Effects of Dietary Fishmeal Replacement by Poultry By-Product Meal and Hydrolyzed Feather Meal on Liver and Intestinal Histomorphology and on Intestinal Microbiota of Gilthead Seabream (Sparus aurata)
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Feeding Trials and Experimental Diets
2.2. Histological Analysis and Measurements
2.3. Microbiota Analysis-DNA Extraction, Bioinformatics and Data Analysis
2.4. Statistical Analysis
3. Results
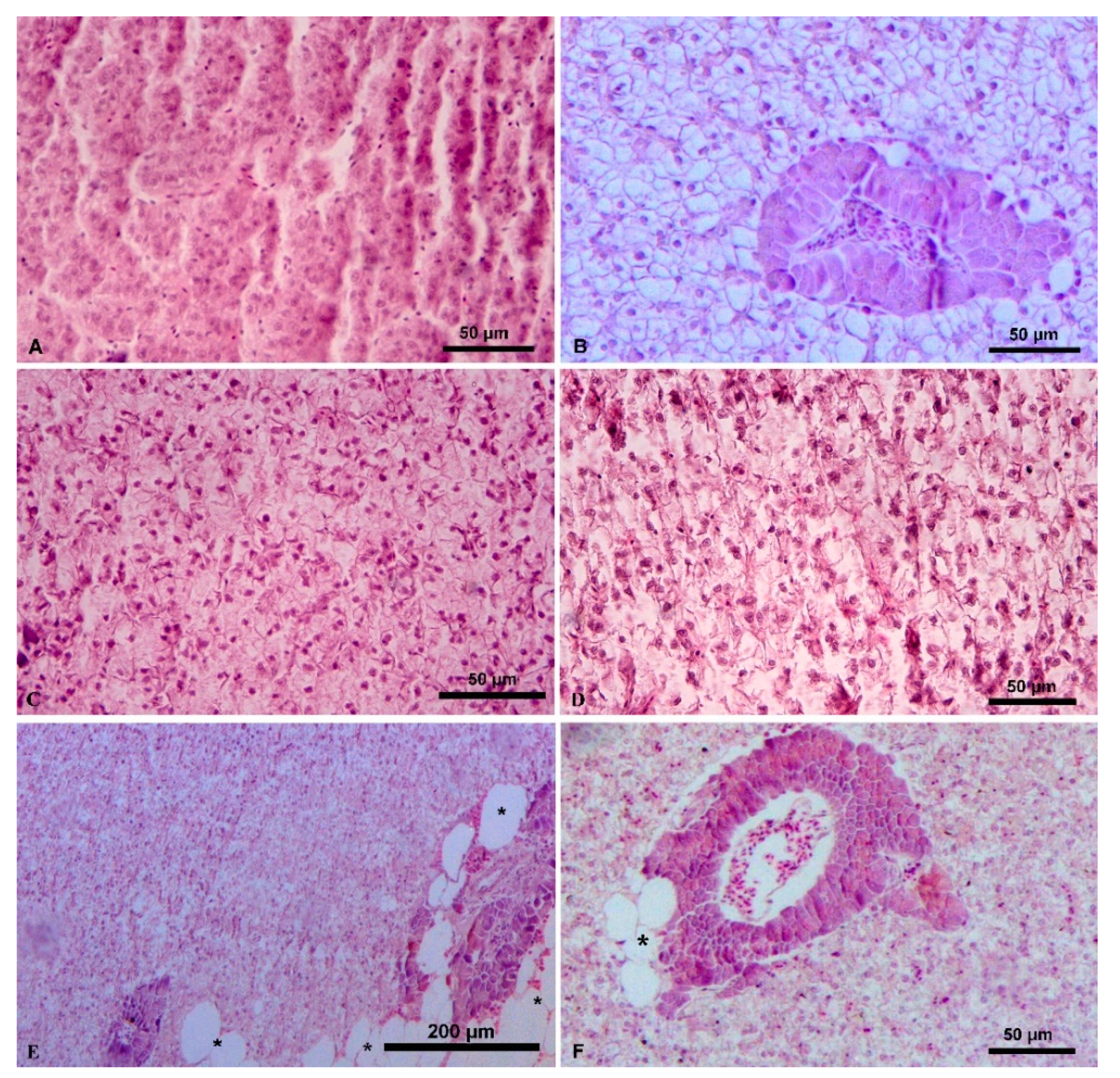
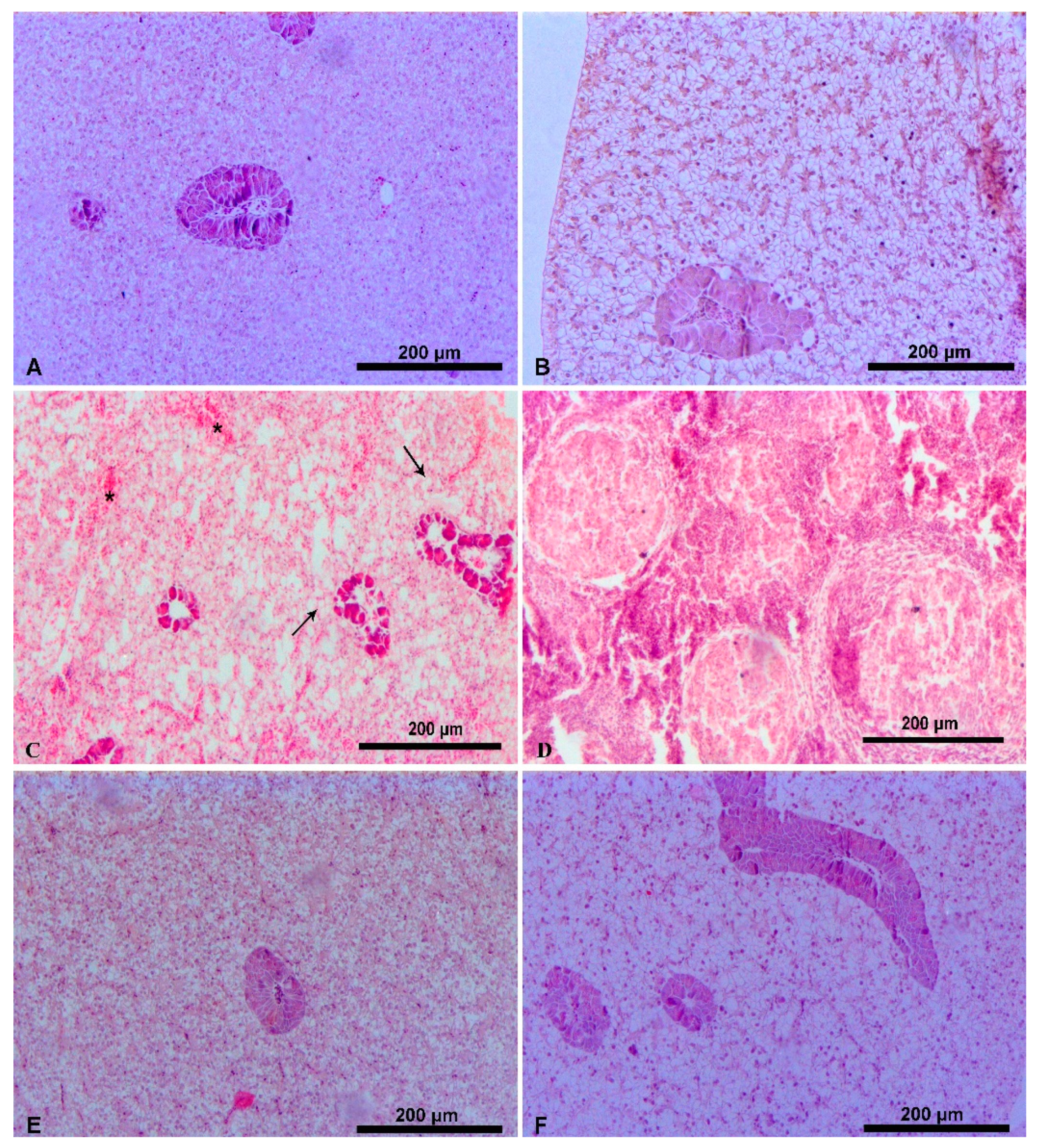
3.1. Liver Histology
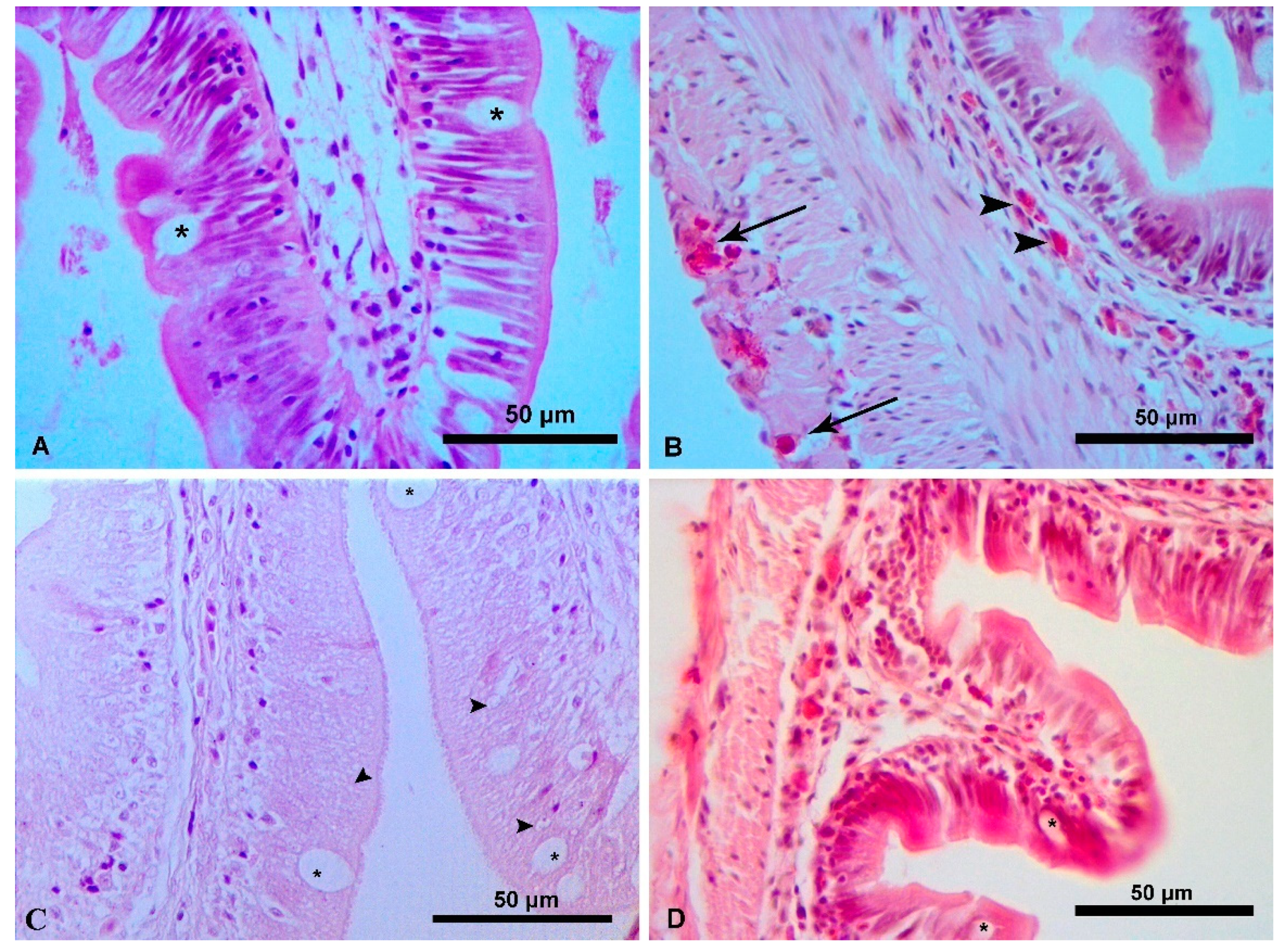
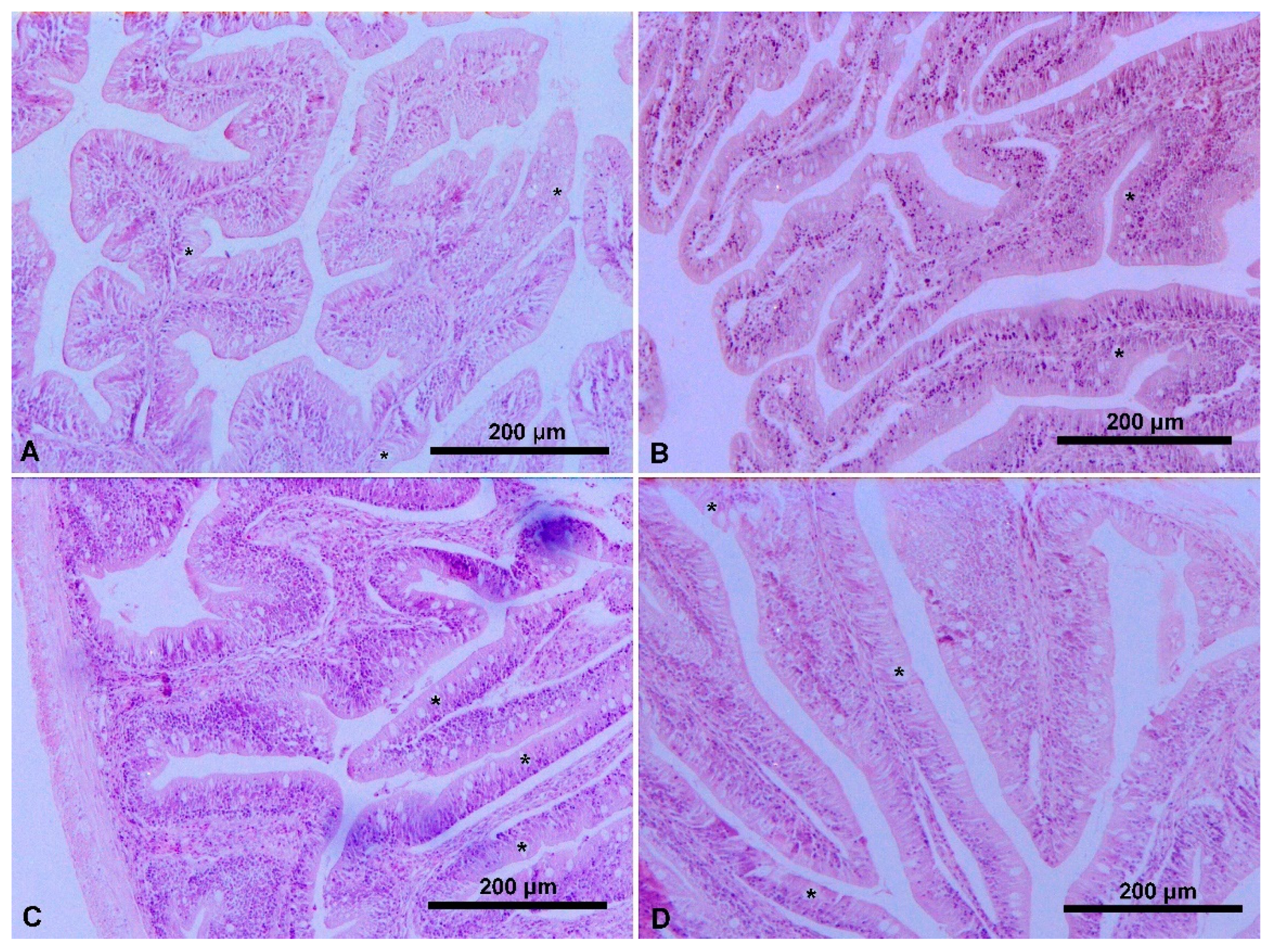
3.2. Intestinal Histology
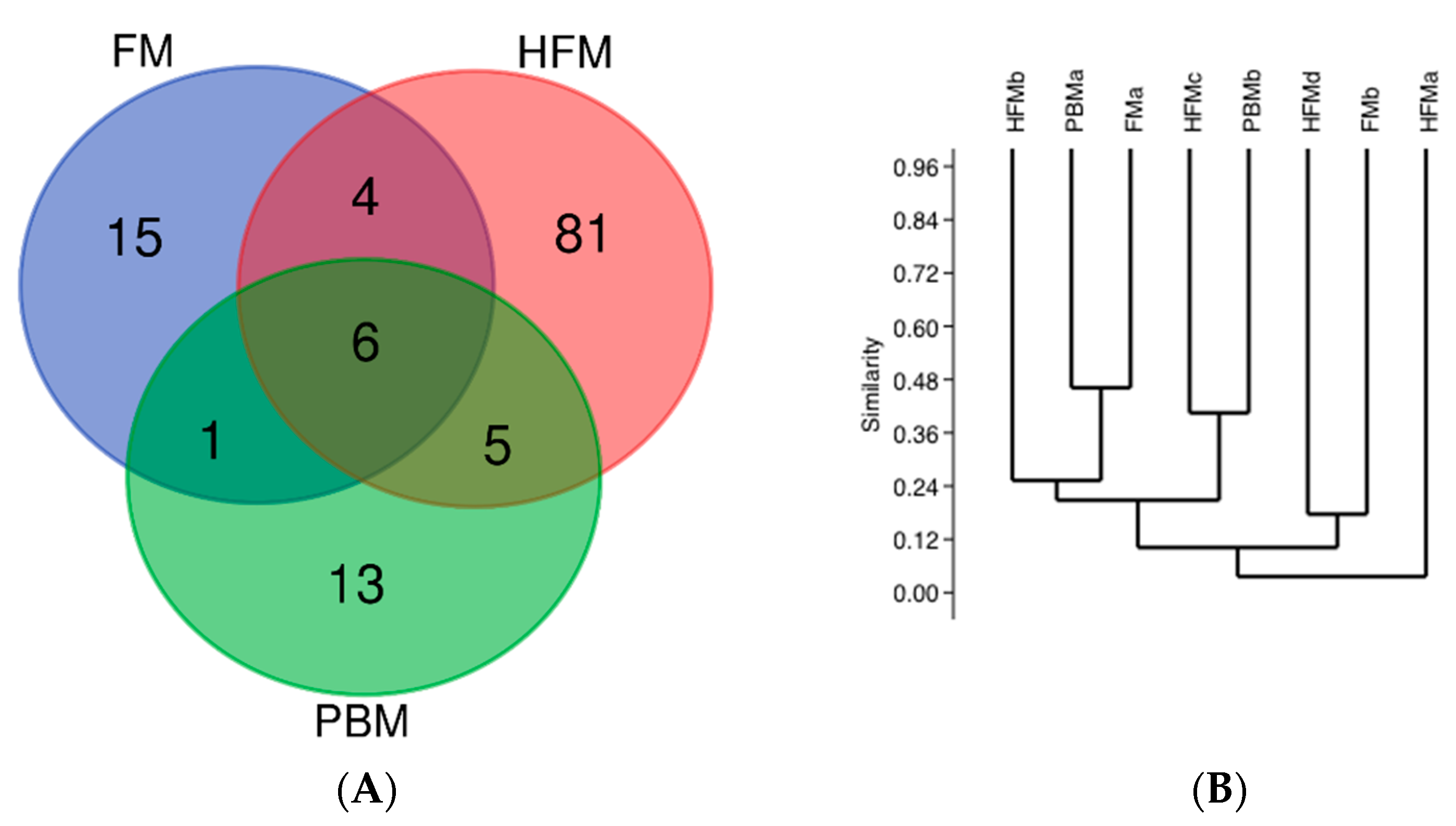
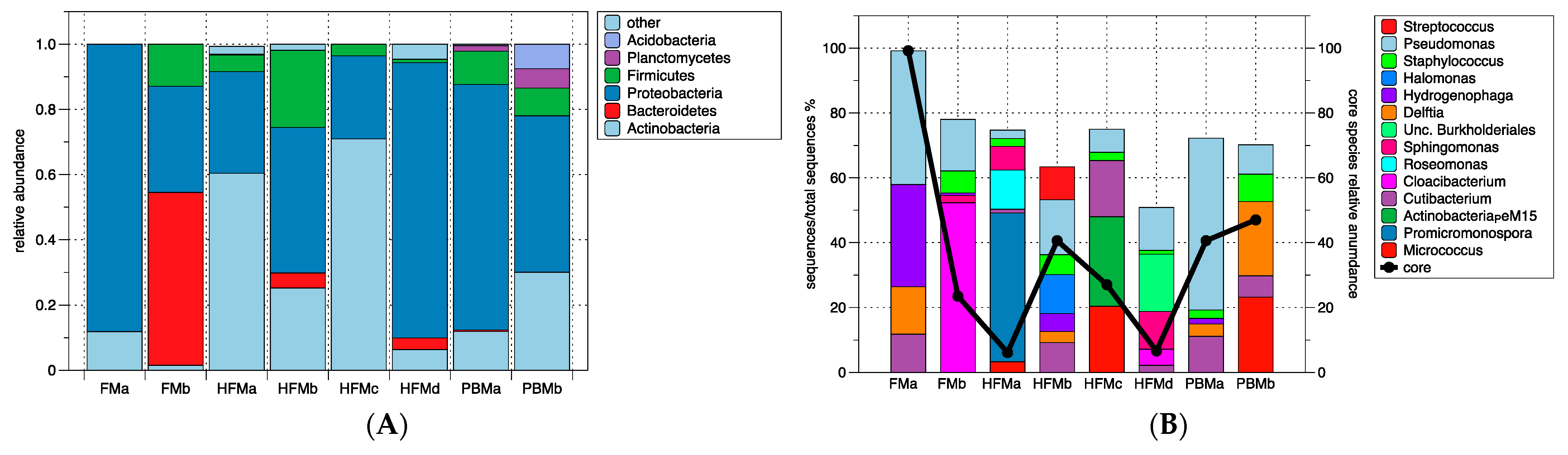
3.3. Intestinal Microbiota
4. Discussion
4.1. Liver Histology
4.2. Intestinal Histology
4.3. Intestinal Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fishery and Aquaculture 2020 (SOFIA); Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [CrossRef]
- FAO. Cultured Aquatic Species Information Programme. Sparus aurata (Linnaeus, 1758). Available online: http://www.fao.org/fishery/culturedspecies/Sparus_aurata/en (accessed on 24 July 2021).
