1. Introduction
Cancer treatment costs account for a substantial part of health care expenditure [
1]. The costs have increased due to advancements in novel drugs and therapy innovations, such as genomic testing, in the last decade [
2]. It is difficult to compare results from different countries directly as the absolute cost of cancer treatment is influenced by each country’s socioeconomic environment, health care system and income level [
3]. Still, administrative health data allows exploration by type of service use and comparison of reimbursement policies between countries. In addition to policy-making at the national level, the economic aspects of the disease could also affect individual treatment decisions as well as duration of treatment.
Lung cancer, one of the most common cancers worldwide, is diagnosed frequently in advanced stage and in older patients with comorbidities [
4]. In a large population-based cost analysis across the EU, the economic cost varied by cancer type, with lung cancer having the greatest overall economic burden (€18.8 billion, 15% of overall cancer costs), followed by breast cancer (12%), colorectal cancer (10%) and prostate cancer (7%) [
5]. With regard to the type of cost, inpatient care was the major component in lung cancer (€2.87 billion, 68%) and colorectal cancer (€4.04 billion, 73%), whereas drugs were the major component in breast cancer (46%) and prostate cancer (57%) across the EU. Substantial differences were observed between EU countries in absolute costs per citizen, and the costs varied by type of health care service.
The mortality of lung cancer is high, accounting for nearly 20% of all cancer deaths in 2018 globally [
4]. In addition to tumour type, total cancer-related costs vary by stage, with the highest costs incurred in the final year of life. In a retrospective cohort study, Jeon et al. evaluated direct medical expenditures in patients with lung cancer in South Korea [
3]. Surgically treated patients had the highest absolute expenditure (
$36,013) due to the high cost in the initial phases of the disease, but the monthly average expenditure was the lowest. In advanced lung cancer, monthly medical expenditure was highest for the last three months of life, with anti-cancer treatment being the greatest cost driver in this period. In the United States, cancer end-of-life (EOL) costs are burdensome, the 5–6% of Medicare beneficiaries who died consumed 27–30% of the annual cancer payments and 78% of costs accrued from acute care in the final 30 days of life [
6,
7]. Mrad et al. demonstrated that aggressive care, as well as hospitalization costs due to intensive care unit (ICU) admissions, chemotherapy and radiotherapy at EOL, has steadily increased from 1998 to 2014 in the United States [
8]. Nearly half of new lung cancer cases are in advanced stage, and approximately half of patients will develop distant metastases after treatment for local disease. Systemic anti-cancer treatment (SACT) is the primary treatment option for these patients. Platinum-based combination chemotherapy has been cornerstone in lung cancer for decades. Recent developments include immunotherapy and targeted treatment in carriers of specific genetic alterations, particularly in EGFR and ALK gene.
In Estonia, approximately 800 people annually are newly diagnosed with lung cancer [
9]. The five-year overall survival (OS) of lung cancer is estimated at 10–15% worldwide [
10]. The poor prognosis means that there is an ultimate need for EOL care services. Estonia has universal insurance coverage and oncology care is delivered only by the national health care system. In this study, we aimed to characterize the patterns of EOL care and use of health care resources in the final 100 and 30 days of life in patients with advanced stage lung cancer based on data from the North Estonia Medical Centre’s Thoracic Oncology Database. Understanding current state of palliative care is fundamental to improve organization of support at the final weeks of life, both for patients and caregivers.
2. Materials and Methods
2.1. Patients and Data Sources
This is a retrospective analysis of the health care data of lung cancer patients diagnosed with advanced disease at North Estonia Medical Centre between 1 January 2015 and 31 December 2017. North Estonia Medical Centre is the single provider of all types of oncology services for a population of 800,000 and its Thoracic Oncology Database has covered all lung cancer patients since 2015. During the study period, a multidisciplinary tumour board had confirmed the treatment decisions of 1485 lung cancer patients.
To identify the impact of palliative SACT, we excluded lung cancer patients with local disease whose primary treatment was either surgery or radiotherapy, irrespective of whether this was combined with SACT. Patients with local disease receive curative treatment. We included patients with advanced disease, defined as patients with distant metastases or locally advanced in chest not amenable to curative treatment. The final cohort contained 778 patients, of which 489 received SACT (SACT group) and 289 did not receive SACT (no-SACT group). The study was approved by the Tallinn Ethics Committee for Medical Research (No. 1972). Patient characteristics such as age, sex and date of treatment decision were extracted from the Thoracic Oncology Database. The patient’s national identification code from the Thoracic Oncology Database was linked to the electronic database of billing data of the Estonian Health Insurance Fund. This database incorporates detailed data on all medical services used during a hospital stay and any outpatient visits, including each cycle of SACT provided. Data on the death of patients was retrieved from the National Death Registry. The data cut-off date was 31 July 2018.
