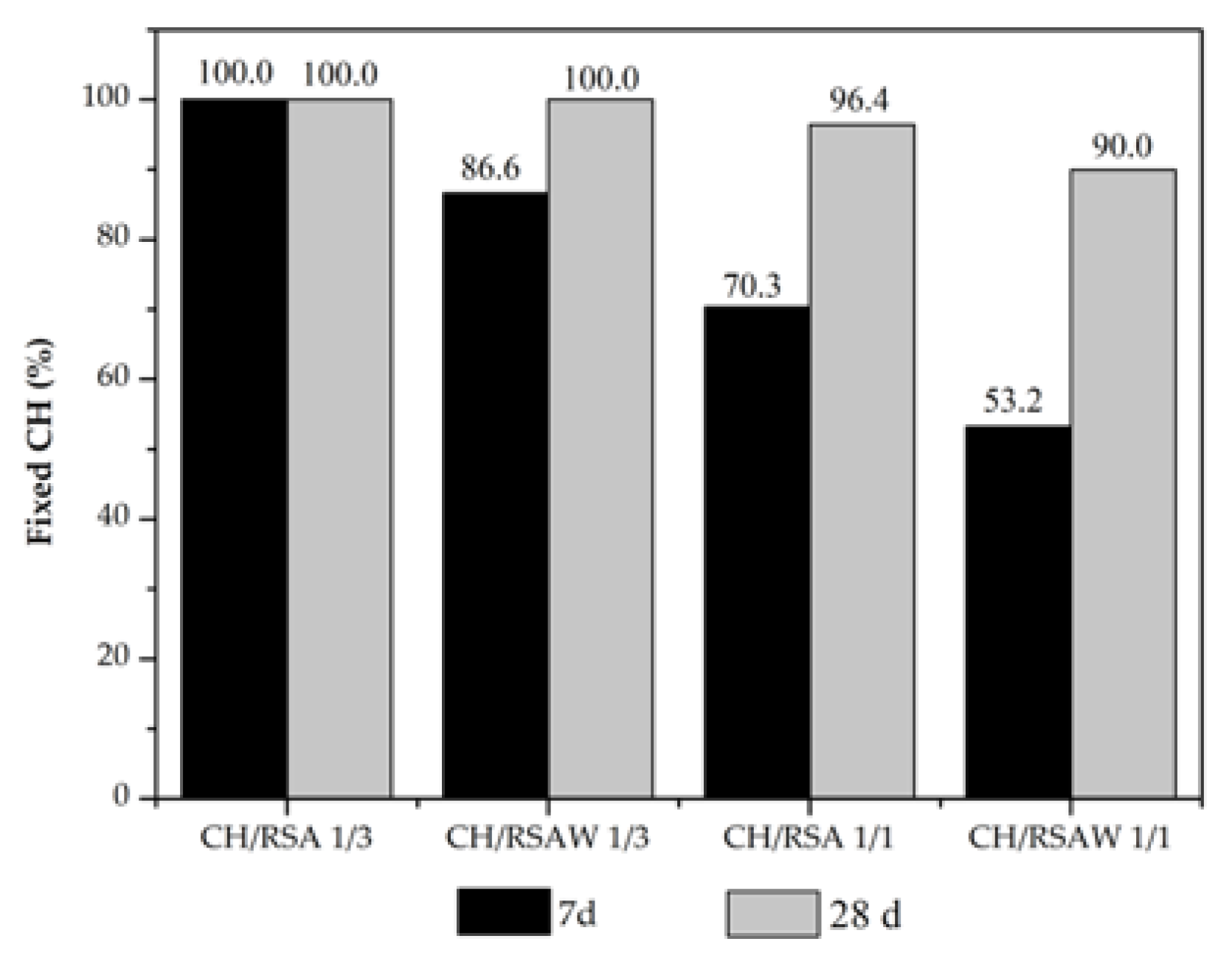
3.2.3. Thermogravimetry Analysis
- CHo is the initial quantity of CH in paste CH/RSA
- CHp,t is the quantity of CH present in paste CH/RSA after a given reaction time (t)
- CHC is the amount of portlandite present in the control paste for time t
- CHp is the amount of portlandite present in the paste with RSA for time t
- %C is the proportion of RSA present in the paste (e.g., 0.7 is for a PC/RSA paste with a 7/3 mass ratio
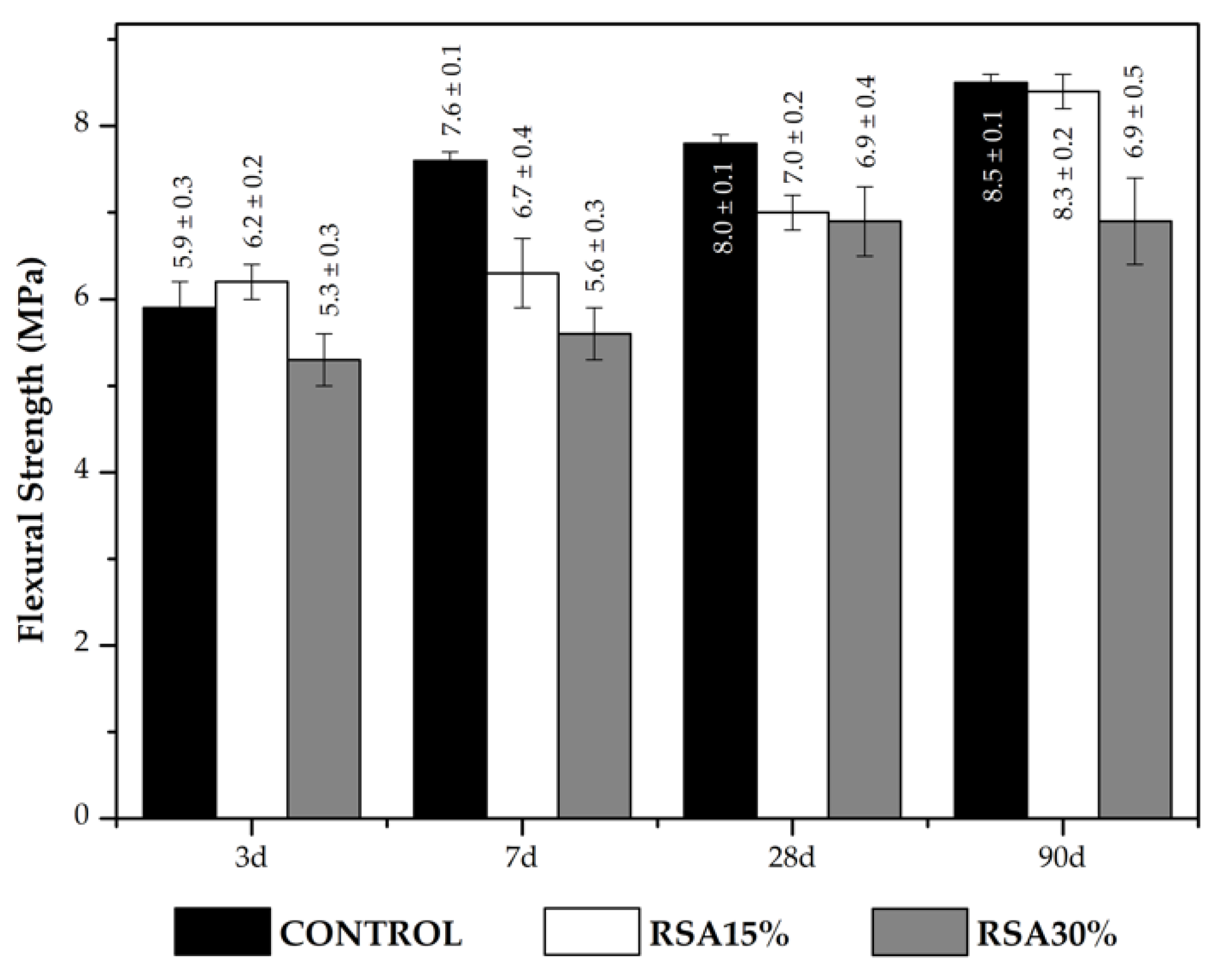
3.2.4. Mechanical Studies about Mortars
4. Conclusions
- -
- Chemical composition shows silica content to be about 52%, with high percentages of potassium oxide (12%) and chloride (3.5%). These chlorides can be reduced by washing the sample with hot water.
- -
- The analysis of crystalline and amorphous silica indicates that RSA is mainly of an amorphous nature.
- -
- A positive result is obtained in the Frattini test, and the pH and electrical conductivity behavior of the CH/ash suspensions demonstrated that RSA is considered a low-activity pozzolan. When RSA is washed, greater ash reactivity takes place, which is classified as a medium-reactivity pozzolan.
- -
- The thermogravimetric analysis showed marked RSA reactivity, and high CH fixation values were obtained in the CH and cement pastes with RSA.
- -
- In mechanical strength terms, good pozzolanic reactivity was found, along with major contribution to compressive strength for the 28–90 day period.
- -
- RSA can be used as a pozzolanic material and is a good option for valorizing the resulting ash after biomass combustion. Only the high chloride content should be taken into account for use in applications in which the content of this chemical parameter is limited (e.g., steel-reinforced concrete or pre-stressed concrete). The use of RSA would not be considered in the EHE-08 standard.