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Yu, H.-R.; Zhang, Q.; Cao, H.; Wang, X.-Z.; Huang, G.-Q.; Zhang, B.-R.; Fan, J.-J.; Liu, S.-W.; Li, W.-Z.; Cui, Y. Apparent digestibility coefficients of selected feed ingredients for juvenile snakehead, Ophiocephalus argus. Aquac. Nutr. 2012, 19, 139–147. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; García, V.; Fuertes, J.B. Evaluation of poultry by-product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L.). Aquac. Res. 2014, 47, 1612–1621. [Google Scholar] [CrossRef]
- Campos, I.; Matos, E.; Marques, A.; Valente, L.M. Hydrolyzed feather meal as a partial fishmeal replacement in diets for European seabass (Dicentrarchus labrax) juveniles. Aquaculture 2017, 476, 152–159. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Psofakis, P.; Mente, E.; Malandrakis, E.; Golomazou, E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac. Nutr. 2019, 25, 3–14. [Google Scholar] [CrossRef]
- Psofakis, P.; Karapanagiotidis, I.; Malandrakis, E.; Golomazou, E.; Exadactylos, A.; Mente, E. Effect of fishmeal replacement by hydrolyzed feather meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and growth-related gene expression of gilthead seabream (Sparus aurata). Aquaculture 2020, 521, 735006. