Abstract
Potato starch was extruded and roasted with apple distillery wastewater to produce starch esters substituted with malic acid residues. The starch esterification degree was higher at the higher roasting temperatures. Starch modification contributed to its darker color, its increased resistance to the action of amylolytic enzymes, and its decreased solubility and heat of phase transition. The changes in the other starch properties examined depended on the extrusion and roasting temperatures. The process, which was conducted without a chemical agent—in this case, the process of starch extrusion and roasting with apple distillery wastewater—should be deemed a novel method for resistant starch production.
1. Introduction
Resistant starch is a starch fraction that is not digested in the human digestive tract. Being a prebiotic, it is a source of carbon to symbiotic bacteria that naturally colonize the large intestine [1]. The proper development of these bacteria increases the synthesis of short chain fatty acids, whose presence prevents the formation of colon cancer. In addition, resistant starch stabilizes the insulin level, while it reduces the blood levels of glucose and triglycerides and also the energy value of foods [2]. Due to its health-promoting properties, there is a need to increase its content in food. This can be achieved by, e.g., its addition to commonly consumed foods, such as bread and pasta. Resistant starch preparations are obtained through various modifications, including: genetic (high-amylose varieties), physical (e.g., annealing at elevated humidity and temperature, freezing, extruding), physicochemical (complexation with lipids), and chemical (e.g., esterification, roasting of starch saturated with iron ions, roasting with glycine) [3,4]. The chemical modifications applied in the food production process are those that involve chemical reactions that require the use of specific chemical substances and occur without a biological agent [5]. The low-substituted modified preparations obtained in this way are used as food additives and are increasingly not accepted by consumers. However, it should be remembered that, during food production processes, the native starch of plant materials enters into contact with other substances and—under favorable conditions—may undergo natural chemical modifications under their influence. These compounds include organic acids that have a carboxyl group that can link with the hydroxyl group of starch. Organic acids are mainly found in fruits, and their contents vary widely and depend, among other things, on the species, variety, and degree of ripeness of the fruit; vegetation conditions; as well as storage time or conditions [6]. Most of the fruits contain mainly malic acid [7,8], which is the major acid in apples [9]. Ripe apples also have traces of citric and tartaric acids [10]; however, their acidity mainly depends on the metabolism of malic and citric acids [2,11]. There are numerous publications in the literature regarding the modification of starch with acids. However, they concern the so-called chemical modifications carried out with the use of modifying reagents. Researchers also describe the effect of various additives on the physicochemical properties of starch, with particular emphasis put on the impact of these additives on the rheological properties of prepared pastes. However, our faculty has recently undertaken research in the area of so-called “green chemistry”, in which the substrates naturally occurring in waste raw materials from the biotechnology industry were used for starch modification [12,13]. This work is part of the undertaken research direction.
The purpose of this study was to produce starch esters characterized by reduced susceptibility to amylolysis via potato starch extrusion and roasting with apple distillery wastewater, and to determine the effect of temperatures in regard to both of these processes on selected properties of the modified preparations.
2. Materials and Methods
2.1. Materials
The initial analytical material was Superior Standard potato starch produced by PEPEES Łomża in 2019 and apple concentrate produced by GOMAR Pińczów in 2019.
2.2. Production of Modified Starch Preparations
An apple concentrate (80°Bx) was diluted to 20°Bx and fermented for 30 days with Saccharomyces bayanus CH158 yeast. The resulting solution was cooked to remove ethanol and then concentrated to 50°Bx in an air-dryer at a temperature of 35 °C. The pH value of the apple distillery wastewater concentrate was adjusted to pH 3.5 using 10 M NaOH. The concentrate of apple distillery wastewater was applied onto potato starch in the amount of 30 g distillery dry matter per 100 g starch dry matter. The sample was then mixed and conditioned at a temperature of 20 °C for 24 h and afterward air-dried to a moisture content of 16 g/100 g. The mixture was extruded in a Brabender 20DN single-screw extruder at the following temperatures (in consecutive sequences): 60–70–80; 80–90–100; 100–110–120, or 120–130–140 °C. The extrusion process was performed with a nozzle 4 mm in diameter and a screw with a compression rate of 1:1. Feeder speed was 50 rpm and screw speed was 120 rpm. The obtained extrudates were roasted at temperatures of 80, 100, 120, or 140 °C for 3 h. Next, they were soaked in distilled water for 24 h and homogenized in a Thermomix multifunctional device (Vorvex). Then, ethyl alcohol was added in the amount ensuring final alcohol concentration at 65%. The sample was left to stand until a clear solution was obtained, which was then decanted from above the precipitate. Next, the starch was again poured with a 65% ethanol solution. The rinsing process was repeated 30 times. The purified preparations were air-dried at a temperature of 35 °C, ground in the Thermomix, and sieved through a screen with mesh size of 400 µm. The preparations produced acc. to the analogous procedure but without apple distillery wastewater were rinsed only once and served as the control sample.
