The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Technological Methods
2.1.1. Materials
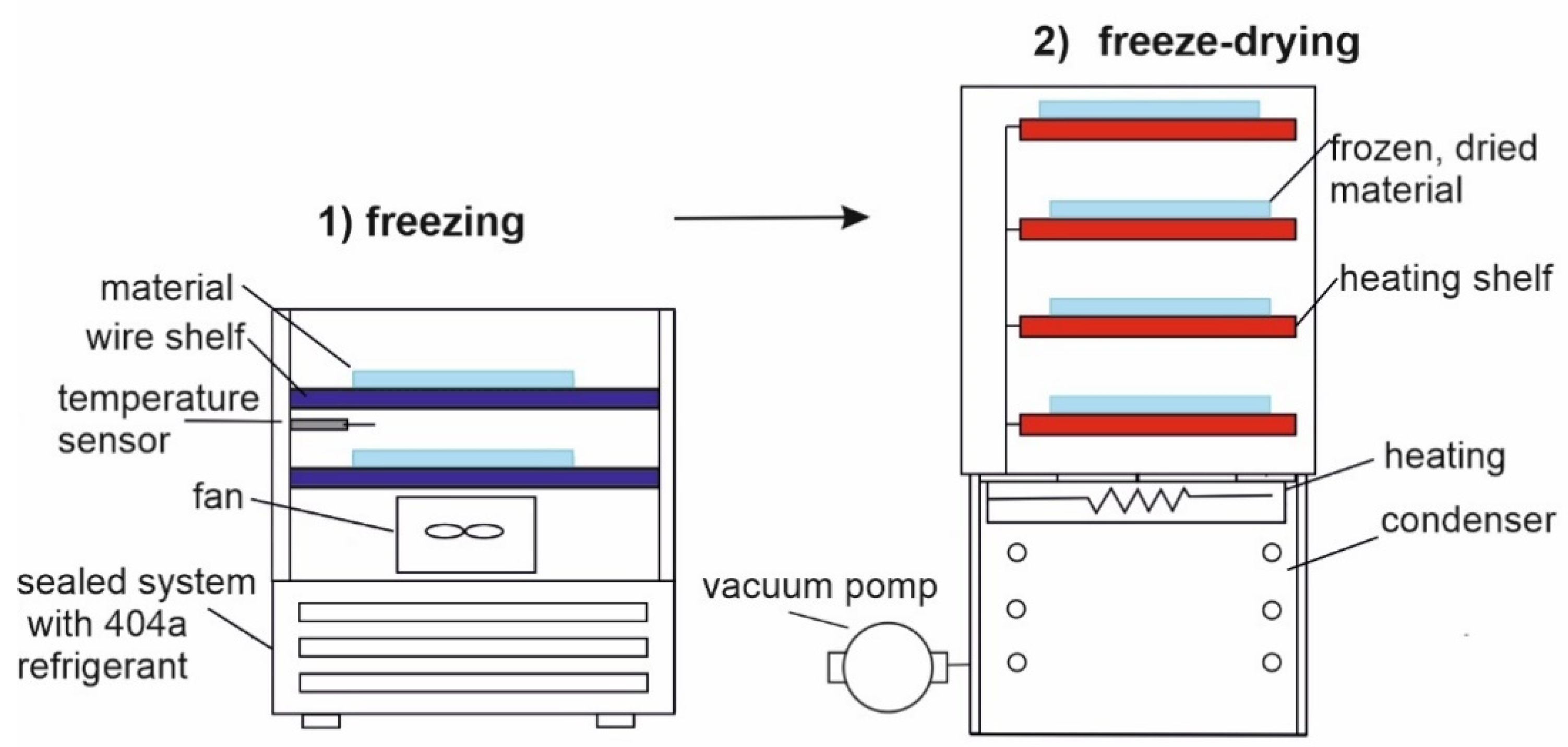
2.1.2. Freeze-Drying
2.2. Methodology for the Determination of the Selected Properties of Soups
2.2.1. Water Content and Water Activity
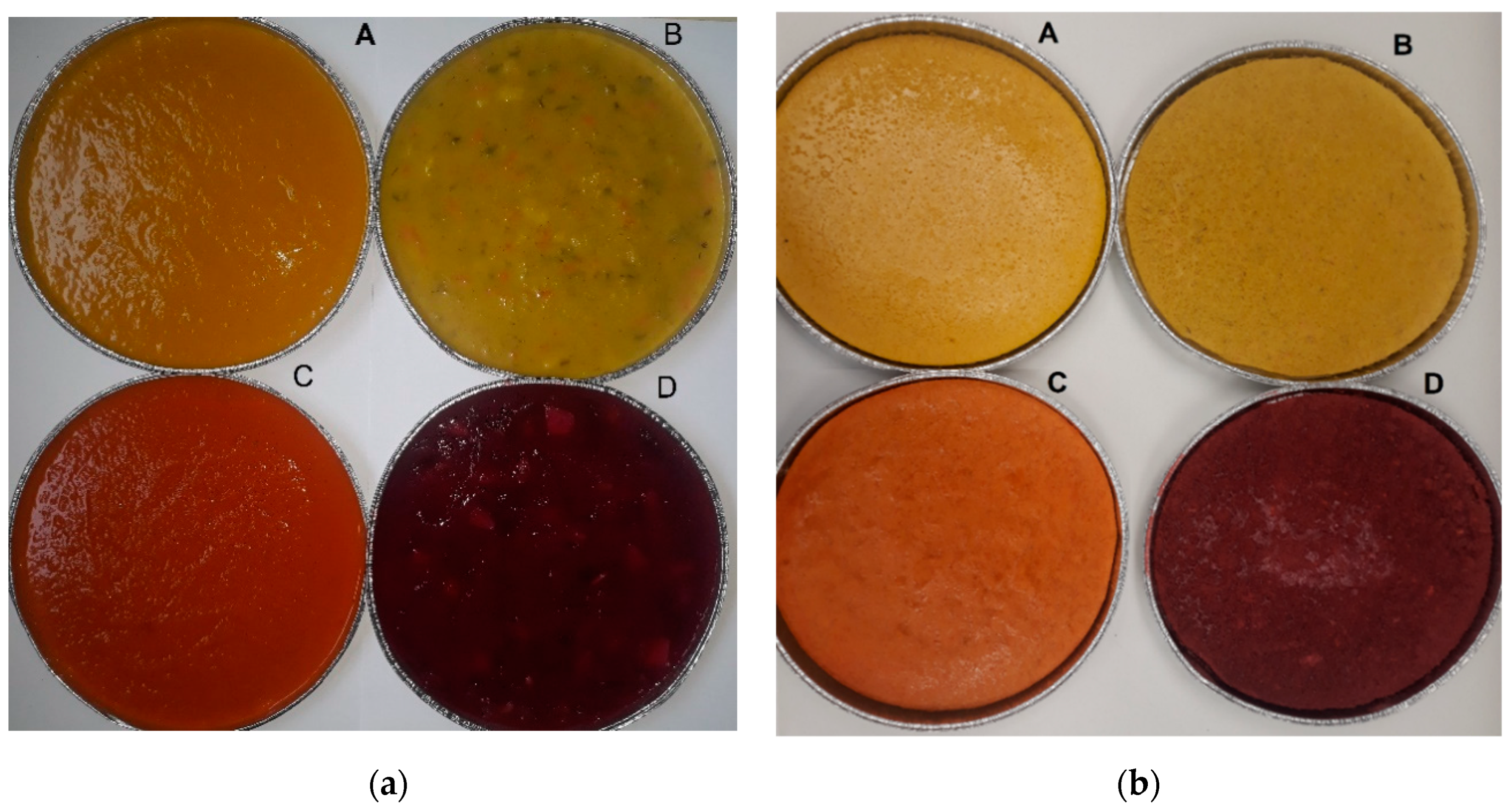
2.2.2. Color
2.2.3. pH
2.2.4. Antioxidant Capacity and the Content of Polyphenolic Compounds
2.2.5. Sensory Analysis
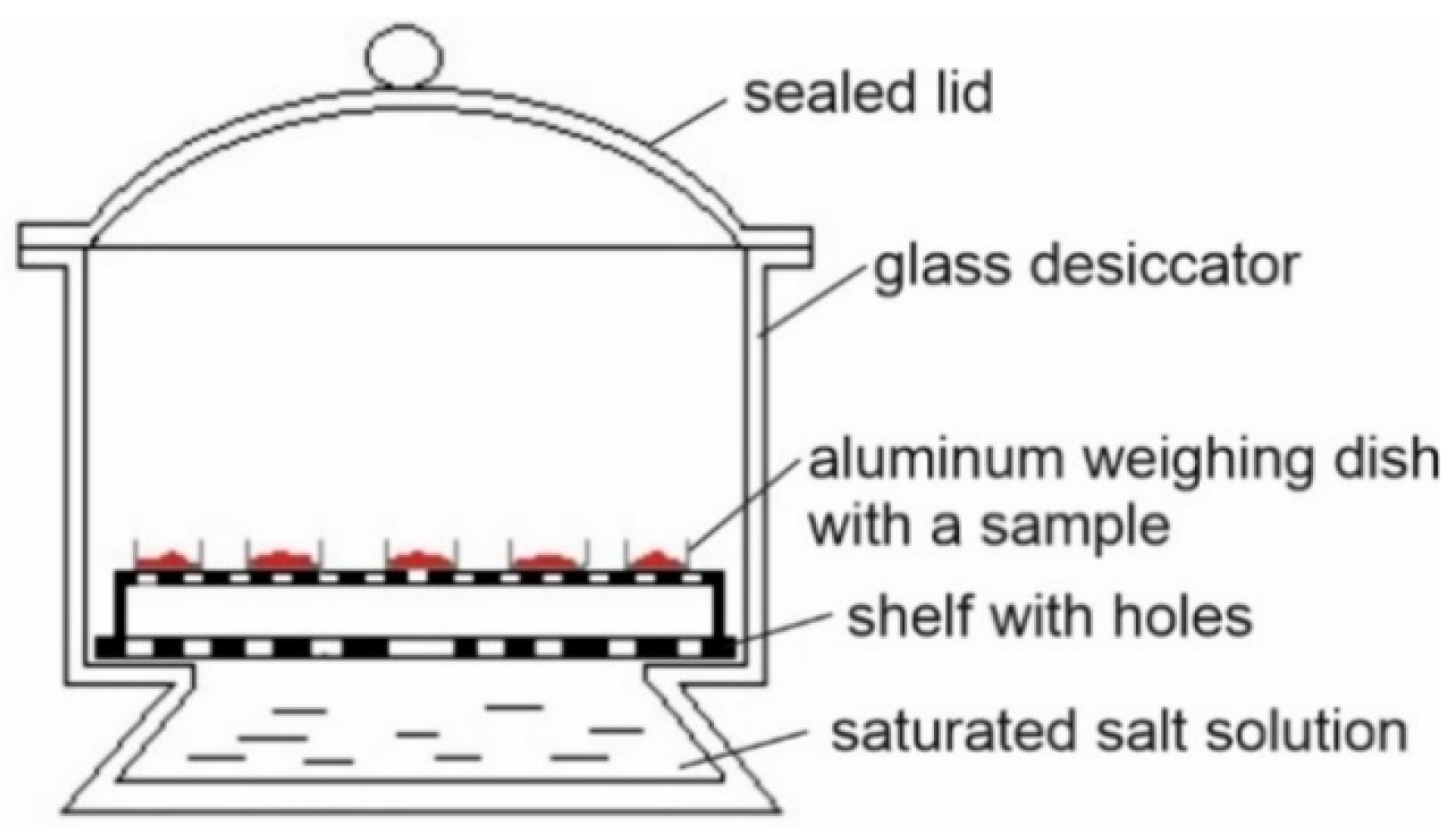
2.2.6. Sorption Isotherms—Static Method
Experimental Determination of Moisture Sorption Isotherms
Modelling of Moisture Sorption Isotherms
2.2.7. Sorption Isotherms—Dynamic Vapor Sorption-Method (DVS)
2.2.8. Water Sorption Kinetics
2.3. Statistical Analysis
3. Results and Discussion
3.1. Characteristic of Physical, Biochemical, and Sensory Properties of Soups before and after Freeze-Drying
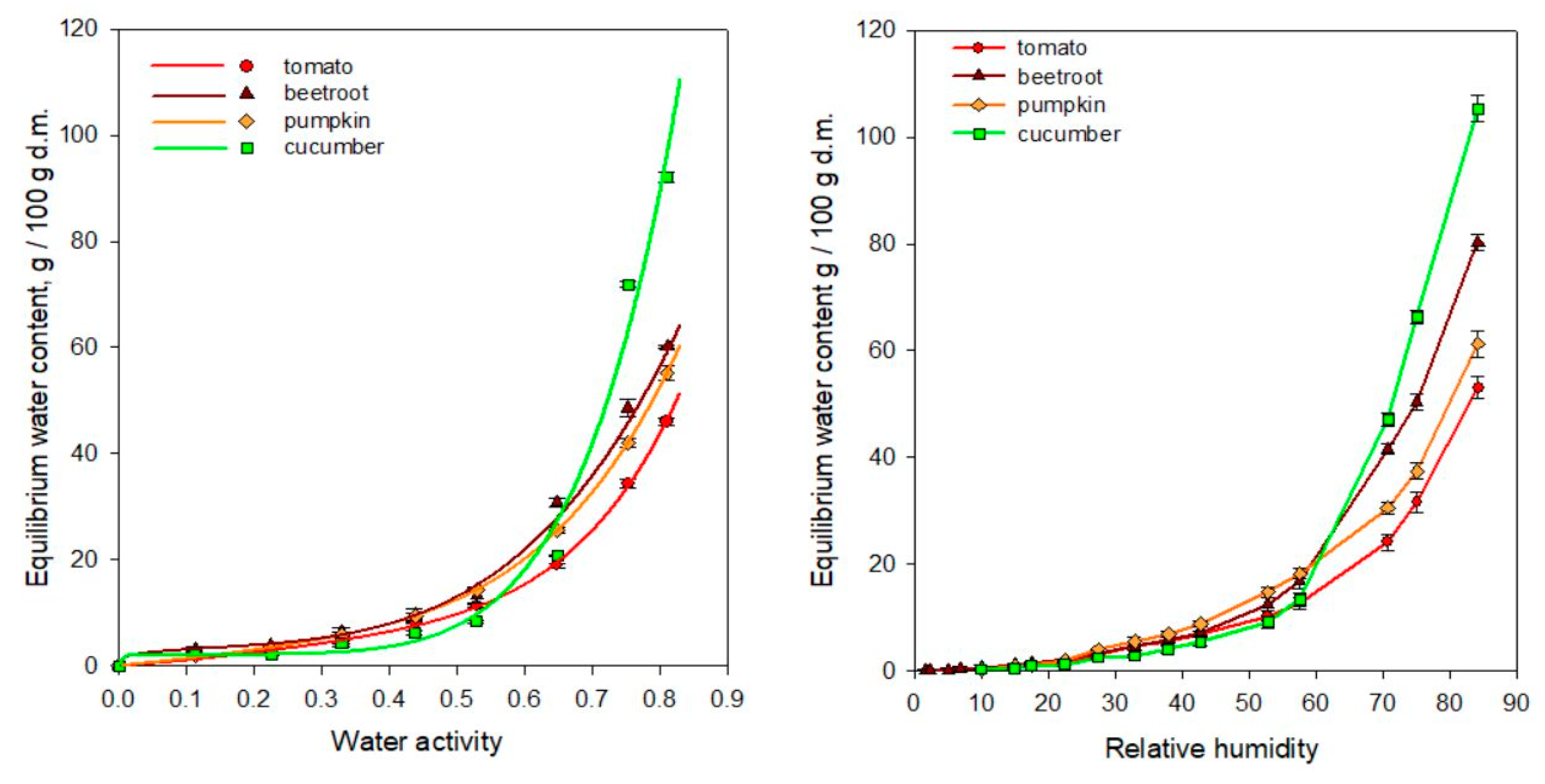
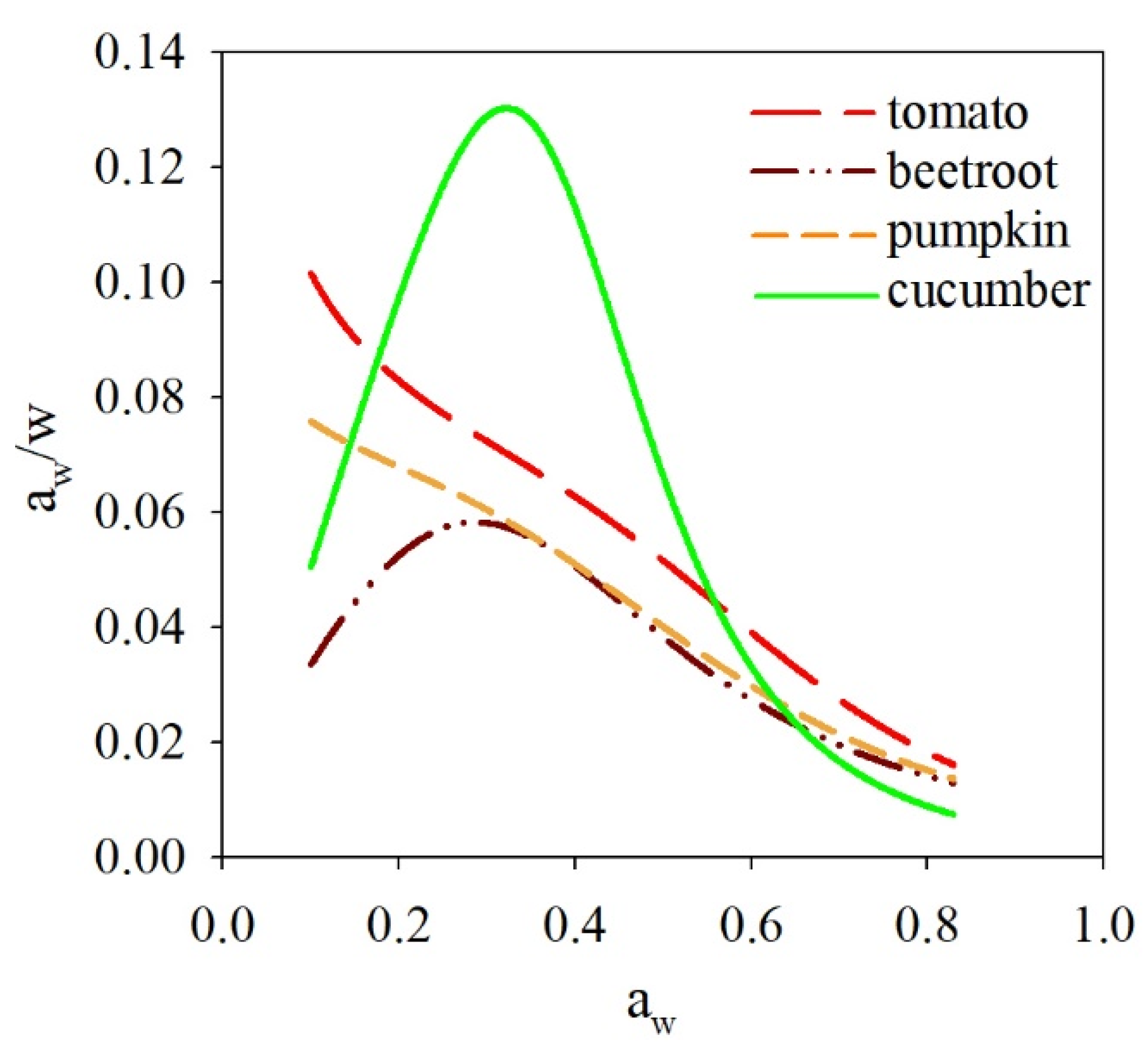
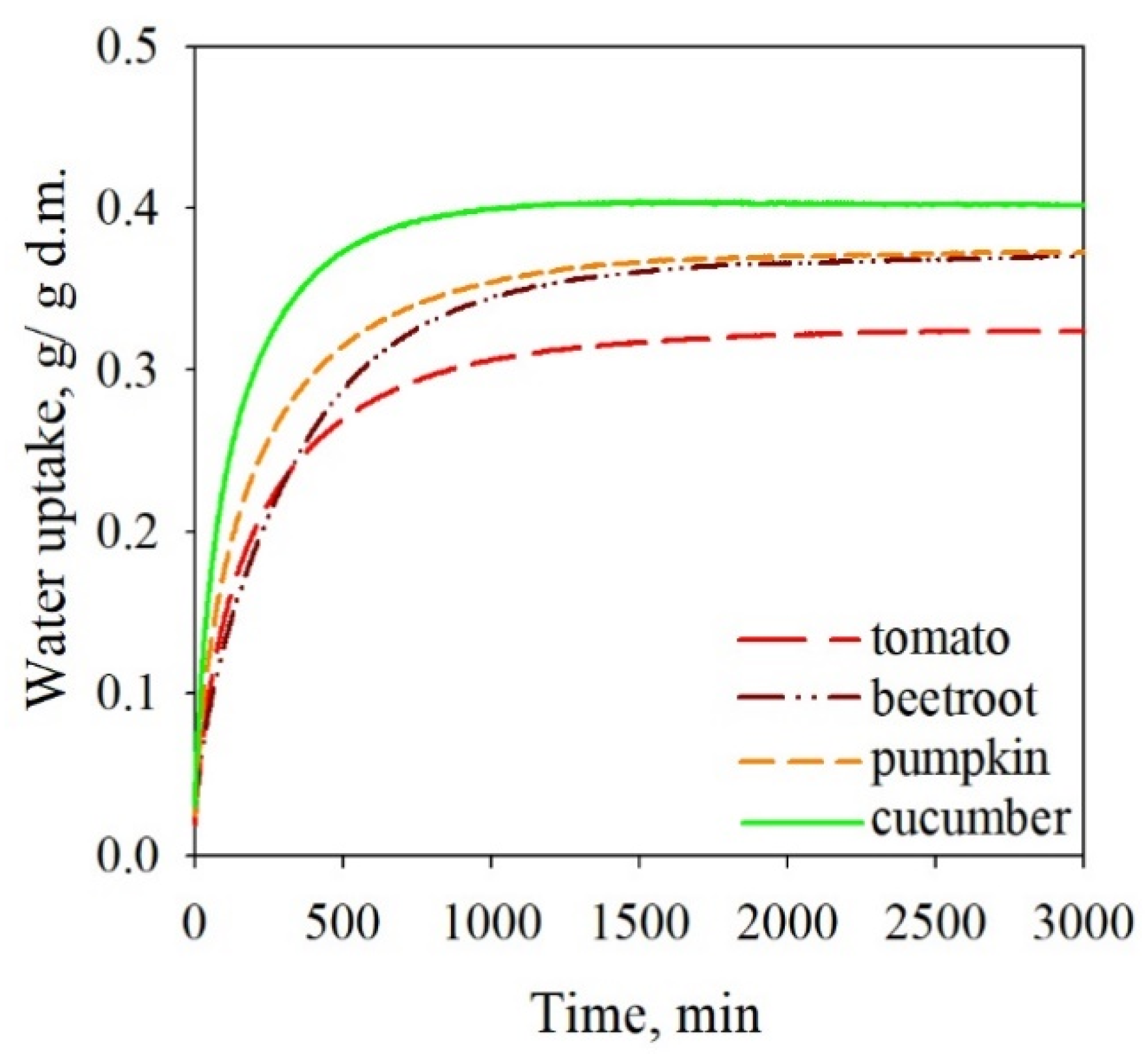
3.2. The Sorption Properties of Freeze-Dried Soups
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Ancos, B.; Sánchez-Moreno, C. Soups. In Innovative Technologies in Beverage Processing, 1st ed.; Aguiló-Aguayo, L., Plaza, L., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 249–273. [Google Scholar]
- Erkekoglu, P.; Sipahi, H.; Baydar, T. Evaluation of Nitrite in Ready-Made Soups. Food Anal. Methods 2009, 2, 61–65. [Google Scholar] [CrossRef]
- Raitio, R.; Orlien, V.; Skibsted, L.H. Storage stability of cauliflower soup powder: The effect of lipid oxidation and protein degradation reactions. Food Chem. 2011, 128, 371–379. [Google Scholar] [CrossRef]
- Rekha, M.N.; Yadav, A.R.; Dharmesh, S.; Chauhan, A.; Ramteke, R.S. Evaluation of antioxidant properties of dry soup mix extracts containing dill (Anethum sowa L.) leaf. Food Bioprocess Technol. 2008, 3, 441–449. [Google Scholar] [CrossRef]
- Farzana, T.; Mohajan, S.; Saha, T.; Hossain, N.; Haque, Z. Formulation and nutritional evaluation of a healthy vegetable soup powder supplemented with soy flour, mushroom, and moringa leaf. Food Sci. Nutr. 2017, 5, 911–920. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.M.H.; Omran, A.A. Preparation of dried vegetarian soup supplemented with some legumes. Food Nutr. Sci. 2014, 5, 2274–2285. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Djantou, E.B.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum L. var. Hungarian). Food Chem. 2009, 117, 647–653. [Google Scholar] [CrossRef]