- -
- Using this type of cement containing RSA, precast concrete elements such as blocks, bricks, tubes, pavements and tiles, without steel reinforcement could be manufactured.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, M.; Barcelo, L.; Blair, B.; Cail, K.; Delagrave, A.; Kazanis, K. Lowering the carbon footprint of concrete by reducing clinker content of cement. Trans. Res. Rec. 2012, 2290, 99–104. [Google Scholar] [CrossRef]
- Proaño, L.; Sarmiento, A.T.; Figueredo, M.; Cobo, M. Techno-economic evaluation of indirect carbonation for CO2 emissions capture in cement industry: A system dynamic approach. J. Clean. Prod. 2020, 263, 121457. [Google Scholar]
- Celik, K.; Meral, C.; Gursel, A.P.; Mehta, P.K.; Horvath, A.; Monteiro, P.J.M. Mechanical properties, durability, and life-cycle assessment of self-consolidating concrete mixtures made with blended Portland cements containing fly ash and limestone powder. Cem. Concr. Compos. 2015, 56, 59–72. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Abd Elaty, M.A.A.; Ghazy, M.F. Performance of Portland cement mixes containing silica fume and mixed with lime-water. HBRC J. 2014, 10, 247–257. [Google Scholar] [CrossRef]
- Radlinski, M.; Olek, J. Investigation into the synergistic effects in ternary cementitious systems containing Portland cement, fly ash and silica fume. Cem. Concr. Compos. 2012, 34, 451–459. [Google Scholar] [CrossRef]
- Langan, B.W.; Weng, K.; Ward, M.A. Effect of silica fume and fly ash on heat of hydration of Portland cement. Cem. Concr. Res. 2002, 32, 1045–1051. [Google Scholar] [CrossRef]
- Nunes, L.R.J.; Loureiro, L.M.E.F.; Sa, L.C.R.; Silva, H.F.C. Evaluation of the potential for energy recovery from olive oil industry waste: Thermochemical conversion technologies as fuel improvement methods. Fuel 2020, 279, 118536. [Google Scholar]
- Reid, W.V.; Ali, M.K.; Field, C.B. The future of bioenergy. Glob. Chang. Biol. 2020, 26, 274–286. [Google Scholar] [CrossRef]
- Dong, L.; Wu, X.; Wang, Q.; Cao, G.; Zhou, C.; Ren, N. Evaluation of a novel pretreatment of NaOH/Urea at outdoor cold-winter conditions for enhanced enzymatic conversion and hythane production from rice straw. Sci. Total Environ. 2020, 744, 140900. [Google Scholar] [CrossRef]
- Ribó, M.; Albiach, R.; Pomares, F.; Canet, R. Alternativas de Gestión de la Paja de Arroz en la Albufera de València; Instituto Valencia de Investigaciones Agrarias (IVIA): Generalitat Valenciana, Valencia, 2017. (In Spanish) [Google Scholar]
- Martirena, F.; Monzó, J. Vegetable ashes as supplementary cementitious materials. Cem. Concr. Res. 2018, 114, 57–64. [Google Scholar] [CrossRef]
- Ahsan, M.B.; Hossain, Z. Supplemental use of rice husk ash (RHA) as a cementitious material in concrete industry. Constr. Build. Mater. 2018, 178, 1–9. [Google Scholar] [CrossRef]
- Joshaghani, A.; Moeini, M.A. Evaluating the effects of sugarcane-bagasse as and rice-husk ash on the mechanical and durability properties of mortar. J. Mater. Civ. Eng. 2018, 30, 04018144. [Google Scholar] [CrossRef]
- Adesina, P.; Olutoge, F.A. Structural properties of sustainable concrete developed using rice husk ash and hydrated lime. J. Build. Eng. 2019, 25, 100804. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.G.; Moon, J. The use of rice husk ash as reactive filler in ultra-high performance concrete. Cem. Concr. Res. 2019, 115, 389–400. [Google Scholar] [CrossRef]
- Miller, S.A.; Cunningham, P.R.; Harvey, J. Rice-based ash in concrete: A review of past work and potential environmental sustainability. Resour. Conserv. Recycl. 2019, 146, 416–430. [Google Scholar] [CrossRef]
- Roselló, J.; Soriano, L.; Santamarina, M.P.; Akasaki, J.L.; Monzó, J.; Payá, J. Rice straw ash A potential pozzolanic supplementary material for cementing systems. Ind. Crops Prod. 2017, 103, 39–50. [Google Scholar] [CrossRef]
- Sung, C.Y.; Lee, H.M.; Kim, Y.I.; Kim, K.-T.; Seo, D.S.; Nam, K.S. Engineering properties of concrete with rice straw ash. J. Agric. Sci. Technol. 1998, 25, 285–292. [Google Scholar]
- Munshi, S.; Dey, G.; Sharma, R.P. Use of rice straw ash as pozzolanic material in cement mortar. Int. J. Eng. Technol. 2013, 5, 603–606. [Google Scholar] [CrossRef]
- Munshi, S.; Sharma, R.P. Experimental investigation on strength and water permeability of mortar incorporate with rice straw ash. Adv. Mater. Sci. Eng. 2016, 3, 9696505. [Google Scholar] [CrossRef]
- Munshi, S.; Sharma, R.P. Investigation on the pozzolanic properties of rice straw ash prepared at different temperatures. Mater. Express 2018, 8, 157–164. [Google Scholar] [CrossRef]
- Agwa, I.S.; Omar, O.M.; Tayeh, B.A.; Abdelsalam, B.A. Effects of using rice straw and cotton stalk ashes on the properties of lightweight self-compacting concrete. Constr. Build. Mater. 2020, 235, 117541. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, B. Effects of rice straw ash and micro silica on mechanical properties of pavement quality concrete. J. Build. Eng. 2019, 26, 100889. [Google Scholar] [CrossRef]
- Bouzón, N. Activadores Alcalinos Alternativos a Partir de la Ceniza de Cáscara de Arroz Para la Fabricación de Geopolímeros. Ph.D. Thesis, Universitat Politécnica de Valéncia, València, Spain, 2015. (In Spanish). [Google Scholar]
- Tashima, M.M.; Soriano, L.; Borrachero, M.V.; Akasaki, J.L.; Payá, J. New method to assess the pozzolanic reactivity of mineral admixtures by means of pH and conductivity measurements in lime: Pozzolan suspensions. Mater. Constr. 2014, 64, e032. [Google Scholar]
- UNE-EN 196-5. Methods of Testing Cement—Part 5: Pozzolanicity Test for Pozzolanic Cement; AENOR: 2011. Available online: https://infostore.saiglobal.com/preview/is/en/2011/i.s.en196-5-2011.pdf?sku=1461445 (accessed on 15 November 2020).