2.2. Use of Health Care Services and Resources
We calculated the use of all health care services and their cost in lung cancer patients who received or did not receive SACT and created a composite measure of cumulative resource use comprised from the following: outpatient care (including general practitioner and specialist care visit), emergency department visit, inpatient care, admission to ICU, nursing care (including nurse home visit and nursing home stay) and prescriptions. We then aimed to study the differences in the use of health care resources during the last 100 and 30 days of life. Deceased SACT patients were divided into three groups: patients who received SACT within 30 last days of life—end-of-life (EOL) SACT group; patients who received their last SACT more than 100 days before death (>100 SACT group); and patients who received their last SACT within 31 to 100 days prior to death (31–100 SACT). We hypothesized that the >100 SACT group receives care similar to the no-SACT patients, whereas the EOL-SACT group with treatment-related complications and/or rapidly progressing disease receive more intensive care.
2.3. Intensity of Health Care
As prices of health care services vary greatly from country to country, the presentation of price-based costs is not comparable. We created cost-based weights, weights are more accurate measures of relative resource intensity. It provides a way of comparing and valuing various health care services between countries. We first retrieved actual average daily cost per service from the Estonian Health Fund and rounded the actual cost to zero (e.g., to the closest 50 or 100 euros). To capture the relative resource intensity, we assigned a specific cost-based weight (50 euros equals 1 weight) to each category of health service. All actual health costs per capita were recalculated using the allocated weight, and the sum of the weights was expressed as an intensity of care index. To compensate for the differences in survival times between SACT and no-SACT patients, the number of months lived with the diagnoses was obtained for each patient, the total number of person-months was calculated for a study arm and, finally, health service use and costs were expressed per person-month.
2.4. Statistical Analysis
To describe the baseline clinical data of the study population and health care use at EOL, frequencies and percentages were used for categorical data and mean values with standard deviations (SD) or median values with quartiles (Q25–Q75) for numeric data. To compare the characteristics of SACT and no-SACT patients, the Mann–Whitney U test (numeric variables), Fisher test or z-test (categorical variables) was used. Patient survival was calculated from the date of multidisciplinary tumour board to the end of life (or to the end of follow-up on 31 July 2018). Difference in survival between SACT and no-SACT groups were compared with univariate Cox regression, results are presented as hazard ratios (HR) and 95% confidence interval. All significance tests were two sided with an α-level of 0.05. All analyses were conducted using Stata 14.2 software.
3. Results
3.1. Study Population
Of the 778 patients with advanced lung cancer, 489 were assigned to receive SACT and 289 patients not eligible to SACT were classified as the no-SACT group; two patients with no insurance were excluded (
Table 1). There were no statistically significant differences in sex and cancer stage distribution between the two groups—28% of SACT and no-SACT patients had stage III and 72% had stage IV lung cancer. The patients in the no-SACT group were older and had more cardiac comorbidities, whereas the patients in the SACT group had more treatment-related complications [
11]. The median overall survival of no-SACT patients was 1.3 months; in patients who received at least one cycle of SACT, 9.1 months (HR = 4.23; 95%CI 3.6–5.0), with a median time from the last cycle of SACT until death of 75 days (Q25–Q75 38–141).
3.2. Use of Health Care Services and Resources
For all health care services, the percentage of patients who used the respective service was much lower for the no-SACT than the SACT arm (
Table 2). A similar trend was seen when the frequency of health care service use was calculated per length of follow-up. Twenty-six (9%) patients in the no-SACT arm had at least one ED visit compared with 337 patients (69%) in the SACT arm. The average cost of an ED visit leading to a hospital stay was higher in the no-SACT arm (
Table 3), likely due to poor baseline health and chronic concomitant diseases demanding more investigations. On average, patients in the SACT arm had one general practitioner visit (0.95 visit per month) and one specialist doctor visit (0.97) per month. In addition to median survival and the length of follow-up being shorter in the no-SACT arm, there was less medical contact during the time lived with diagnoses (general practitioner visit 0.21 vs. 0.95 visit, specialist doctor visit 0.17 vs. 0.9 visit, home nurse visit 0.05 vs. 0.22 visit, hospital inpatient 0.15 vs. 0.97 days per patient month).