- Kerdpiboon, S.; Devahastin, S.; Kerr, W.L. Comparative fractal characterization of physical changes of different food products during drying. J. Food Eng. 2007, 83, 570–580. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Heindl, A.; Müller, J. Assessment of convection, hot-air combined with microwave-vacuum and freeze-drying methods for mushrooms with regard to product quality. Int. J. Food Sci. Technol. 2011, 46, 333–342. [Google Scholar] [CrossRef]
- Liu, C.; Pirozzi, A.; Ferrari, G.; Vorobiev, E.; Grimi, N. Impact of pulsed electric fields on vacuum drying kinetics and physicochemical properties of carrot. Food Res. Int. 2020, 137, 109658. [Google Scholar] [CrossRef]
- Yaghmaee, P.; Durance, T.D. Efficacy of vacuum microwave drying in microbial decontamination of dried vegetables. Dry. Technol. 2007, 25, 1099–1104. [Google Scholar] [CrossRef]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying kinetics and bioactivity of beetroot slices pretreated in concentrated chokeberry juice and dried with vacuum microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Bouraoui, M.; Richard, P.; Durance, T. Microwave and convective drying of potato slices. J. Food Process. Eng. 1994, 17, 353–363. [Google Scholar] [CrossRef]
- Seyhan, F.G.; Evranuz, Ö. Low Temperature mushroom (A. bisporus) drying with desiccant dehumidifiers. Dry. Technol. 2000, 18, 433–445. [Google Scholar] [CrossRef]
- Nagaya, K.; Li, Y.; Jin, Z.; Fukumuro, M.; Ando, Y.; Akaishi, A. Low-temperature desiccant-based food drying system with airflow and temperature control. J. Food Eng. 2006, 75, 71–77. [Google Scholar] [CrossRef]
- Garcia-Perez, J.; Cárcel, J.A.; Riera, E.; Mulet, A. Influence of the applied acoustic energy on the drying of carrots and lemon peel. Dry. Technol. 2009, 27, 281–287. [Google Scholar] [CrossRef]