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of Test Methods to Assess Pozzolanic Activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V. Fluid catalytic cracking catalyst residue (FC3R): An excellent mineral by-product for improving early-strength development of cement mixtures. Cem. Concr. Res. 1999, 29, 1773–1779. [Google Scholar] [CrossRef]
- UNE-EN 450-1. Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria; AENOR: Madrid, Spain, 2013; Available online: https://infostore.saiglobal.com/preview/98701122690.pdf?sku=871436_SAIG_NSAI_NSAI_20720611461445 (accessed on 15 November 2020).
- Kubiliute, R.; Kaminskas, R.; Kazlauskaite, A. Mineral wool production waste as an additive for Portland cement. Cem. Concr. Compos. 2018, 88, 130–138. [Google Scholar] [CrossRef]
- Chen, X.; Bi, Y.; Zhang, H.; Wang, J. Chlorides removal and control though water-washing process on MSWI fly ash. Procedia Environ. Sci. 2016, 31, 560–566. [Google Scholar] [CrossRef]
- Moraes, M.J.B.; Moraes, J.C.B.; Tashima, M.M.; Akasaki, J.L.; Soriano, l.; Borrachero, M.V.; Payá, J. Production of bamboo leaf ash by auto-combustion for pozzolanic and sustainable use in cementitious matrices. Constr. Build. Mater. 2019, 208, 369–380. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Toledo Filho, R.D.; De Moraes Rego Fairbairn, E. Use of ultrafine rice husk ash with high-carbon content as pozzolan in high performance concrete. Mater. Struct. 2009, 42, 983–992. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Velázquez, S. Chemical activation of pozzolanic reaction of fluid catalytic cracking catalyst residue (FC3R) in lime pastes: Thermal analysis. Adv. Cem. Res. 2004, 16, 123–130. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Velázquez, S. Evaluation of the pozzolanic activity of fluid catalytic cracking catalyst residue (FC3R). Thermogravimetric analysis studies on FC3R-Portland cement pastes. Cem. Concr. Res. 2003, 33, 603–609. [Google Scholar] [CrossRef]
- Tashima, M.M.; Fioriti, C.F.; Akasaki, J.L.; Payá, J.; Sousa, L.C.; Melges, J.L.P. High reactive rice husk ash (RHA): Production method and pozzolanic reactivity. Ambiente Construido 2012, 12, 151–163. [Google Scholar] [CrossRef]