Median cost per person-month was EUR 215 in the no-SACT arm and EUR 992 in the SACT arm (
Table 3). In both study arms, the largest proportion of costs was related to inpatient stay. The average cost per person-month in the no-SACT arm was higher for intensive care (297 vs. 118 euros) and inpatient stays (250 vs. 157 euros), likely due to poor baseline health and initial non-cancer treatment. Approximately one-third of all costs were related to the administration of SACT in the respective group. Costs of specialist doctor visits were higher in the SACT arm (241 vs. 111 euros), likely due to diagnostic procedures for regular disease assessment. Seventy-eight per cent of no-SACT patients had at least one prescription compared with 98 percent of SACT patients, although the median number of prescriptions per person-month did not differ significantly.
3.3. Emergency Department Visits
78% (N = 384, median 2 visits per patient) of SACT patients and 34% (N = 108, median 1 visit per patient) of no-SACT patients had at least one ED visit since diagnosis of lung cancer, including the visit leading to the diagnosis. For SACT patients, the most common diagnoses in the ED after lung cancer (52% patients) were pneumonia (11%), severe infection and sepsis (3%) and pleural effusion (3%). No-SACT patients visited the ED mainly due to lung cancer (48%), atrial fibrillation (4%) and constipation, abdominal pain (3%). In both study arms, approximately 10% of patients died on the same day as or day following the ED visit.
In total, 384 SACT patients had 885 ED visits, nearly half were managed as an outpatient ED visit and half led to hospitalization. Among SACT patients who were hospitalized from the ED, 27% had a brain scan and 57% had any other CT scan performed, whereas SACT patients managed as outpatients, 13% and 29%, respectively, had a brain scan and any other CT scan performed. For all other health care activities, such as laboratory tests, ECG and chest X-ray, no difference was seen between hospitalized and outpatient ED claims.
3.4. Place of Death
By the study cut-off date, 77% of SACT patients (
N = 376) had died compared with 97% of no-SACT patients (
N = 278). Among the patients who died during the study period, only 15% of patients in the no-SACT group died in an acute care hospital and 7% in a nursing hospital, whereas 38% of the SACT group deaths occurred in an acute care hospital and 24% in a nursing hospital (
Table 4). The majority of deaths in an acute care hospital occurred after the patients had been admitted via the ED.
Among deceased SACT patients, 72 patients received SACT within 30 last days of life (EOL-SACT group), 162 patients more than 100 days before death (>100 SACT) and 142 patients received their last SACT within 31 to 100 days prior to death (31–100 SACT).
The majority of no-SACT patients (78%) died out-of-hospital, whereas the majority of deaths in the SACT group (62%) occurred under medical supervision in an acute care or nursing hospital. The probability of dying in a hospital setting was highest in the EOL-SACT group and decreased in parallel with the time elapsed from the last SACT administered. We hypothesized that the SACT > 100 group received care similar to that of no-SACT patients, but more no-SACT patients died out-of-hospital and less in nursing hospitals compared with the SACT > 100 group.
3.5. Intensity of Health Care in Relation to Time before Death