- Garcia-Perez, J.; Cárcel, J.A.; De La Fuente-Blanco, S.; De Sarabia, E.R.-F. Ultrasonic drying of foodstuff in a fluidized bed: Parametric study. Ultrasonics 2006, 44, e539–e543. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Paniwnyk, L.; Lelas, V. Accelerated drying of button mushrooms, Brussels sprouts and cauliflower by applying power ultrasound and its rehydration properties. J. Food Eng. 2007, 81, 88–97. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, B.; Guo, X.; Ding, F.; Pan, Z.; Ma, H. Effects of power ultrasound enhancement on infrared drying of carrot slices: Moisture migration and quality characterizations. LWT 2020, 126, 109312. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Yu, H. Effect of far-infrared drying on the water state and glass transition temperature in carrots. J. Food Eng. 2014, 136, 42–47. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Abozed, S.S.; El-Damhougy, S.; Salama, M.F.; Hussein, M.M. Efficiency of newly formulated functional instant soup mixtures as dietary supplements for elderly. Heliyon 2020, 6, e03197. [Google Scholar] [CrossRef] [PubMed]
- George, J.P.; Datta, A. Development and validation of heat and mass transfer models for freeze-drying of vegetable slices. J. Food Eng. 2002, 52, 89–93. [Google Scholar] [CrossRef]
- Torringa, E.; Esveld, E.; Scheewe, I.; Berg, R.V.D.; Bartels, P. Osmotic dehydration as a pre-treatment before combined microwave-hot-air drying of mushrooms. J. Food Eng. 2001, 49, 185–191. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M.; Kakuda, Y.; Liptay, A.; Niekamp, F. Lycopene degradation and isomerization in tomato dehydration. Food Res. Int. 1999, 32, 15–21. [Google Scholar] [CrossRef]
- Cui, Z.-W.; Xu, S.-Y.; Sun, D.-W.; Chen, W. Temperature Changes during microwave-vacuum drying of sliced carrots. Dry. Technol. 2005, 23, 1057–1074. [Google Scholar] [CrossRef]
- Roratto, T.B.; Monteiro, R.L.; Carciofi, B.A.; Laurindo, J.B. An innovative hybrid-solar-vacuum dryer to produce high-quality dried fruits and vegetables. LWT 2021, 140, 110777. [Google Scholar] [CrossRef]
- Mondal, I.H.; Rangan, L.; Uppaluri, R.V. A robust and novel methodology for the optimal targeting of leafy vegetable mix soup formulations. LWT 2020, 134, 110152. [Google Scholar] [CrossRef]
- Singh, S.; Ghosh, S.; Patil, G.R. Development of a mushroom-whey soup powder. Int. J. Food Sci. Technol. 2003, 38, 217–224. [Google Scholar] [CrossRef]