- Xu, W.; Yiu Lo, T.; Wang, W.; Ouyang, D.; Wang, P.; Xing, F. Pozzolanic reactivity of silica fume and ground rice husk ash as reactive silica in a cementitious system: A comparative study. Materials 2016, 9, 146. [Google Scholar] [CrossRef]
- Kramar, S.; Ducman, V. Evaluation of ash pozzolanic activity by means of the strength activity index, Frattini test and DTA/TG analysis. Tech. Gaz. 2018, 25, 1746–1752. [Google Scholar]


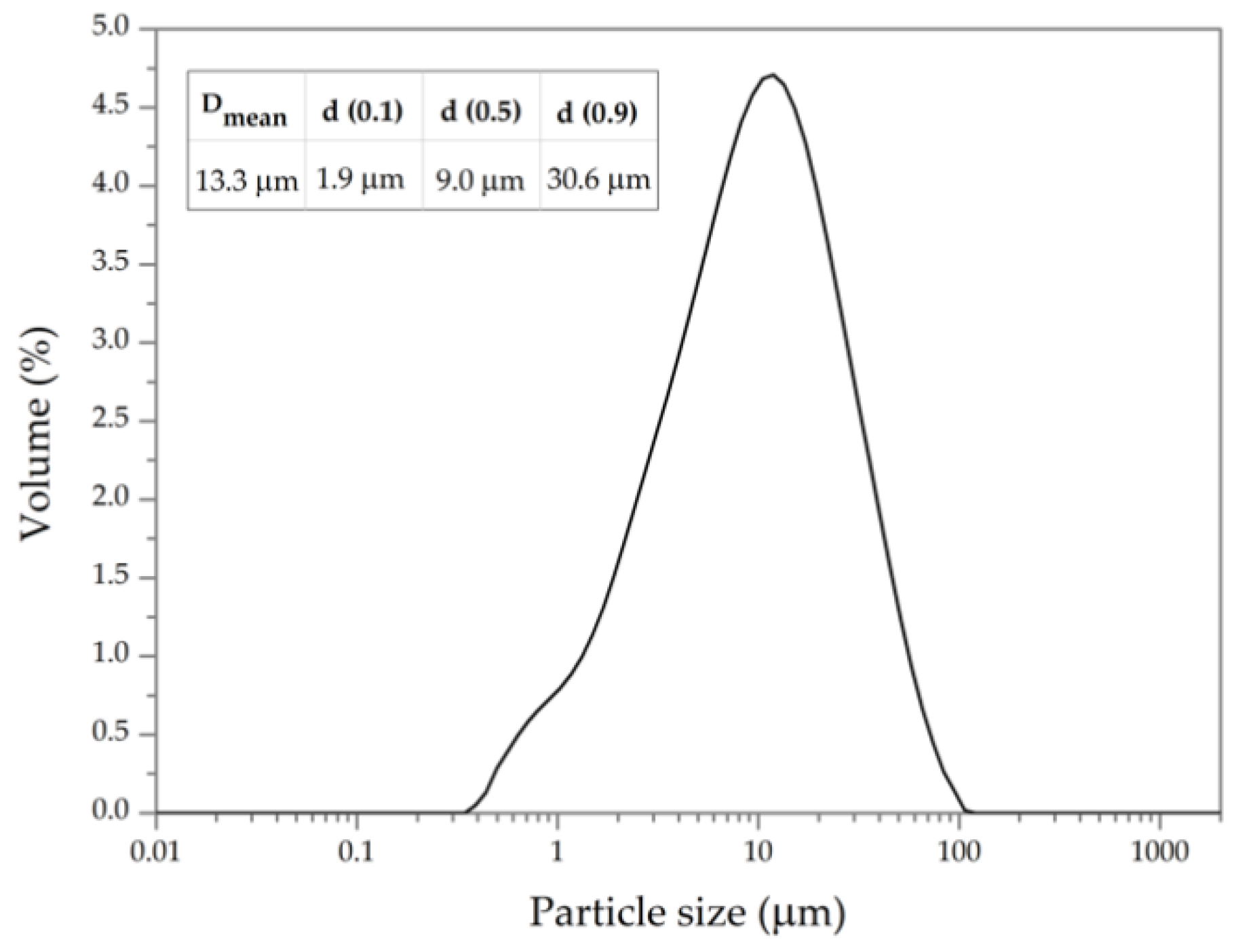

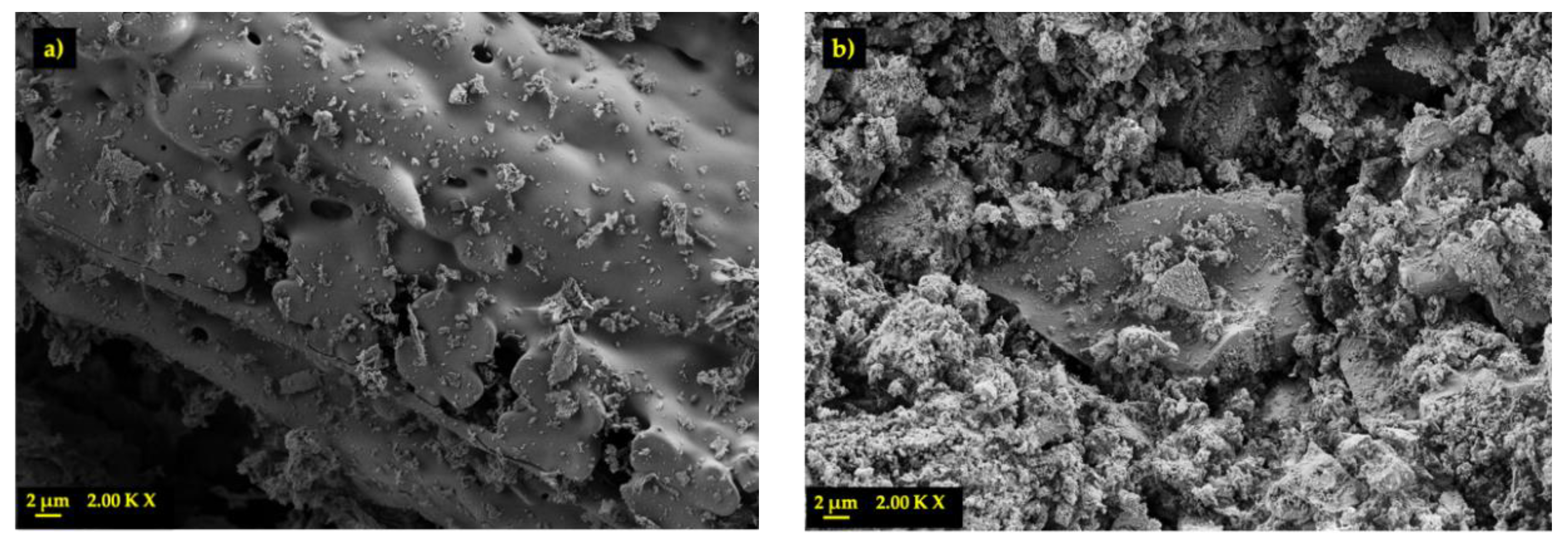
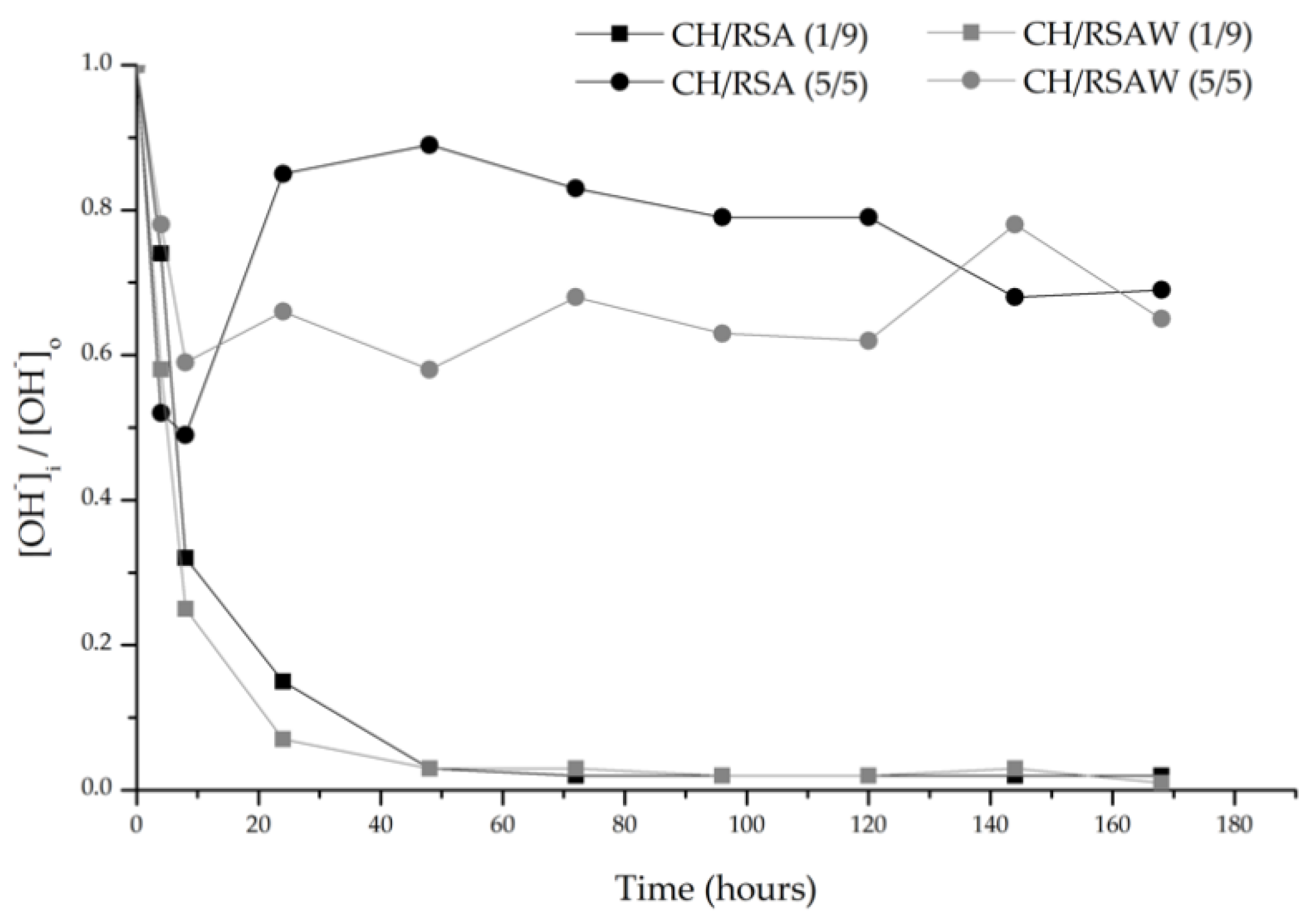
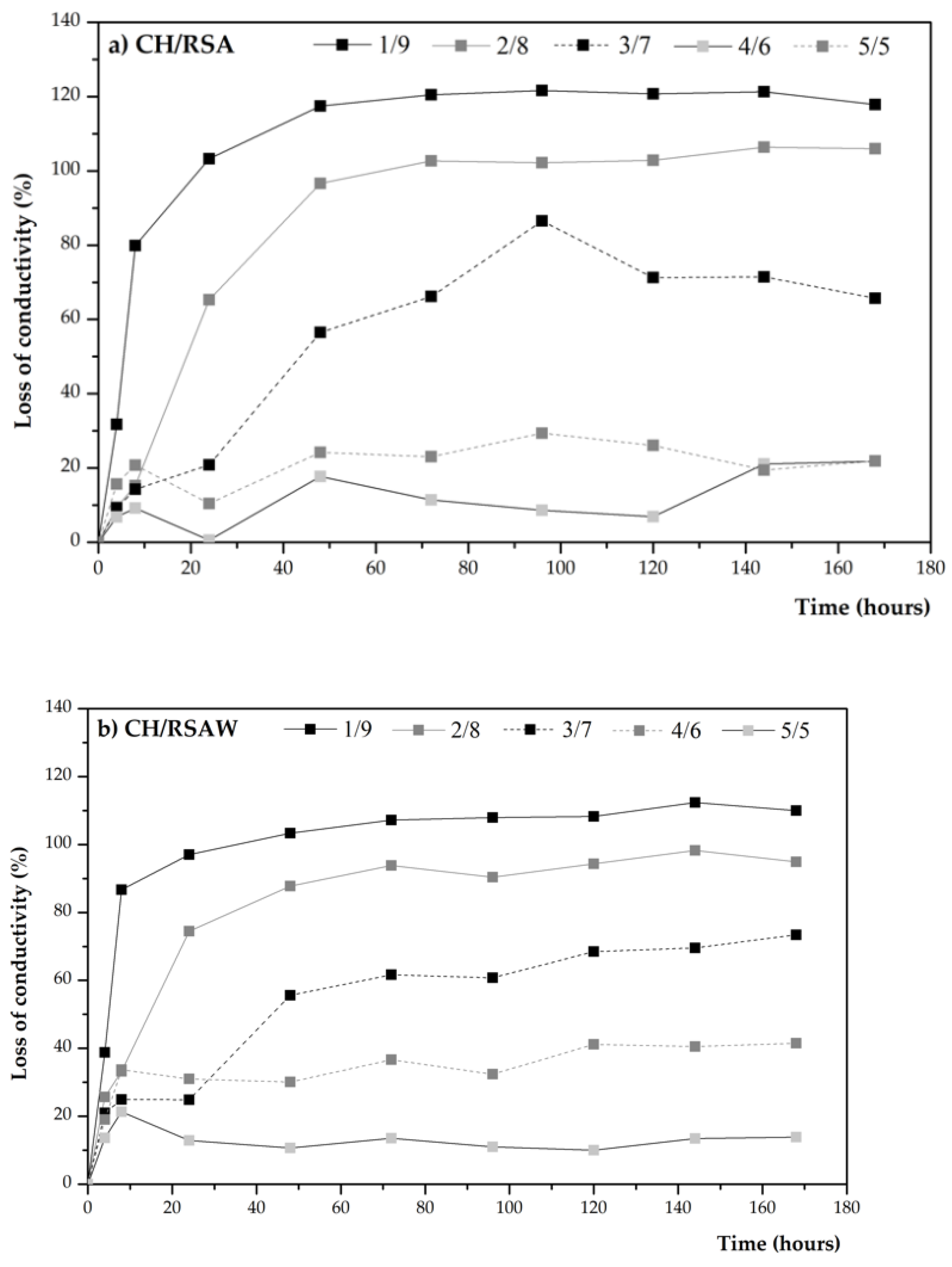

| Mortar | PC (g) | RSA (g) | Water (g) | Sand (g) |
|---|---|---|---|---|
| Control | 450.0 | - | 225.0 | 1350.0 |
| RSA 15% | 382.5 | 67.5 | 225.0 | 1350.0 |
| RSA 30% | 315.0 | 135.0 | 225.0 | 1350.0 |
| % | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | P2O5 | Cl− | Other | L.O.I |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSA | 52.44 | 0.47 | 0.17 | 8.01 | 2.71 | 2.26 | 12.05 | 0.89 | 2.58 | 3.52 | 0.29 | 14.60 |