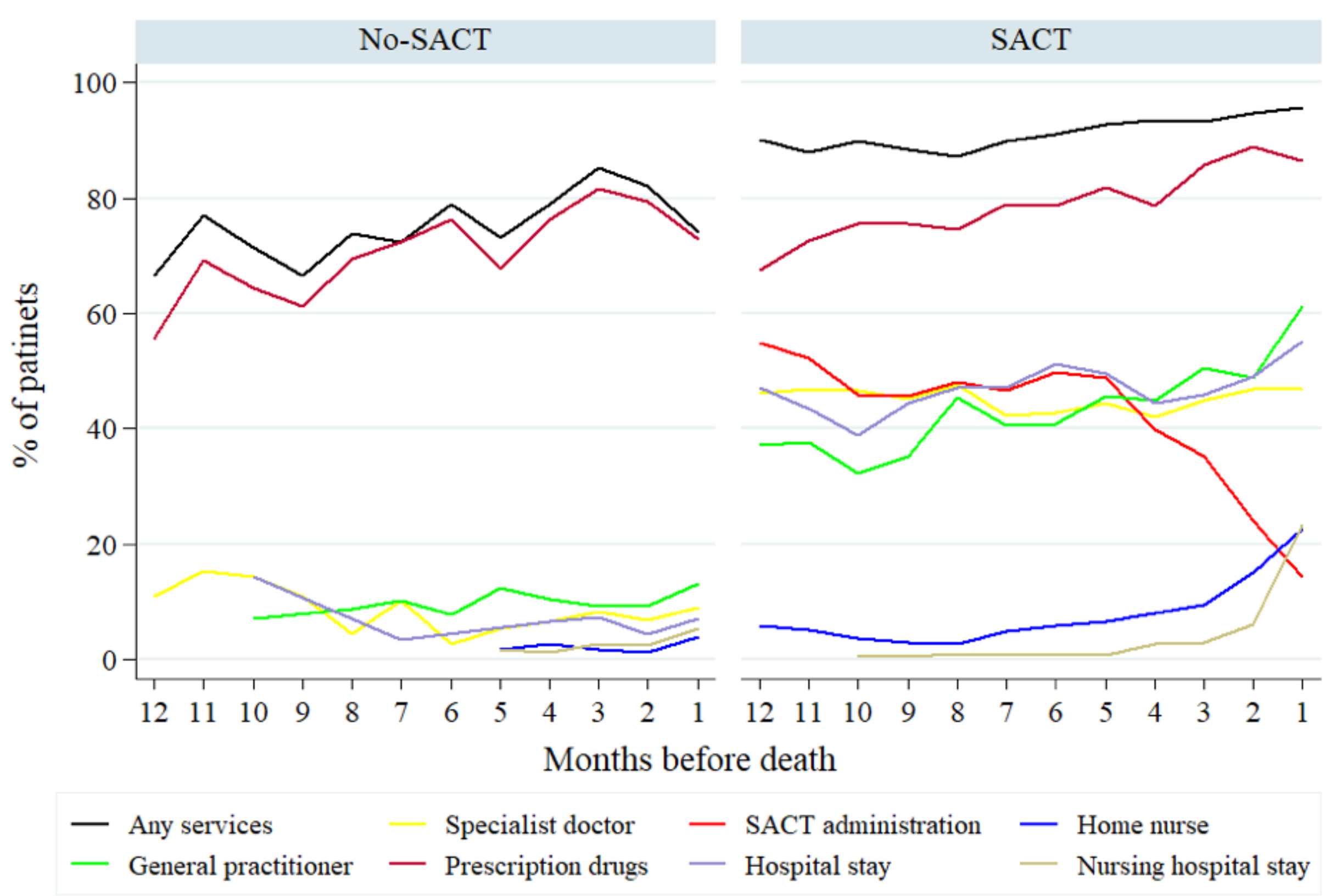
The type of health care services and related costs were calculated for each month lived with the diagnosis until death (
Figure 1). In the no-SACT arm, the vast majority of patients received only prescription drugs, regardless of time lived. Over time, there was no significant change in the users of all services combined. In the SACT arm, the number of patients with home nursing, a nursing hospital stay and prescription drugs increased near death, whereas the use of SACT dropped significantly five months before death. Still, the proportion of patients who received nursing services remained below 20% even in the final month of life.
Actual average daily cost per service was retrieved from the Estonian Health Fund and rounded to the closest 50 or 100 (
Table 5). The proposed weights were obtained from the prices of health care services in our country and their interrelationship (
Table 5). This is the first presentation of such an index.
Using the allocated weight, all actual health costs per capita were recalculated and the sum of the weights expressed as intensity of care index (
Table 6). Overall, the no-SACT patients received considerably less medical attention compared with the SACT patients, and large differences were seen in the type of service received by the study groups.
4. Discussion
Advanced stage lung cancer inevitably leads to death. Up to 60% of patients develop brain metastases, 40% develop bone metastases and nearly all have breathing difficulties, making early palliative and supportive care particularly important [
12]. Timely initiation of palliative care paves the way for planned hospice care and improved quality of life at EOL [
13,
14]. This study focused on health care services reimbursed by the national insurer in Estonia in patients with advanced lung cancer. Use of the resources was analysed in relation to time before death in patients who received SACT compared with those who were ineligible for SACT, e.g., amenable to supportive care only.
An international study of place of death reported that in most of the 40 populations studied covering all deaths, the median percentage of all deaths occurring in hospitals was 54%, in residential homes 18% and other locations 28% [
15]. In Estonia, the respective figures for 2010 were 54%, 11% and 35%, i.e., very close to the international average. In the current study, the available data did not allow to discriminate between residential and private homes, and the category out-of-hospital includes both residential care and private home deaths. We found that 62% of deaths in the SACT group occurred in a hospital, close to the average of 55% of all deaths in Estonia (
Table 4). Only 22% of deaths in the no-SACT group occurred in a hospital setting. This can be interpreted as less medical attention being given to lung cancer patients who are ineligible for SACT and other modalities of active oncological care. Patients who died early after SACT (EOL-SACT) likely died in an acute care hospital due to treatment related complications and/or rapidly progressing disease. The likelihood of dying out-of-hospital increased as the time from the last SACT until death was prolonged. Still, we observed a substantial difference in the place of death between the no-SACT arm and patients who died >100 days after the last SACT. Hence, EOL care is affected by the receipt of previous active oncology treatment.