- Fernández-Pérez, V.; Tapiador, J.; Martín, A.; De Castro, M.L. Optimization of the drying step for preparing a new commercial powdered soup. Innov. Food Sci. Emerg. Technol. 2004, 5, 361–368. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, M.; Mujumdar, A.S. Effect of food ingredient on microwave freeze drying of instant vegetable soup. LWT 2010, 43, 1144–1150. [Google Scholar] [CrossRef]
- Raji, A.O.; Akinoso, R.; Aruna, T.E.; Raji, M.O. Reconstitution potentials and moisture sorption isotherms of selected freeze dried Nigerian soups. J. Food Process. Eng. 2016, 40, e12516. [Google Scholar] [CrossRef]
- Raji, A.O.; Rahman, A.; Maimuna, S.; Oyewale, R.M. Sensory properties and stability of selected freeze-dried nigerian soups stored at ambient condition. In Proceedings of the 4th Regional Food Science and Technology Summit (Refosts) Akure, Ondo State, Nigeria, 6–7 June 2018; pp. 323–330. [Google Scholar]
- Liu, W.; Zhang, M.; Devahastin, S.; Wang, W. Establishment of a hybrid drying strategy for instant cream mushroom soup based on starch retrogradation behavior. Int. J. Biol. Macromol. 2020, 147, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, M.; Mujumdar, A.S.; Sun, J.-C. Microwave freeze–drying characteristics and sensory quality of instant vegetable soup. Dry. Technol. 2009, 27, 962–968. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic, T.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Nindo, C.; Sun, T.; Wang, S.; Tang, J.; Powers, J. Evaluation of drying technologies for retention of physical quality and antioxidants in asparagus (Asparagus officinalis L.). LWT 2003, 36, 507–516. [Google Scholar] [CrossRef]
- Goula, A.M.; Karapantsios, T.D.; Achilias, D.S.; Adamopoulos, K.G. Water sorption isotherms and glass transition temperature of spray dried tomato pulp. J. Food Eng. 2008, 85, 73–83. [Google Scholar] [CrossRef]
- Garbalińska, H.; Bochenek, M.; Malorny, W.; Von Werder, J. Comparative analysis of the dynamic vapor sorption (DVS) technique and the traditional method for sorption isotherms determination—Exemplified at autoclaved aerated concrete samples of four density classes. Cem. Concr. Res. 2017, 91, 97–105. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, M.J. Effect of drying method on the moisture sorption isotherms for Inonotus obliquus mushroom. LWT 2008, 41, 1478–1484. [Google Scholar] [CrossRef]