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; Report EUR 26255; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Maiolo, S.; Parisi, G.; Biondi, N.; Lunelli, F.; Tibaldi, E.; Pastres, R. Fishmeal partial substitution within aquafeed formulations: Life cycle assessment of four alternative protein sources. Int. J. Life Cycle Assess. 2020, 25, 1455–1471. [Google Scholar] [CrossRef]
- den Hartog, L.A.; Sijtsma, S.R. Sustainable feed ingredients. In Proceedings of the 12th International Symposium of Australian Renderers Association “Rendering for Sustainability”, Victoria, Australia, 23–26 July 2013; pp. 18–26. [Google Scholar]
- Maiolo, S.; Cristiano, S.; Gonella, F.; Pastres, R. Ecological sustainability of aquafeed: An emergy assessment of novel or underexploited ingredients. J. Clean. Prod. 2021, 294, 126266. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Baeza-Ariño, R.; Nogales-Mérida, S.; Jover-Cerdá, M.; Tomás-Vidal, A. Carob seed germ meal as a partial substitute in gilthead sea bream (Sparus aurata) diets: Amino acid retention, digestibility, gut and liver histology. Aquaculture 2012, 338-341, 124–133. [Google Scholar] [CrossRef]
- Hartviksen, M.A.B.; Vecino, J.L.G.; Ringo, E.; Bakke, A.M.; Wadsworth, S.; Krogdahl, A.; Ruohonen, K.; Kettunen, A. Alternative dietary protein sources for Atlantic salmon (Salmo salar L.) effect on intestinal microbiota, intestinal and liver histology and growth. Aquac. Nutr. 2014, 20, 381–398. [Google Scholar] [CrossRef]
- Baeza-Ariño, R.; Martínez-Llorens, S.; Nogales-Mérida, S.; Jover-Cerdá, M.; Tomás-Vidal, A. Study of liver and gut alterations in sea bream, Sparus aurata L., fed a mixture of vegetable protein concentrates. Aquac. Res. 2014, 47, 460–471. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Peña-Llopis, S.; Requeni, P.G.; Médale, F.; Kaushik, S.; Sánchez, J.P. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 2005, 249, 387–400. [Google Scholar] [CrossRef]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Rigos, G.; Henry, M.; Alexis, M.; Kentouri, M. Effects of Fish Meal Replacement by a Soybean Protein on Growth, Histology, Selected Immune and Oxidative Status Markers of Gilthead Sea Bream, Sparus aurata. J. World Aquac. Soc. 2015, 46, 115–128. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An up-date on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Hu, L.; Yun, B.; Xue, M.; Wang, J.; Wu, X.; Zheng, Y.; Han, F. Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance, flesh quality and liver histology of Japanese seabass (Lateolabrax japonicus). Aquaculture 2013, 372–375, 52–61. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Su, N.; Wang, A.; Tan, X.; Sun, Z.; Zou, C.; Liu, Q.; Ye, C. Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 511, 734203. [Google Scholar] [CrossRef]
- Zhou, Z.; Yao, W.; Ye, B.; Wu, X.; Li, X.; Dong, Y. Effects of replacing fishmeal protein with poultry by-product meal protein and soybean meal protein on growth, feed intake, feed utilization, gut and liver histology of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 2020, 516, 734503. [Google Scholar] [CrossRef]
- Chaklader, R.; Siddik, M.A.B.; Fotedar, R. Total replacement of fishmeal with poultry by-product meal affected the growth, muscle quality, histological structure, antioxidant capacity and immune response of juvenile barramundi, Lates calcarifer. PLoS ONE 2020, 15, e0242079. [Google Scholar] [CrossRef]
- Yu, R.; Cao, H.; Huang, Y.; Peng, M.; Kajbaf, K.; Kumar, V.; Tao, Z.; Yang, G.; Wen, C. The effects of partial replacement of fishmeal protein by hydrolysed feather meal protein in the diet with high inclusion of plant protein on growth performance, fillet quality and physiological parameters of Pengze crucian carp (Carassius auratus var. Pengze). Aquac. Res. 2019, 51, 636–647. [Google Scholar] [CrossRef]
- Perry, W.B.; Lindsay, E.; Payne, C.J.; Brodie, C.; Kazlauskaite, R. The role of the gut microbiome in sustainable teleost aquaculture. Proc. R. Soc. B 2020, 287, 20200184. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Ghosh, K.; Ringø, E. Enzyme-producing bacteria isolated from fish gut: A review. Aquac. Nutr. 2012, 18, 465–492. [Google Scholar] [CrossRef]
- Wu, S.; Ren, Y.; Peng, C.; Hao, Y.; Xiong, F.; Wang, G.; Li, W.; Zou, H.; Angert, E.R. Metatranscriptomic discovery of plant biomass-degrading capacity from grass carp intestinal microbiomes. FEMS Microbiol. Ecol. 2015, 91, fiv107. [Google Scholar] [CrossRef] [PubMed]
- Foysal, J.; Fotedar, R.; Tay, C.-Y.; Gupta, S.K. Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 2019, 7, e6891. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Wolf, J.; Braunbeck, T. OECD Guidance Document for the Diagnosis of Endocrine-Related Histopathology of Fish Gonads; Organization for Economic Co-operation and Development 2009; p. 96. Available online: https://www.oecd.org/chemicalsafety/testing/42140701.pdf (accessed on 14 April 2020).