2.3. Qualitative and Quantitative Analyses of Starch Esters with the High-Performance Liquid Chromatography (HPLC) Technique
The chromatographic analysis of organic acids was carried out in refined and de-esterified preparations [12]. Roasted extrudates were prepared for determinations through refining, which allows assuming that all free acids were removed from the sample. Twenty grams of the preparation was transferred to a conical flask containing 300 mL of distilled water. The suspension was brought to the boiling point and stirred until the preparation dissolved. After cooling, 1 L of rectified ethanol was added to the flask to allow for starch precipitation. After 24 h, the clear solution was decanted from above the precipitate. The refining procedure was repeated two more times, and the resulting preparation was air-dried at a temperature of 30 °C and ground. Next, it underwent base de-esterification. To this end, 2 g of the preparation (per dry matter basis) and 100 mL of 0.5 M NaOH were stirred at a temperature of 35 °C for 12 h. Then, 400 mL of rectified ethanol was added to a clear solution to allow for starch precipitation. The filtrate obtained was concentrated to the volume of 10 mL by evaporation on a BUCHI rotary evaporator.
The concentrates produced were analyzed for the content of organic acids with the HPLC liquid chromatography method using a Hewlett-Packard 1100 chromatograph (Hewlett Packard, Wilmington, DE, USA). The degree of substitution was expressed as the percentage content of acid residues in the preparations.
2.4. Swelling Power and Solubility of Starch Preparations in Water Having a Temperature of 80 °C
In brief, 200 mL of an aqueous suspension containing 1 g starch preparations or modified starch preparations per 100 g solution were prepared in a round-bottom flask. The flask was placed in a water bath with shaking at a temperature of 80 °C. It was kept under these conditions for 30 min until the water bath temperature had been obtained inside it. Afterward, the flask was cooled to a temperature of 20 °C, and water evaporated during heating was supplemented. Next, 50 g of the starch suspension was weighed into centrifuge tubes, which were then centrifuged in a Biofuge 28RS Heraeus Sepatech centrifuge at 14,500 rpm and 20 °C for 30 min. Next, the supernatant was decanted and its dry matter content was determined with the air-dry method at a temperature of 105 °C. The precipitate left in the tubes was weighed [14].
2.5. Determination of the Characteristics of Phase Transitions of Starch Preparations with Differential Scanning Calorimetry (DSC)
This determination was conducted using a DSC 822E differential scanning calorimeter (Mettler Toledo) [15]. Pre-tests were performed in a temperature range of 25–100 °C. Aluminum crucibles (100 μL) with lids were used for analyses. A 10-g starch sample (per starch dry matter) was weighed and placed on crucible’s bottom, then distilled water was added in the ratio of 3:1 respective to sample weight. The measuring crucible was covered with a lid, conditioned at 20 °C for 30 min. Next, it was transferred to an oven chamber having a temperature of 25 °C and heated to a temperature of 100 °C at the heating rate of 4 °C/min.
2.6. Rheological Properties of Starch Pastes
Analyses were carried out using an RS 6000 oscillating-rotating viscosimeter (Haake, Germany) for starch suspensions contained 5 g starch preparations or modified starch preparations per 100 g solution that were heated at 96 °C for 30 min under continuous stirring [16,17,18]. The properties of the prepared pastes were determined based on the flow curves.