- Witczak, T.; Socha, R.; Stępień, A.; Grzesik, M. Candied orange peel produced in solutions with various sugar compositions: Sugar composition and sorption properties of the product. J. Food Process. Eng. 2016, 40, 12. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Jakubczyk, E.; Górska, A.; Wirkowska-Wojdyła, M.; Bryś, J. The use of moisture sorption isotherms and glass transition temperature to assess the stability of powdered baby formulas. J. Therm. Anal. Calorim. 2014, 118, 911–918. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Ostrowska-Ligęza, E.; Gondek, E. Moisture sorption characteristics and glass transition temperature of apple puree powder. Int. J. Food Sci. Technol. 2010, 45, 2515–2523. [Google Scholar] [CrossRef]
- Amaral, I.C.; Silva, M.T.S.; Pereira, D.F.; Silva, E.K.; Resende, J.V.; Júnior, R.A.B. Effect of carrier agents on the physical and thermal stability of freeze-dried passion fruit (Passiflora edulis f. flavicarpa) pulp. Dry. Technol. 2016, 34, 713–722. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, X.; Zhao, Y.; Fang, Z. Glass transition and state diagram for freeze-dried Agaricus bisporus. J. Food Eng. 2012, 111, 667–674. [Google Scholar] [CrossRef]
- Moraga, G.; Talens, P.; Moraga, M.; Martínez-Navarrete, N. Implication of water activity and glass transition on the mechanical and optical properties of freeze-dried apple and banana slices. J. Food Eng. 2011, 106, 212–219. [Google Scholar] [CrossRef]
- Pavan, M.A.; Schmidt, S.J.; Feng, H. Water sorption behavior and thermal analysis of freeze-dried, refractance window-dried and hot-air dried açaí (Euterpe oleracea Martius) juice. LWT 2012, 48, 75–81. [Google Scholar] [CrossRef]
- Pakowski, Z.; Mujumdar, A.S. Drying of pharmaceutical products. In Handbook of Industrial Drying; Mujumdar, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Volume 2, pp. 689–712. [Google Scholar]
- Operating Instructions: Vaccum Drying Oven VO 200. Available online: https://www.memmert.com/index.php?eID=dumpFile&t=f&f=4699&token=480573c96b64ba6a645bdb0ca725e95a0c7e5be3 (accessed on 3 November 2020).