- Nikouli, E.; Meziti, A.; Antonopoulou, E.; Mente, E.; Kormas, K.A. Gut Bacterial Communities in Geographically Distant Populations of Farmed Sea Bream (Sparus aurata) and Sea Bass (Dicentrarchus labrax). Microorganisms 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Iwai, S.; Weinmaier, T.; Schmidt, B.L.; Albertson, D.G.; Poloso, N.J.; Dabbagh, K.; DeSantis, T.Z. Piphillin: Improved Prediction of Metagenomic Content by Direct Inference from Human Microbiomes. PLoS ONE 2016, 11, e0166104. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Ordination methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. 2021. Available online: http://outputs.worldagroforestry.org/cgi-bin/koha/opac-detail.pl?biblionumber=39504 (accessed on 27 July 2021).
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Zheng, Z.X.; Jiang, R.L.; Xie, N.X. Replacing fishmeal with rendered animal protein ingredients in diets for Malabar grouper, Epinephelus malabaricus, reared in net pens. J. World Aquac. Soc. 2009, 40, 67–75. [Google Scholar] [CrossRef]
- Hernãndez, C.; Olvera-Novoa, M.; Hardy, R.; Hermosillo, A.; Reyes, C.; Gonzãlez, B. Complete replacement of fish meal by porcine and poultry by-product meals in practical diets for fingerling Nile tilapia Oreochromis niloticus: Digestibility and growth performance. Aquac. Nutr. 2010, 16, 44–53. [Google Scholar] [CrossRef]
- Hernández, C.; Sanchez-Gutierrez, Y.; Hardy, R.W.; Benitez-Hernández, A.; Domínguez-Jimenez, P.; González-Rodríguez, B.; Osuna-Osuna, L.; Tortoledo, O. The potential of pet-grade poultry by-product meal to replace fish meal in the diet of the juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquac. Nutr. 2014, 20, 623–631. [Google Scholar] [CrossRef]
- Fuertes, J.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; Gonzalez-Rodriguez, A. Effects of fishmeal replacement by feather meal in practical diets for juvenile crayfish (Pacifastacus leniusculus Dana, Astacidae). Aquac. Nutr. 2013, 20, 36–43. [Google Scholar] [CrossRef]
- Zapata, D.B.; Lazo, J.P.; Herzka, S.Z.; Viana, M.T. The effect of substituting fishmeal with poultry by-product meal in diets for Totoaba macdonaldi juveniles. Aquac. Res. 2016, 47, 1778–1789. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; et al. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef]
- Gajardo, K.; Jaramillo-Torres, A.; Kortner, T.M.; Merrifield, D.L.; Tinsley, J.; Bakke, A.M.; Krogdahl, Å. Alternative Protein Sources in the Diet Modulate Microbiota and Functionality in the Distal Intestine of Atlantic Salmon (Salmo salar). Appl. Environ. Microbiol. 2017, 83, 83. [Google Scholar] [CrossRef]
- Sabbagh, M.; Schiavone, R.; Brizzi, G.; Sicuro, B.; Zilli, L.; Vilella, S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture 2019, 511, 734220. [Google Scholar] [CrossRef]
- Spisni, E.; Tugnoli, M.; Ponticelli, A.; Mordenti, T.; Tomasi, V. Hepatic steatosis in artificially fed marine teleosts. J. Fish Dis. 1998, 21, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.B.; Chungu, P.; Fotedar, R.; Howieson, J. Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch). PLoS ONE 2019, 14, e0215025. [Google Scholar] [CrossRef]
- Panicz, R.; Żochowska-Kujawska, J.; Sadowski, J.; Sobczak, M. Effect of feeding various levels of poultry by-product meal on the blood parameters, filet composition and structure of female tenches (Tinca tinca). Aquac. Res. 2017, 48, 5373–5384. [Google Scholar] [CrossRef]
- Aydín, B.; Gümüş, E.; Balci, B.A. Effect of dietary fish meal replacement by poultry by-product meal on muscle fatty acid composition and liver histology of fry of Nile tilapia, Oreochromis niloticus (Actinopterygii: Perciformes: Cichlidae). Acta Ichthyol. Piscat. 2015, 45, 343–351. [Google Scholar] [CrossRef]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef]
- Berillis, P.; Mente, E. Histology of Goblet Cells in the Intestine of the Rainbow Trout Can Lead to Improvement of the Feeding Management. J. Fish. 2017, 11, 32–33. [Google Scholar] [CrossRef][Green Version]
- Ferguson, H.W. Systemic pathology of fish. In A Text and Atlas of Comparative Tissue Responses in Diseases of Teleosts, 2nd ed.; Scotian Press: London, UK, 2006. [Google Scholar]