| Sample | Percentage of Replacement (%) | 8 Days | 15 Days |
|---|---|---|---|
| PC/RSA | 10% | Negative | Negative |
| 20% | Positive | Positive | |
| 25% | Positive | Positive | |
| PC/RSAW | 10% | Negative | Negative |
| 20% | Positive | Positive | |
| 25% | Positive | Positive |
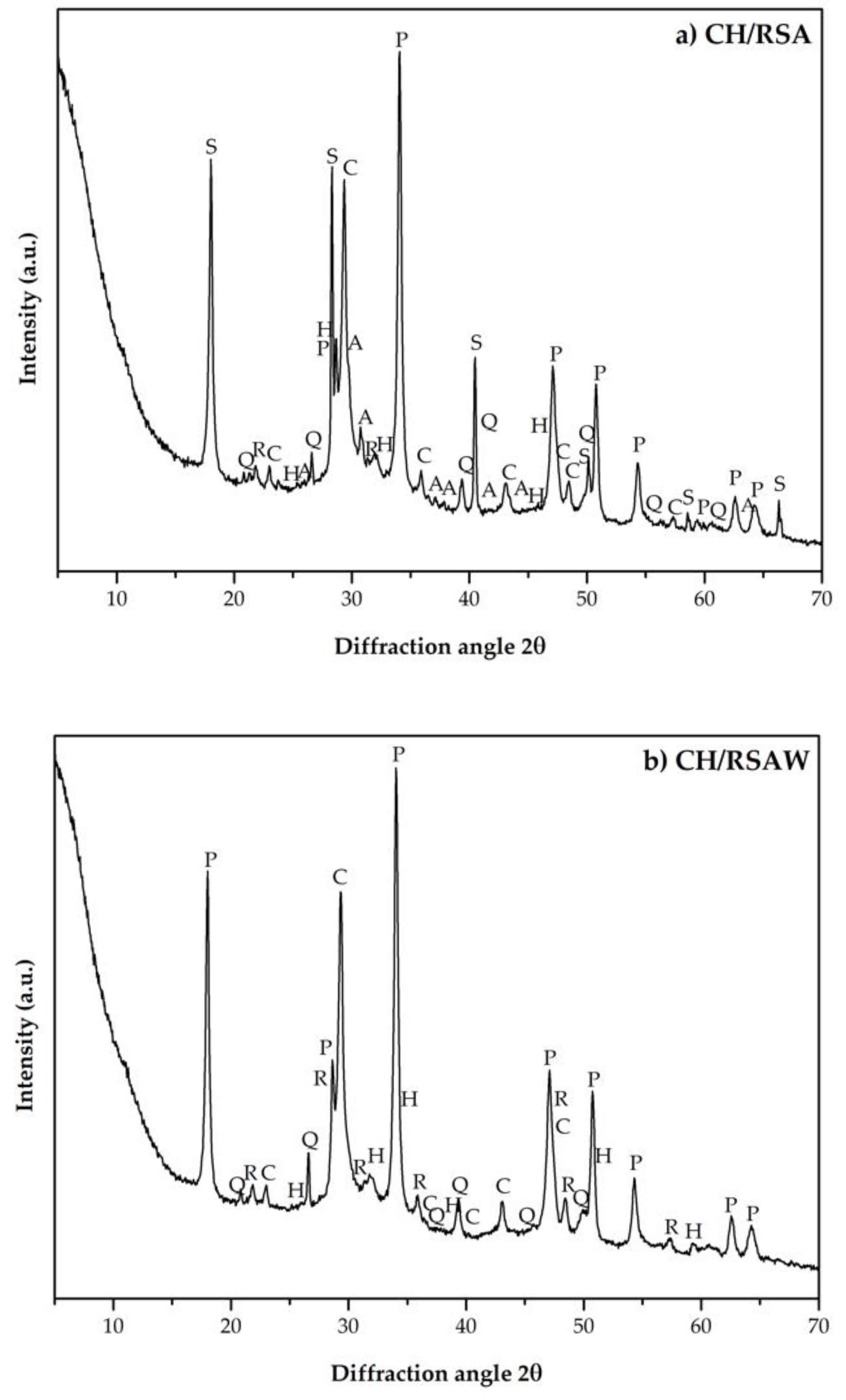
| Key | Mineral Phase | Chemical Formula | PDFcard |
|---|---|---|---|
| Q | Quartz | SiO2 | 331161 |
| R | Cristobalite | SiO2 | 391425 |
| C | Calcite | CaCO3 | 050586 |
| A | Arkanite | K2SO4 | 050613 |
| P | Portlandite | Ca(OH)2 | 040733 |
| H | Hydrotalcite | Mg6Al2CO3(OH)16.4H2O | 140191 |
| S | Hydrated potassium carbonate | K2CO3.1.5H2O | 110655 |
| 3 Days | 7 Days | 28 Days | 90 Days | |
|---|---|---|---|---|
| PC/RSA 7/3 | 60.9% | 64.8% | 81.5% | 90.7% |
| PC/RSA 7.5/2.5 | n.d * | 62.1% | 69.5% | n.d |
| PC/RSA 8/2 | n.d | 59.2% | 61.1% | n.d |
| PC/RSA 8.5/1.5 | 37.8% | 45.1% | 49.8% | 55.7% |
| AGE (Days) | RSA 15% | RSA 30% |
|---|---|---|
| 3 | 97.45 | 80.79 |
| 7 | 98.16 | 83.41 |
| 28 | 105.97 | 93.15 |
| 90 | 108.95 | 107.54 |
| Mortar | α | β | R2 |
|---|---|---|---|