- Brito, V.; Gonçalves, T.D. Drying kinetics of porous stones in the presence of NaCl and NaNO3: Experimental assessment of the factors affecting liquid and vapour transport. Transp. Porous Media 2013, 100, 193–210. [Google Scholar] [CrossRef]
- Fullerton, A.; Fischer, T.; Lahti, A.; Wilhelm, K.-P.; Takiwaki, H.; Serup, J. Guidelines for measurement skin colour and erythema: A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1996, 35, 1–10. [Google Scholar] [CrossRef]
- Nowacka, M.; Śledź, M.; Wiktor, A.; Witrowa-Rajchert, D. Changes of radical scavenging activity and polyphenols content during storage of dried apples. Int. J. Food Prop. 2014, 17, 1317–1331. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dadan, M.; Rybak, K.; Wiktor, A.; Nowacka, M.; Żubernik, J.; Witrowa-Rajchert, D. Selected chemical composition changes in microwave-convective dried parsley leaves affected by ultrasound and steaming pre-treatments—An optimization approach. Food Chem. 2018, 239, 242–251. [Google Scholar] [CrossRef] [PubMed]
- International Standard ISO 13299: Sensory Analysis-Methodology-General Guidance for Establishing a Sensory Profile; International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- Basu, S.; Shivhare, U.S.; Mujumdar, A.S. Models for sorption isotherms for foods: A review. Dry. Technol. 2006, 24, 917–930. [Google Scholar] [CrossRef]
- Lewicki, P.P. A Three Parameter Equation For Food Moisture Sorption Isotherms. J. Food Process. Eng. 1998, 21, 127–144. [Google Scholar] [CrossRef]
- Peleg, M. Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J. Food Process. Eng. 1993, 16, 21–37. [Google Scholar] [CrossRef]
- Blahovec, J.; Yanniotis, S. Modified classification of sorption isotherms. J. Food Eng. 2009, 91, 72–77. [Google Scholar] [CrossRef]
- Polish Standard. Soups; PN-V-74015:2001; Polish Committee for Standardization: Warsaw, Poland, 2001; Available online: https://sklep.pkn.pl/pn-v-74015-2001p.html (accessed on 3 November 2020).
- Mayor, L.; Sereno, A. Modelling shrinkage during convective drying of food materials: A review. J. Food Eng. 2004, 61, 373–386. [Google Scholar] [CrossRef]
- Davis, E.A. Functionality of sugars: Physicochemical interactions in foods. Am. J. Clin. Nutr. 1995, 62, 170S–177S. [Google Scholar] [CrossRef]
- Therdthai, N.; Zhou, W. Characterization of microwave vacuum drying and hot air drying of mint leaves (Mentha cordifolia Opiz ex Fresen). J. Food Eng. 2009, 91, 482–489. [Google Scholar] [CrossRef]
- Antigo, J.L.D.; Bergamasco, R.D.C.; Madrona, G.S. Effect of ph on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. 2017, 38, 72–77. [Google Scholar] [CrossRef]
- Kerr, W.L.; Varner, A. Chemical and physical properties of vacuum-dried red beetroot (Beta vulgaris) powders compared to other drying methods. Dry. Technol. 2020, 38, 1165–1174. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.J.; Camacho, M.D.M.; Martínez-Navarrete, N. The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree. Foods 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.; Barroca, M.J. Effect of drying treatments on texture and color of vegetables (pumpkin and green pepper). Food Bioprod. Process. 2012, 90, 58–63. [Google Scholar] [CrossRef]
- Valdenegro, M.; Almonacid, S.; Henríquez, C.; Lutz, M.; Fuentes, L.; Simpson, R. The effects of drying processes on organoleptic characteristics and the health quality of food ingredients obtained from goldenberry fruits (Physalis peruviana). Open Access Sci. Rep. 2013, 2, 1–7. [Google Scholar]
- Sagrin, M.S.; Chong, G. Effects of drying temperature on the chemical and physical properties of Musa acuminata Colla (AAA Group) leaves. Ind. Crop. Prod. 2013, 45, 430–434. [Google Scholar] [CrossRef]
- Carrillo, C.; Wilches-Pérez, D.; Hallmann, E.; Kazimierczak, R.; Rembiałkowska, E. Organic versus conventional beetroot. Bioactive compounds and antioxidant properties. LWT 2019, 116, 108552. [Google Scholar] [CrossRef]
- Seremet, L.; Nistor, O.V.; Andronoiu, D.G.; Mocanu, G.D.; Barbu, V.V.; Maidan, A.; Rudi, L.; Botez, E. Development of several hybrid drying methods used to obtain red beetroot powder. Food Chem. 2020, 310, 125637. [Google Scholar] [CrossRef]
- Șeremet, L.; Nistor, O.V.; Andronoiu, D.G.; Rudi, L.; Botez, E. Influence of different drying methods on the physicochemical properties of red beetroot (Beta vulgaris L. var. Cylindra). Food Chem. 2017, 236, 59–67. [Google Scholar] [CrossRef]
- Raharitsifa, N.; Ratti, C. Foam-mat freeze-drying of apple juice part 2: Stability of dry products during storage. J. Food Process. Eng. 2010, 33, 341–364. [Google Scholar] [CrossRef]