The flow curves were plotted for the pastes at a measurement temperature of 50 °C and a shear rate of 1–300 s−1. A hot paste was placed in a system of coaxial cylinders (Z38AL type) of an RS 6000 rheometer, then cooled and relaxed at the measurement temperature for 15 min. The flow curves plotted were described using the following equations:
Ostwald de Waele:
Casson:
where: τ—shear stress (Pa), K—consistency coefficient (Pa∙sn), —shear rate (s−1), n—flow index, τoc—yield point (Pa), ηc—Casson’s plastic viscosity (Pa∙s).
2.7. Color Determination of Starch Preparations
Color difference (darkening) ΔE was calculated from Hunter color scale values (L, a, b) and determined with a Konica Minolta CR–5 chronometer in reference to extruded starch [19]. Color difference was calculated with the following formula:
ΔE = (ΔL2 + Δa2 + Δb2)½
2.8. Resistance of Starch Preparations to the Action of Amyloglucosidase
A 0.36 g starch preparations or modified starch preparations per 100 g solution was prepared in a conical flask, which was then kept at a boiling temperature for 5 min. The suspension was then cooled, evaporated water was completed to the weight of 38 g, and 34 mL of an acetate buffer (pH 4.5) was added. The flask was placed in a water bath with a shaker at a temperature of 37 °C, and 4 mL of an amyloglucosidase solution was added (enzyme to acetate buffer ratio was at 1:100). After 20 min, and 1, 2, or 3 h (or till the maximal saccharification of the preparation), 1 mL of the hydrolysate was collected to a centrifuge tube and centrifuged at 5000 rpm for 5 min in an MPW 312 centrifuge. Then, 10 μL of the supernatant was collected from the centrifuge sample, transferred to a microcuvette containing 1 mL of BIOSYSTEM reagent, stirred, and incubated at a temperature of 20 °C for 15 min. Absorbance was measured at a wavelength of λ = 500 nm using a CECIL CE 2010 colorimeter against a blank sample made of the reagent with the acetate buffer. The content of glucose was read out from the standard curve [16,17].
2.9. Data Computation and Presentation
Laboratory analyses were carried out at least in three replications. Tables and Figures present mean values from these determinations.
Results were subjected to statistical analysis with Statistica 13.0 PL software. Two-way analysis of variance with Duncan’s test (at a significance level of p ≤ 0.05) was performed to determine significant differences between mean values.
3. Results and Discussion
Potato starch extrusion with apple distillery wastewater followed by roasting led to obtaining starch esters substituted with malic acid residues and with trace amounts of other acids (Figure 1).

Figure 1.
Degree of substitution of esters produced by starch extrusion with apple distillery wastewater and roasting (EW).
The effectiveness of the esterification process was mainly affected by the roasting temperature of the starch extrudates. The preparations roasted at the highest temperature tested (140 °C) had an almost five-fold higher degree of substitution than those heated at 80 °C. The effect of temperature on the intensity of chemical reactions is commonly known. In the present study, the impact of the extrusion temperature was negligible, probably because of the short period of preparation exposure to its action. The low effectiveness of the starch extrusion process has been reported by other authors [20,21]. The relatively good outcome of the esterification reaction achieved in the present study can be due to a high concentration of reacting substances, their very well mixing, and the roasting process applied [22].
The extrusion of starch increases its solubility in water and decreases its swelling power, with the intensity of changes being dependent on the type and moisture content of the raw material, and process parameters [23]. The roasting of native starch also increases its solubility, which is associated with carbohydrate thermolysis [24]. The solubility of the extruded and roasted starch in water increased along with an increasing extrusion temperature and decreased along with an increasing roasting temperature (Figure 2a).
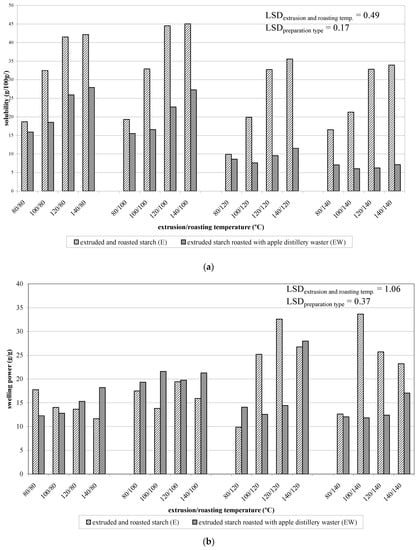
Figure 2.