| Control | 39.20 | 3.72 | 0.94 |
| RSA 15% | 35.48 | 5.64 | 0.99 |
| RSA 30% | 25.54 | 7.31 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, S.; Soriano, L.; Monzó, J.; Payá, J.; Font, A.; Borrachero, M.V. Evaluation of Rice Straw Ash as a Pozzolanic Addition in Cementitious Mixtures. Appl. Sci. 2021, 11, 773. https://doi.org/10.3390/app11020773
Hidalgo S, Soriano L, Monzó J, Payá J, Font A, Borrachero MV. Evaluation of Rice Straw Ash as a Pozzolanic Addition in Cementitious Mixtures. Applied Sciences. 2021; 11(2):773. https://doi.org/10.3390/app11020773
Chicago/Turabian StyleHidalgo, Samantha, Lourdes Soriano, José Monzó, Jordi Payá, Alba Font, and Mª Victoria Borrachero. 2021. "Evaluation of Rice Straw Ash as a Pozzolanic Addition in Cementitious Mixtures" Applied Sciences 11, no. 2: 773. https://doi.org/10.3390/app11020773
APA StyleHidalgo, S., Soriano, L., Monzó, J., Payá, J., Font, A., & Borrachero, M. V. (2021). Evaluation of Rice Straw Ash as a Pozzolanic Addition in Cementitious Mixtures. Applied Sciences, 11(2), 773. https://doi.org/10.3390/app11020773