- Getahun, E.; Gabbiye, N.; Delele, M.A.; Fanta, S.W.; Gebrehiwot, M.G.; Vanierschot, M. Effect of maturity on the moisture sorption isotherm of chili pepper (Mareko Fana variety). Heliyon 2020, 6, e04608. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.L.; Romanelli, P.; Barboza, S.; Gabas, A.; Romero, J.T. Sorption isotherms of alligator’s meat (Caiman crocodilus yacare). J. Food Eng. 2002, 52, 201–206. [Google Scholar] [CrossRef]
- Rohvein, C.; Santalla, E.; Gely, M.C. Note: Estimation of sorption isotherm and the heat of sorption of quinoa (Chenopodium quinoa Willd.) seeds. Food Sci. Technol. Int. 2004, 10, 409–413. [Google Scholar] [CrossRef]
- Lewicki, P. The applicability of the GAB model to food water sorption isotherms. Int. J. Food Sci. Technol. 1997, 32, 553–557. [Google Scholar] [CrossRef]
- Stępień, A.; Witczak, M.; Witczak, T. Moisture sorption characteristics of food powders containing freeze dried avocado, maltodextrin and inulin. Int. J. Biol. Macromol. 2020, 149, 256–261. [Google Scholar] [CrossRef]
- Giovanelli, G.; Zanoni, B.; Lavelli, V.; Nani, R. Water sorption, drying and antioxidant properties of dried tomato products. J. Food Eng. 2002, 52, 135–141. [Google Scholar] [CrossRef]
- Kiranoudis, C.; Maroulis, Z.; Tsami, E.; Marinos-Kouris, D. Equilibrium moisture content and heat of desorption of some vegetables. J. Food Eng. 1993, 20, 55–74. [Google Scholar] [CrossRef]
- Yanniotis, S.; Blahovec, J. Model analysis of sorption isotherms. LWT 2009, 42, 1688–1695. [Google Scholar] [CrossRef]
- Gruyaert, E.; Dieleman, C.; De Belie, N. Influence of sample preparation and the addition of BFS on water vapour sorption isotherms. In Proceedings of the International Conference on Durability of Building Materials and Components, Porto, Portugal, 12–15 April 2011; pp. 1–8. [Google Scholar]
| - | Tomato Soup (Cream) | Beetroot Soup | Pumpkin Soup (Cream) | Cucumber Soup |
|---|---|---|---|---|
| Main ingredients: | - | - | - | - |
| - | Tomato pulp, tomato paste and diced tomato (64%) water, a mixture of chopped vegetables, rapeseed oil | Water, red beetroot (31%), string and red beans, a mixture of chopped vegetables, rapeseed oil | Water, pumpkin (35%), a mixture of chopped vegetables, rapeseed oil | Water, pickled cucumbers (21%), a mixture of chopped vegetables, rapeseed oil |
| Concentration of solid constituents (g/100 g): | ||||
| Carbohydrates | 3.7 | 7.8 | 3.7 | 2.3 |
| -including sugars | 3.6 | 4.3 | 3.1 | 0.9 |
| Fiber | 1.6 | 1.2 | <0.5 | 0.9 |
| Protein | 1.1 | 0.7 | 0.7 | 0.5 |
| Fat | 1.9 | <0.5 | 1.5 | <0.5 |
| Salt | 0.63 | 0.89 | 0.61 | 1 |
| Attributes | Definition | Scale-Boundary Conditions |
|---|---|---|
| Color | Color typical for the main vegetable ingredient of a selected soup: tomato (red), pumpkin (yellow), beetroot (red-violet), pickled cucumber (green) | 1-very bright color 10-dark, deep color |
| Consistency | Consistency from thin, watery, to thick | 1-thin 10-thick |
| Taste | Taste typical for the main ingredient of the soup | 1-no perceptible flavor 10-intensive perceptible flavor |
| Aroma | Aroma typical of the main vegetable ingredient of the soup | 1-no perceptible aroma 10-Intensive perceptible aroma |
| Overall quality | Overall evaluation | 1-low 10-high |
| Concentration of Main Solid Compounds g/100 g | Tomato Soup | Beetroot Soup | Pumpkin Soup | Cucumber Soup |
|---|---|---|---|---|
| Sugars | 33.6 ± 3.3 | 40.6 ± 1.5 | 44.5 ± 1.9 | 13.9 ± 0.5 |
| Salt | 5.7 ± 0.6 | 8.4 ± 0.3 | 8.8 ± 0.4 | 15.4 ± 0.6 |
| Fiber | 8.4 ± 0.8 | 8.5 ± 0.3 | - | 13.9 ± 0.5 |
| Kind of Soup | Product | Water Content (%) | Water Activity |
|---|---|---|---|
| Tomato soup | fresh | 89.5 ± 0.4 a* | 0.976 ± 0.001 a |
| dried | 2.0 ± 0.2 c | 0.206 ± 0.006 c | |
| stored | 3.6 ± 0.2 b | 0.268 ± 0.051 b | |
| Beetroot soup | fresh | 89.7 ± 0.2 a | 0.980 ± 0.002 a |
| dried | 2.6± 0.1 c | 0.221 ± 0.002 c | |
| stored | 4.1± 0.4 b | 0.286 ± 0.011 b | |
| Pumpkin soup | fresh | 93.2 ± 0.1 a | 0.980 ± 0.001 a |
| dried | 2.3± 0.1 c | 0.197 ± 0.001 c | |
| stored | 3.9 ± 0.3 b | 0.279 ± 0.009 b | |
| Cucumber soup | fresh | 93.7 ± 0.1 a | 0.973 ± 0.004 a |
| dried | 2.9 ± 0.1 c | 0.199 ± 0.011 c | |
| stored | 3.7 ± 0.3 b | 0.271 ± 0.021 b |
| Product | L* | +a* | +b* | Chroma C* | Hue h° | Total Color Change ΔE*ab |
|---|---|---|---|---|---|---|
| Tomato soup | ||||||
| fresh | 44.67 ± 0.08 c* | 33.98 ± 0.40 a | 59.84 ± 0.24 a | 68.81 ± 0.40 a | 60.41 ± 0.20 a | - |