Solubility (a) and swelling power (b) of extruded and roasted starch (E) and of starch extruded with apple distiller wastewater and roasted (EW).
Each time, an increase in the extrusion temperature by 20 °C caused an increase in the solubility by several to a few dozen percent on average (from ca. 16 to ca. 39 g/100 g). In the case of roasting, the differences resulted from exceeding the roasting temperature of 100 °C. At roasting temperatures of 80 and 100 °C, the solubility was at ca. 35 g/100 g on average, but, when the temperature of 100 °C had been exceeded, it decreased to ca. 25 g/100 g. These differences could be caused by the starch re-polymerization with the low-molecular-weight products of its depolymerization observed at higher temperatures. The re-polymerization process could, additionally, be intensified by low-molecular-weight dextrins formed during the extrusion process and present from the very beginning of the roasting process. This hypothesis can be corroborated by the results of analyses of extruded and roasted starch resistance to amylolysis (which will be discussed further in the Discussion portion of this section), which was exceptionally high (Figure 3).

Figure 3.
Resistance of extruded and roasted starch (E) and of starch extruded with apple distiller wastewater and roasted (EW) to the action of amyloglucosidase.
The starch esters produced in all of the experimental variants were characterized by lower solubility than the control samples. At roasting temperatures up to 100 °C, the value of this trait decreased by several dozen percent on average (from ca. 35 to 21 g/100 g), whereas, at higher roasting temperatures, it reached only a few percent (6–9 g/100 g on average). An analogous tendency was observed during starch esterification with citric acid [25] or malic acid [26]. It should also be remembered that the process of esterification was performed in an acidic medium, which catalyzes starch hydrolysis processes, thereby increasing the content of the water-soluble fraction. The low solubility of starch esters can only be explained by the crosslinking (attachment) of the soluble starch fraction with starch chains by means of malic acid. The mechanism of crosslinking of starch roasted with citric acid was described in our previous work [25].
The extrusion of starch decreases its swelling power, with the decrease being greater along with an increasing extrusion temperature [27]. In the case of starch preparations additionally subjected to the roasting process, this dependency could be noticed only at the lowest of the tested temperatures (Figure 2b). The roasting of extruded starch at temperatures exceeding 100 °C led to a significant increase in its swelling power. In contrast, a lower swelling power was determined in the preparations extruded at the lowest tested temperature (80 °C). Due to a low water content, starch undergoes only partial pasting during the extrusion process. The mechanical and heat energies cause the de-structuring of its remaining part [28]. When the extrusion process had been performed at a relatively low temperature (80 °C), the supplied energy could be insufficient to cause the complete destruction of the starch structures, and, therefore, the obtained modified preparations, having different structures from those of the other preparations, exhibited different properties. The significant increase in the swelling power of the remaining preparations was, most likely, due to transformations of the starch caused by its roasting (thermolysis, depolymerization, transglucosidation) [29]. Most of the starch esters produced differed significantly in their swelling power from the starch preparations obtained without the apple distillery wastewater (Figure 2b). The starch extrusion and roasting with the apple distillery wastewater led to its esterification, which—especially at higher temperatures—was inevitably accompanied by the thermolysis and acidic hydrolysis of the starch. The hydrolysis process was, somehow, counteracted by the intermolecular crosslinking process of the starch chains. Hence, the properties of individual preparations depended on the degree of starch esterification and hydrolysis, which, in turn, were affected by the experimental conditions.
The thermal characteristics of the extruded and roasted starch fell within a wide range of temperatures (42–76 °C), at the heat of phase transition ranging from 4 to 9 J/g (Table 1).

Table 1.
Thermal characteristics of extruded and roasted starch (E) and of starch extruded with apple distiller wastewater and roasted (EW).