- Hu, H.; Kortner, T.M.; Gajardo, K.; Chikwati, E.M.; Tinsley, J.; Krogdahl, Å. Intestinal Fluid Permeability in Atlantic Salmon (Salmo salar L.) Is Affected by Dietary Protein Source. PLoS ONE 2016, 11, e0167515. [Google Scholar] [CrossRef]
- Tran-Ngoc, K.T.; Haidar, M.N.; Roem, A.J.; Sendão, J.; Verreth, J.; Schrama, J.W. Effects of feed ingredients on nutrient digestibility, nitrogen/energy balance and morphology changes in the intestine of Nile tilapia (Oreochromis niloticus). Aquac. Res. 2019, 50, 2577–2590. [Google Scholar] [CrossRef]
- Nikouli, E.; Meziti, A.; Smeti, E.; Antonopoulou, E.; Mente, E.; Kormas, K.A. Gut Microbiota of Five Sympatrically Farmed Marine Fish Species in the Aegean Sea. Microb. Ecol. 2021, 81, 460–470. [Google Scholar] [CrossRef]
- El-Rhman, A.M.A.; Khattab, Y.A.; Shalaby, A.M. Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2009, 27, 175–180. [Google Scholar] [CrossRef]
- Akayli, T.; Albayrak, G.; Ürkü, Ç.; Çanak, Ö.; Yörük, E. Characterization of Micrococcus luteus and Bacillus marisflavi Recovered from Common Dentex (Dentex dentex) Larviculture System. Mediterr. Mar. Sci. 2015, 17, 163. [Google Scholar] [CrossRef]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-Ya, K.; Omura, S.; Cane, D.-E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef]
- Dvergedal, H.; Sandve, S.R.; Angell, I.L.; Klemetsdal, G.; Rudi, K. Association of gut microbiota with metabolism in juvenile Atlantic salmon. Microbiome 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Perlot, T.; Penninger, J.M. ACE2—From the renin–angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013, 15, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Ma, K.L.; Ruan, X.Z.; Liu, B.C. Intestinal dysbiosis activates renal renin-angiotensin system contributing to incipient diabetic nephropathy. Int. J. Med. Sci. 2018, 15, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.; Vaishnava, S. Vitamin A at the interface of host-commensal-pathogen interactions. PLoS Pathog. 2019, 15, e1007750. [Google Scholar] [CrossRef] [PubMed]


| FM | PBM25 | PBM25+ | PΒM50 | PBM50+ | PBM100 | HFM25 | HFM25+ | HFM50 | HFM50+ | HFM100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity score | |||||||||||
| Liver | 1 | 1 | 1 | 2 | 2 | 3 | 1 | 1 | 3 | 2 | 4 |
| Intestine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver fat (%) | 38.0 | 36.2 | 40.6 | 42.0 | 43.2 | 42.5 | 35.7 | 36.9 | 42.7 | 40.8 | 7.8 |
| FMa | FMb | HFMa | HFMb | HFMc | HFMd | PBMa | PBMb | |
|---|---|---|---|---|---|---|---|---|
| Richness | 14 | 14 | 48 | 29 | 14 | 28 | 15 | 16 |
| Sequences | 2283 | 132 | 2206 | 325 | 196 | 181 | 234 | 319 |
| Shannon | 1.32 | 1.71 | 2.27 | 2.97 | 2.05 | 2.87 | 2.10 | 2.23 |
| Cumulative abundance >1% | 99.21 | 96.21 | 92.84 | 97.54 | 97.45 | 97.24 | 98.72 | 98.12 |
| Coverage | 1.00 | 0.95 | 0.99 | 0.99 | 0.97 | 0.97 | 0.99 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psofakis, P.; Meziti, A.; Berillis, P.; Mente, E.; Kormas, K.A.; Karapanagiotidis, I.T. Effects of Dietary Fishmeal Replacement by Poultry By-Product Meal and Hydrolyzed Feather Meal on Liver and Intestinal Histomorphology and on Intestinal Microbiota of Gilthead Seabream (Sparus aurata). Appl. Sci. 2021, 11, 8806. https://doi.org/10.3390/app11198806
Psofakis P, Meziti A, Berillis P, Mente E, Kormas KA, Karapanagiotidis IT. Effects of Dietary Fishmeal Replacement by Poultry By-Product Meal and Hydrolyzed Feather Meal on Liver and Intestinal Histomorphology and on Intestinal Microbiota of Gilthead Seabream (Sparus aurata). Applied Sciences. 2021; 11(19):8806. https://doi.org/10.3390/app11198806
Chicago/Turabian StylePsofakis, Pier, Alexandra Meziti, Panagiotis Berillis, Eleni Mente, Konstantinos A. Kormas, and Ioannis T. Karapanagiotidis. 2021. "Effects of Dietary Fishmeal Replacement by Poultry By-Product Meal and Hydrolyzed Feather Meal on Liver and Intestinal Histomorphology and on Intestinal Microbiota of Gilthead Seabream (Sparus aurata)" Applied Sciences 11, no. 19: 8806. https://doi.org/10.3390/app11198806
APA StylePsofakis, P., Meziti, A., Berillis, P., Mente, E., Kormas, K. A., & Karapanagiotidis, I. T. (2021). Effects of Dietary Fishmeal Replacement by Poultry By-Product Meal and Hydrolyzed Feather Meal on Liver and Intestinal Histomorphology and on Intestinal Microbiota of Gilthead Seabream (Sparus aurata). Applied Sciences, 11(19), 8806. https://doi.org/10.3390/app11198806