| dried | 52.09 ± 0.05 b | 32.46 ± 0.20 b | 54.40 ± 0.12 b | 63.35 ± 0.20 b | 59.17 ± 0.10 b | 9.32 ± 0.08 b |
| stored | 60.05 ± 0.95 a | 28.74 ± 0.91 c | 45.64 ± 0.93 c | 53.93 ± 1.27 c | 57.90 ± 0.32 c | 21.64 ± 0.35 a |
| Beetroot soup | ||||||
| fresh | 23.05 ± 0.04 b | 39.64 ± 0.60 a | 10.72 ± 0.40 b | 41.06 ± 0.63 a | 15.25 ± 0.35 c | - |
| dried | 55.07 ± 0.02 a | 21.91 ± 0.30 b | 13.97 ± 0.30 a | 25.98 ± 0.41 b | 32.65 ± 0.30 a | 36.74 ± 0.11 a |
| stored | 54.79 ± 0.75 a | 21.05 ± 0.82 b | 10.05 ± 2.83 b | 22.42 ± 2.11 b | 25.55 ± 5.45 b | 33.34 ± 1.40 a |
| Pumpkin soup | ||||||
| fresh | 56.13 ± 0.03 c | 11.13 ± 0.20 a | 64.66 ± 0.11 a | 65.61 ± 0.16 a | 80.29 ± 0.20 c | - |
| dried | 62.53 ± 0.03 b | 6.92 ± 0.05 b | 60.05 ± 0.70 b | 60.45 ± 0.70 b | 83.50 ± 0.09 b | 8.94 ± 0.23 b |
| stored | 68.04 ± 0.61 a | 4.04 ± 0.08 c | 45.21 ± 0.25 c | 45.39 ± 0.25 c | 84.92 ± 0.10 a | 23.88 ± 0.10 a |
| Cucumber soup | ||||||
| fresh | 56.38 ± 0.07 c | 6.01 ± 0.02 a | 44.53 ± 0.70 b | 44.94 ± 0.63 b | 82.50 ± 0.10 c | - |
| dried | 66.03 ± 0.03 b | 2.66 ± 0.04 b | 49.05 ± 0.12 a | 49.12 ± 0.12 a | 86.90 ± 0.07 b | 11.18 ± 0.39 b |
| stored | 72.08 ± 0.82 a | 1.01 ± 0.11c | 35.04 ± 0.81 c | 35.05 ± 0.81 c | 88.45± 0.20 a | 19.01 ± 0.54 a |
| Product | pH | Content of Polyphenolic Compounds (TPC) (mg GAE/100 g d.m.) | EC 50 DPPH• (mg/mL) |
|---|---|---|---|
| Tomato soup | |||
| fresh | 3.95 ± 0.14 a* | 763 ± 15 a | 2.08 ± 0.14 b |
| dried | 4.17 ± 0.08 a | 543 ± 22 b | 2.38 ± 0.09 a |
| stored | 4.05 ± 0.11 a | 401 ± 29 c | - |
| Beetroot soup | |||
| fresh | 4.74 ± 0.08 a | 595 ± 49 a | 1.26± 0.08 a |
| dried | 4.85 ± 0.10 a | 537 ± 57 a | 1.24 ± 0.09 a |
| stored | 4.80 ± 0.12 a | 521 ± 47 a | - |
| Pumpkin soup | |||
| fresh | 5.59 ± 0.09 a | 556± 42 a | 1.15 ± 0.03 b |
| dried | 5.65 ± 0.07 a | 501 ± 47 ab | 1.26 ± 0.04 a |
| stored | 5.61 ± 0.09 a | 451 ± 47 b | - |
| Cucumber soup | |||
| fresh | 3.89 ± 0.03 b | 569± 50 a | 2.26 ± 0.09 b |
| dried | 4.20 ± 0.07 a | 367 ± 55 b | 3.19 ± 0.04 a |
| stored | 4.02 ± 0.11 ab | 311 ± 41b | - |
| Product | Color | Consistency | Taste | Aroma | Overall Quality |
|---|---|---|---|---|---|
| Tomato soup | 36 ± 3 | 5 ± 1 | 18 ± 2 | 24 ± 2 | 26 ± 3 |
| Beetroot soup | 42 ± 5 | 0 ± 0 | 9 ± 1 | 11 ± 1 | 16 ± 2 |
| Pumpkin soup | 31 ± 2 | 0 ± 0 | 11 ± 1 | 21 ± 2 | 20 ± 1 |
| Cucumber | 35 ± 3 | 4 ± 1 | 1 ± 0 | 17 ± 1 | 18 ± 2 |
| Model | Parameter | Tomato | Beetroot | Pumpkin | Cucumber |
|---|---|---|---|---|---|
| GAB 1 | um | 24.66 | 65.42 | 69.11 | 107.09 |
| C | 0.33 | 0.2 | 0.17 | 0.06 | |
| k | 0.92 | 0.84 | 0.83 | 0.97 | |
| R2 | 0.998 | 0.978 | 0.998 | 0.97 | |
| RMS | 14.11 | 20.05 | 15.24 | 47.35 | |
| P | 8.52 | 14.57 | 9.77 | 37.65 | |
| BET 2 | um | 11.12 | 8.88 | 18.02 | 68.37 |
| c | 0.8 | 2.03 | 0.53 | 0.06 | |
| R2 | 0.996 | 0.96 | 0.992 | 0.972 | |
| RMS | 15.22 | 16.59 | 14.78 | 80.49 | |
| P | 9.77 | 11.78 | 8.49 | 56.34 | |
| Lewicki 1 | F | 3.13 | 16.73 | 13.29 | 5.23 |
| G | 1.59 | 0.89 | 0.96 | 1.78 | |
| H | −35.59 | 4.19 | 3.97 | 6.21 | |
| R2 | 0.966 | 0.989 | 0.998 | 0.966 | |
| RMS | 48.08 | 18.85 | 14.01 | 35.21 | |
| P | 29.9 | 12.96 | 7.6 | 25.7 | |
| Peleg 1 | A | 108.81 | 120.44 | 109.87 | 325.43 |
| B | 19.12 | 5.39 | 17.86 | 2.16 | |
| D | 5.87 | 3.82 | 4.67 | 5.91 | |
| E | 1.29 | 0.26 | 1.13 | 0.04 | |
| R2 | 0.999 | 0.976 | 0.999 | 0.989 | |
| RMS | 13.16 | 13.14 | 11.89 | 21.31 | |
| P | 7.63 | 9.16 | 6.44 | 16.5 | |
| Hasley 1 | g | 4.12 | 5.9 | 5.48 | 3 |
| h | 0.77 | 0.81 | 0.81 | 0.58 | |
| R2 | 0.995 | 0.969 | 0.991 | 0.962 | |
| RMS | 25.51 | 23.32 | 42.68 | 35.69 | |
| P | 15.21 | 17.92 | 23.78 | 29.28 |
| Parameter | Beetroot Soup | Cucumber Soup |
|---|---|---|
| X4 | 5.21 (X4 > 0.1) | 3.61 (X4 > 0.1) |
| D10 | 0.75 (positive) | 1.47 (positive) |
| Rfi | −0.16 (negative) | −0.27 (negative) |
| awm | 0.28 (0.1) | 0.30 (0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, E.; Jaskulska, A. The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups. Appl. Sci. 2021, 11, 654. https://doi.org/10.3390/app11020654
Jakubczyk E, Jaskulska A. The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups. Applied Sciences. 2021; 11(2):654. https://doi.org/10.3390/app11020654
Chicago/Turabian StyleJakubczyk, Ewa, and Aleksandra Jaskulska. 2021. "The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups" Applied Sciences 11, no. 2: 654. https://doi.org/10.3390/app11020654
APA StyleJakubczyk, E., & Jaskulska, A. (2021). The Effect of Freeze-Drying on the Properties of Polish Vegetable Soups. Applied Sciences, 11(2), 654. https://doi.org/10.3390/app11020654