Transitions of this type observed in extruded starch were reported by other researchers [16,30]. The starch roasted at temperatures of 120 or 140 °C had a higher mean heat of phase transition compared to the starch extruded and roasted at lower temperatures (80 or 100 °C). The value of the heat of phase transition is determined by damage caused to the structure of chains arranged in double helices. In turn, the growing range of temperatures is affected by the degradation of the crystalline structures of the starch [31,32]. Similar conclusions were formulated by Forssell et al. based on the thermal characteristics of the pasting of stored extruded starch [33]. The observed changes could, presumably, intensify as a result of the additional roasting of extruded starch because this process contributes to the depolymerization, transglucosidation, and re-polymerization of starch [1], accompanied by the formation of 1,2- or 1,3-glycosidic bonds, which is atypical of starch [34]. For most of the preparations, starch esterification caused an increase in the pasting temperatures and a decrease in the heat of phase transition (except for the preparation extruded and heated at the lowest temperature tested) to the values of 0.13–1.92 J/g noted at the highest roasting temperature used in the study. The disappearance of transitions in the thermal characteristics, along with an increasing degree of substitution of starch esters, has already been reported by many authors [35,36].
The pastes made from extruded and roasted starch (E) or from starch extruded with apple distillery wastewater and roasted (EW) at various temperatures exhibited the properties of shear-thinned non-Newtonian fluids (Table 2 and Table 3).

Table 2.
Parameters of Ostwald de Waele’s model characterizing the rheological properties of pastes made from extruded and roasted starch (E) and from starch extruded with apple distillery wastewater and roasted (EW).

Table 3.
Parameters of Casson’s model characterizing the rheological properties of pastes made from extruded and roasted starch (E) and from starch extruded with apple distillery wastewater and roasted (EW).
In the case of the starch extruded with apple distillery wastewater at all the temperatures tested and roasted at 140 °C, it was impossible to determine the values of the rheological coefficients described according to Ostwald de Waele’s model because of the drastically decreased viscosity of the pastes in the entire course of the flow curves. The viscosity of the pastes made of extruded and roasted starch depended on both the extrusion and roasting temperatures. In all the roasting variants, the highest viscosity in the whole course of the flow curves was demonstrated for the paste prepared from the starch extruded at the lowest temperature tested (80 °C), followed by the starch extruded at 100 °C. In contrast, the pastes prepared from the starch extruded at 120 or 140 °C had the lowest and similar viscosities. An analogous tendency of changes was noted for the rheological coefficients, including those describing the viscosity at the beginning of the shearing, K and τo, as well as ηc describing viscosity at the end of the shearing [17,37]. One of the major causes of the decreased viscosity of starch pastes is a decrease in the molecular weight of starch under the influence of enzymes or physical interactions [38]. The unbeneficial effect of starch extrusion temperature on the viscosity of pastes made of it was reported by many authors [39,40]. As mentioned earlier, the production process of starch esters was, probably, accompanied by processes of both thermal-acidic hydrolysis and crosslinking induced by esterification. Both these processes, having an opposite effect on the molecular weight of the starch, caused changes in the viscosity of the pastes. Therefore, paste viscosity was an outcome of both of these processes. All of the starch esters produced at the highest roasting temperature (140 °C) formed the least viscous pastes despite the highest degree of esterification, which is indicative of very advanced hydrolytic processes of starch. The lower roasting temperatures resulted in the increased viscosity of the pastes made of the starch extruded at the highest temperatures (120 and 140 °C) and in the decreased viscosity (in the entire course of the flow curves) of those prepared from the starch extruded at lower temperatures (80 and 120 °C). An exception was observed for the EW100/100 preparation, whose viscosity increased noticeably.
The color difference (darkening) ΔE of the starch extruded with apple distillery wastewater and roasted (EW) compared to the reference sample (E) increased with increasing roasting temperature, on average from about 9 to 27 (Figure 4). Such a significant color change may result from the caramelization reaction proceeding under these conditions [41] and the Maillard reaction [42].
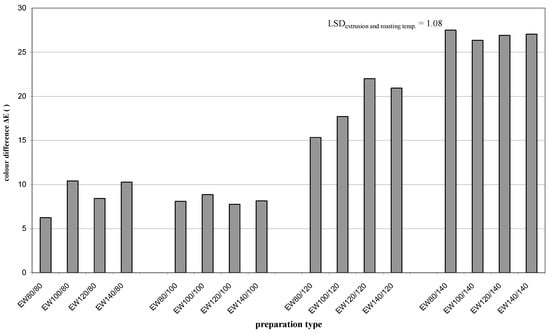
Figure 4.
Color difference ΔE of esters produced by starch extrusion with apple distillery wastewater and roasting (EW).