Inpatient care accounted for the largest proportion of health care services used in our study. Nearly all patients in the SACT group had at least one inpatient hospital stay, whereas only 11% of patients in the no-SACT arm were hospitalized during the course of their disease (
Table 1). Our results are in line with other similar studies across the world. In Japan, most EOL medical costs in lung cancer patients were inpatient costs, and EOL costs surpassed those for initial treatment [
16]. Despite lower utilization in the US, inpatient hospitalization was still the main cost driver both in the US and Canada [
17]. Among EU countries, inpatient care costs varied substantially from 30% in Slovakia to 67% in Ireland—accounting for an average of 56% of cancer-related health care costs for all cancer types combined [
5]. Our study is unique in regard to the EOL data analysis depending on patients’ eligibility for active oncology treatment.
Next, we analysed the use of health care services by month before death. For all service types and for all months, the percentage of patients who used health care service was lower for the no-SACT arm than the SACT arm. Administration of SACT dropped as of the fifth month before death but was still nearly 20% during the last month of life. General practitioner visits increased from 40% in patients using the service in the 12th month before death to 60% in SACT patients in the last month but remained below 20% in no-SACT patients regardless of time before death. The percentage of patients with inpatient care did not change significantly over time during the last year of life. In contrast, in the recent US study, patients receiving acute inpatient care increased from 12.2% in the sixth month before death to 43.8% in the last month [
6]. Similarly, the percentage of patients who received hospice care increased from 0.7% in the sixth month to 35.6% in the last month. In the current study, although the average cost per person-month for nursing care was higher in the no-SACT arm, the frequency of service use per person-month was much lower than in the SACT arm (
Table 2 and
Table 3). Approximately 20% of patients in the SACT arm, compared with only 7% in the no-SACT arm, received nursing care in the final month of life in our study. This clearly demonstrates a deficiency in the organization of hospice care.
In developed countries, approximately one-third of lung cancer cases are diagnosed via an ED visit [
18,
19]. Unplanned ED visits are related to significant clinical and economic burden due to the need for out-of-hours radiologist and specialist care services. In the recent UK study, lung cancer patients diagnosed during ED admission incurred greater costs during the first month and had a worse prognosis compared with outpatient diagnoses [
18,
19]. Although we were not able to discern whether the initial diagnosis was made via ED, the higher than average costs per person-month for intensive care and hospital bed stays in the no-SACT arm indicate that our results are similar to the UK. To exclude costs related to the initial lung cancer diagnosis, costs per person-month were calculated from the day after the multidisciplinary tumour board until death. Our ED claims contained data on whether patients were discharged or admitted to the hospital. Patients may have a CT scan performed during ED admission, but procedures leading to the histology and treatment decision are generally carried out in an outpatient setting. Both the significant proportion of patients who died in acute care hospital admitted via ED and the high frequency of ED service use indicate a need for better organization of out-of-hours oncology care and hospice care covered by the health insurance fund.
In our study, costs related to the administration of SACT accounted for 30% of total health care costs in the SACT arm. Across the EU, drug expenditure accounted for more than €13.5 billion, i.e., 27% of cancer-related health care costs in 2013, for all cancer types combined [
5]. Drug expenditure as a proportion of overall cancer-related health care costs was lowest in Lithuania (15%) and highest in Cyprus (61%). At the time of the study, immunotherapy and targeted treatment, except first generation EGFR tyrosine kinase inhibitors, were not reimbursed in Estonia. In Estonia, total health expenditure on cancer was substantially below the European average in 2014 [
20]. Expenditure on anti-cancer drugs remained constant during 2010–2014, and Estonia was in the group of countries with the lowest spending (less than 99 million euros) on anti-cancer drugs.
The strength of our study is that we analysed resource use at EOL in advanced lung cancer patients in terms of previous active oncology care and in relation to time before death. This study has some limitations. It was a single centre retrospective study. Our results capture all health care services provided, since Estonia has universal insurance coverage. We were not able to analyze care out-of-pocket costs covered by patient or their family members. The study included patients diagnosed with advanced lung cancer from 2015 to 2017 with a data cut off at 31 July 2018. The current landscape of advanced lung cancer treatment has changed. This study was conducted to establish a benchmark for lung cancer costs prior to the wider availability of immunotherapy. In our ongoing work, we aim to analyse costs in patients who receive immunotherapy and targeted treatment and compare costs related to conventional chemotherapy.