The resistance of extruded and roasted starch (E) to the action of amyloglucosidase increased from ca. 14 to 17 g/100 g on average along with the increasing extrusion and roasting temperatures. It was due to the re-polymerization occurring during the heat-treatment of the starch [1,43] (Figure 3). The esterification process (EW) caused an increase in the enzymatic resistance of the starch, with an analogous tendency of changes. The starch esters produced via the extrusion of native potato starch with apple distillery wastewater and roasting revealed high resistance, ranging from ca. 30 to over 41 g/100 g. The starch extruded and roasted at 100 °C was characterized by both high enzymatic resistance (ca. 31 g/100 g on average) and the relatively high viscosity of its pastes in the entire course of the flow curves. Due to these properties, it can presumably find applications as a novel type of resistant starch with texture-forming properties.
The chemical processes employed in food technology include those that are based on chemical reactions that require using certain chemical substances and proceed without a biological factor [44]. Only selected modified starches are permitted for use in food production. They are treated as food additives and denoted with the symbol ‘E’ and a respective number on food labels [45]. In the present study, a natural waste product from the production of dry calvados was used as a novelty in the starch modification process. The study concerned the chemical transformations of potato starch under the influence of organic acids naturally occurring in apple juices during their heat treatment. As a result of these transformations, starch esters were obtained whose main property was the high resistance to the action of amylolytic enzymes.
4. Conclusions
The extrusion and roasting of potato starch with acids contained in the raw material derived from apple distillery wastewater allowed the production of starch esters substituted with malic acid residues. The degree of starch esterification was higher on average upon the use of higher roasting temperatures. The extrusion of starch followed by its roasting with apple distillery wastewater caused its darker color, its increased resistance to amylolytic enzymes, and its lower solubility compared to the starch roasted without the addition of wastewater. The changes observed in the modified starch preparations were significantly greater at roasting temperatures exceeding 100 °C. The thermal characteristics of extruded and roasted starch fell within a wide range of temperatures (42–76 °C), and their changes depended on both the extrusion and roasting temperatures. The heat of phase transition of the starch esters was lower compared the control samples (except for the preparation extruded and roasted at lower temperatures), with the greatest changes observed at the highest temperatures of extrusion and roasting. The viscosity of the pastes made of starch esters depended on the extrusion and roasting temperatures. The starch esters produced at the highest roasting temperature tested (regardless of extrusion temperature) formed less viscous pastes. At the lower roasting temperatures, apparent viscosity increased in the entire course of the flow curves of the pastes prepared from starch extruded at the higher temperatures (120 and 140 °C) and decreased in the case of those made from starch extruded at the lower temperatures (80 and 100 °C), except for the paste formed from the preparation extruded and roasted at a temperature of 100 °C. The process of the extrusion and roasting of starch with apple distillery wastewater, i.e., the process performed without a chemical agent, should be deemed an innovative method for resistant starch production.
Author Contributions
Conceptualization, T.Z.; methodology, A.J. and A.G.; software, A.G. and M.K.-Ż.; validation, T.Z.; formal analysis, D.S. and B.R.; investigation, D.S., B.M. and M.K.-Ż.; resources, Đ.A. and B.M.; data curation, A.G. and A.J.; writing—original draft preparation, T.Z. and J.B.; writing—review and editing, all authors; visualization, A.G. and Đ.A.; supervision, J.B. and D.S.; project administration, M.K.-Ż. and A.J.; funding acquisition, B.R. All authors have read and agreed to the published version of the manuscript.
Funding
The publication is co-financed under the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019. This research was funded by JOSIP JURAJ STROSSMAYER UNIVERSITY OF OSIJEK, under the research project Potential of application a distillery wastewater from the production of apple brandy for the production of naturally modified starches (UNIOS-ZUP 2018-26).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Leszczyński, W. Resistant starch—Classification, structure, production. Pol. J. Food Nutr. Sci. 2004, 54, 37–50. [Google Scholar]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié–A–Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity. A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [Green Version]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jay-Lin, J.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4/6, 587–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Ačkar, D.; Babić, J.; Jozinović, A.; Miličević, B.; Jokić, S.; Miličević, R.; Rajič, M.; Šubarić, D. Starch Modification by Organic Acids and Their Derivatives: A Review. Molecules 2015, 20, 19554–19570. [Google Scholar] [CrossRef] [Green Version]
- Diakou, P.; Svanella, L.; Raymond, P.; Gaudillère, J.P.; Moing, A. Phosphoenolpyruvate carboxylase during grape berry development: Protein level, enzyme activity and regulation. Aust. J. Plant Physiol. 2000, 27, 221–229. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Ma, B.; Zhao, S.; Wu, B.; Wang, D.; Peng, Q.; Owiti, A.; Fang, T.; Liao, L.; Ogutu, C.; Korban, S.S.; et al. Construction of a high density linkage map and its application in the identification of QTLs for soluble sugar and organic acid components in apple. Tree Genet. Genomes 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Cornille, A.; Gladieux, P.; Smulders, M.J.M.; Roldán–Ruiz, I.; Laurens, F.; Le Cam, B.; Nersesyan, A.; Clavel, J.; Olonova, M.; Feugey, L.; et al. New insight into the history of domesticated apple: Secondary contribution of the european wild apple to the genome of cultivated varieties. PLoS Genet. 2012, 8, e1002703. [Google Scholar] [CrossRef] [Green Version]
- Zięba, T.; Solińska, D.; Kapelko-Żeberska, M.; Gryszkin, A.; Babić, J.; Aćkar, D.; Hernández, F.; Lonćarić, A.; Šubarić, D.; Jozinović, A. Properties of Potato Starch Roasted with Apple Distillery Wastewater. Polymers 2020, 12, 1668. [Google Scholar] [CrossRef]
- Zdybel, E.; Zięba, T.; Tomaszewska-Ciosk, E.; Rymowicz, W. Effect of the esterification of starch with a mixture of carboxylic acids from Yarrowia lipolitica fermentation broth on its selected properties. Polymers 2020, 12, 1383. [Google Scholar] [CrossRef]
- Richter, M.; Augustat, S.; Schierbaum, F. Ausgewählte Methoden der Stärkechemie: Isolierung, Charakterisierung und Analytik von Stärkepolysacchariden; Wissenschaftliche Verlagsgesellschaft m.b.H: Stuttgart, Germany, 1968; p. 208. [Google Scholar]
- Zięba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations. Part 1. Structure, susceptibility to amylase, and pasting characteristics. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar] [CrossRef]
- Zięba, T.; Kapelko, M.; Jacewicz, K.; Styczyńska, M. Properties of extruded starch modified with phosphorus and glycin. Pol. J. Food Nutr. Sci. 2007, 57, 633–638. [Google Scholar]
- Zięba, T.; Kapelko, M.; Gryszkin, A. Selected properties of potato starch subjected to multiple physical and chemical modifications. Pol. J. Food Nutr. Sci. 2007, 57, 639–645. [Google Scholar]
- Gryszkin, A.; Zięba, T.; Kapelko-Żeberska, M. Hydrothermal modification of wheat starch. Part 2. Thermal characteristics of pasting and rheological properties of pastes. J. Cereal Sci. 2016, 69, 194–198. [Google Scholar] [CrossRef]
- Clydesdale, F.M. Colorometry—Methodology and Applications. CRC Crit. Rev. Food Sci. Nutr. 1978, 10, 243–301. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yu, L.; Liu, H.; Chen, L. Starch Modification Using Reactive Extrusion. Starch Stärke 2006, 58, 131–139. [Google Scholar] [CrossRef]
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersoon, R.; Johansson, C.; Kuktaite, R.; Jarnstrom, L.; Koch, K. Molecular structure of citric acid cross–linked starch films. Carbohydr. Polym. 2013, 96, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Moad, G. Chemical modification of starch by reactive extrusion. Prog. Polym. Sci. 2011, 36, 218–237. [Google Scholar] [CrossRef]
- Willet, J.L.; Shoren, R.L. Processing and properties of extruded starchpolymer foams. Polymers 2002, 43, 5935–5947. [Google Scholar] [CrossRef]
- Lu, Z.H.; Donner, E.; Liu, Q. Effect of roasted peaflour/starch and encapsulated pea starch incorporationon thein vitrostarch digestibility of pea breads. Food Chem. 2018, 245, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kapelko–Żeberska, M.; Zięba, T.; Pietrzak, W.; Gryszkin, A. Effect of citric acid esterification conditions on the properties of the obtained resistant starch. Int. J. Food Sci. Technol. 2016, 51, 1647–1654. [Google Scholar] [CrossRef]
- Shi, M.; Gao, Q.; Liu, Y. Changes in the Structure and Digestibility of Wrinkled Pea Starch with Malic Acid Treatment. Polymers 2018, 10, 1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapelko, M.; Zięba, T.; Gryszkin, A.; Styczyńska, M.; Wilczak, A. Properties of retrograded and acetylated starch produced via starch extrusion or starch hydrolysis with pullulanase. Carbohydr. Polym. 2013, 97, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–992. [Google Scholar] [CrossRef]
- Tomasik, P.; Wiejak, S.; Pałasiński, M. The thermal decomposition of carbohydrates. Part II. The decomposition of starch. Adv. Carbohydr. Chem. Biochem. 1989, 47, 279–343. [Google Scholar]
- Kim, J.H.; Tanhehco, E.J.; Ng, P.K.W. Effect of extrusion conditions on resistant starch formation from pastry wheat flour. Food Chem. 2006, 99, 718–723. [Google Scholar] [CrossRef]
- Gryszkin, A.; Zięba, T.; Kapelko, M.; Buczek, A. Effect of thermal modifications of potato starch on its selected properties. Food Hydrocoll. 2014, 40, 122–127. [Google Scholar] [CrossRef]
- Singh, J.; Singh, N. Studies on the morphological, thermal and rheological properties of starch separated from same Indian potato cultivars. Food Chem. 2001, 75, 67–77. [Google Scholar] [CrossRef]
- Forssell, P.M.; Mikkilä, J.M.; Moates, G.K.; Parker, R. Phase and glasstransition behaviour of concentrated barley starch–glicerol–water mixtures, a model for thermoplastic starch. Carbohydr. Polym. 1997, 34, 275–282. [Google Scholar] [CrossRef]
- Ohkuma, K.; Matsuda, I.; Katta, Y.; Hanno, Y. Pyrolysis of starch and its digestibility by enzymes–Characterization of indigestible dextrin. Denpun Kagaku 1990, 37, 107–114. [Google Scholar]
- Afolabi, T.A.; Olu–Owolabi, B.I.; Adebowale, K.O.; Lawal, O.S.; Akintayo, C.O. Functional and tableting properties of acetylated and oxidised finger millet Eleusine coracana starch. Starch Stärke 2012, 64, 326–337. [Google Scholar] [CrossRef]
- Bello–Pérez, L.A.; Agama–Acevedo, E.; Zamudio–Flores, P.B.; Mendez–Montealvo, G.; Rodriguez–Ambriz, S.L. Effect of low and high acetylation degree in the morphological, physicochemical and structural characteristics of barley starch. LWT Food Sci. Technol. 2010, 43, 1434–1440. [Google Scholar] [CrossRef]
- Kapelko, M.; Zięba, T. Właściwości skrobi ekstrudowanej modyfikowanej glicyną. Żywność Nauka Technol. Jakość 2007, 54, 21–30. [Google Scholar]
- Wang, S.; Copeland, L. Effect of Acid Hydrolysis on Starch Structure and Functionality: A Review. Food Sci. Nutr. 2015, 55, 1081–1097. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; Liu, W.; Chen, J.; Zeng, Z.; Liu, C. Properties of Starch after Extrusion: A Review. Starch Stärke 2018, 70, 1700110. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.A.; Mason, S.L.; Zheng, H.; Brenna, C.S. Rheological, pasting and microstructural studies of dairy protein–starch interactions and their application in extrusion-based products: A review. Starch Stärke 2017, 69, 160–273. [Google Scholar] [CrossRef]
- Sengar, G.; Sharma, H.K. Food caramels: A review. J. Food Sci. Technol. 2014, 51, 1686–1696. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.I.F.S.; Jongen, W.M.F.; Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modeling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Francis, J.F. Wiley Encyclopedia of Food Science and Technology, 2nd ed.; John Wiley and Sons Inc.: New York, NY, USA, 1999. [Google Scholar]
- Harrison, J.R. International Regulation of Natural Health Products; Universal Publishers: Boca Raton, FL, USA, 2008. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).