Key-Parameters in Chemical Stabilization of Soils with Multiwall Carbon Nanotubes
Abstract
:1. Introduction
2. Methodology
2.1. Testing Plan
2.2. Materials
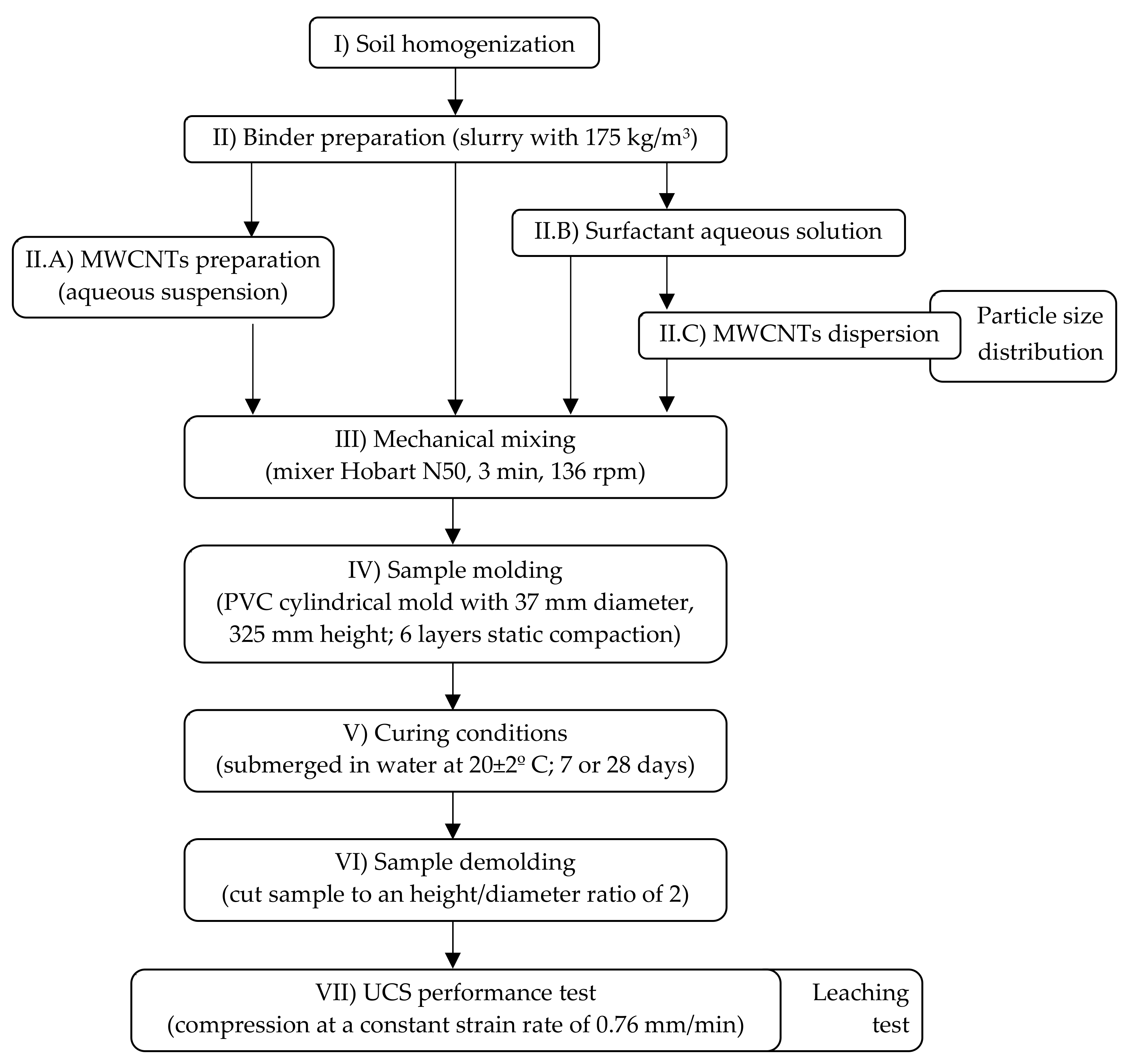
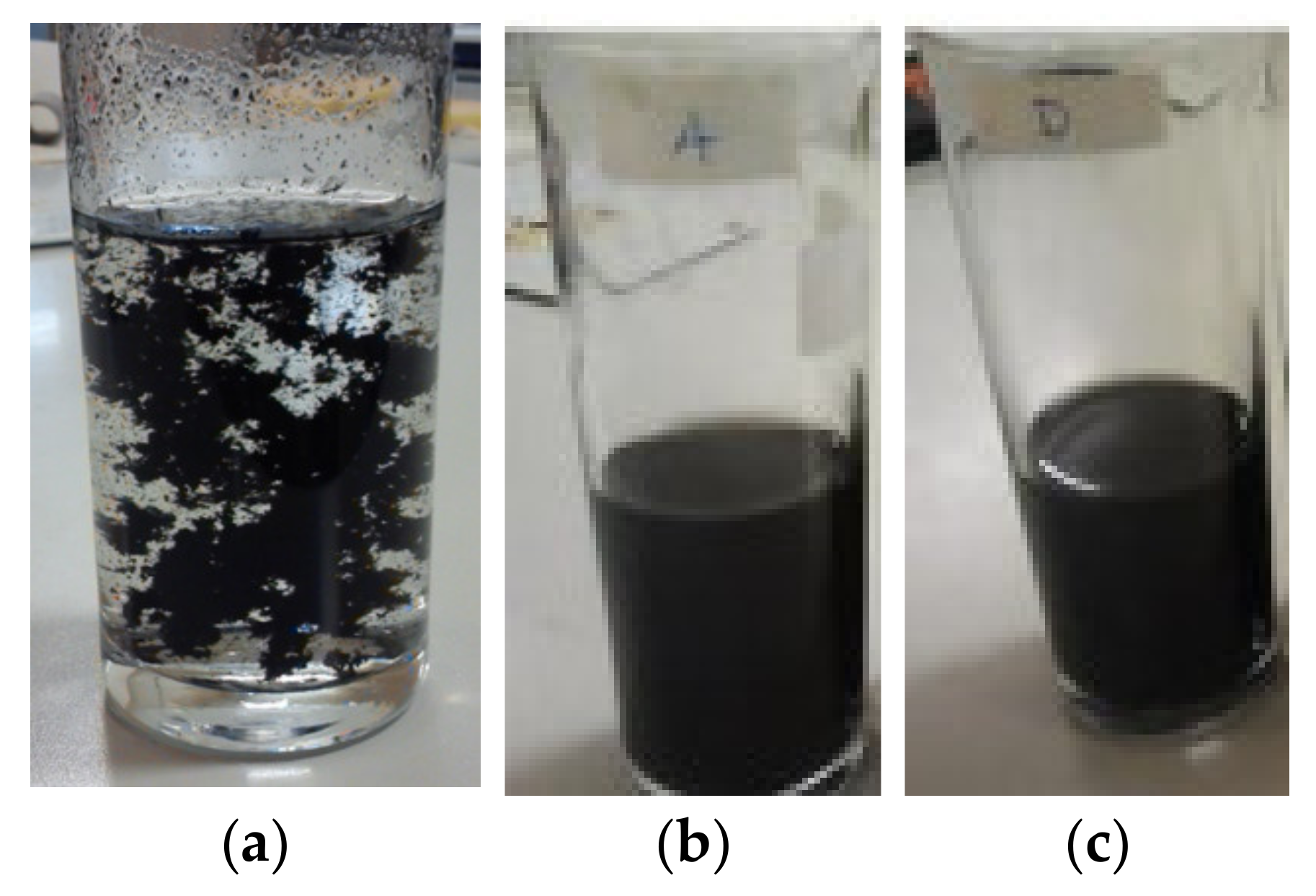
2.3. Sample Preparation and Tests
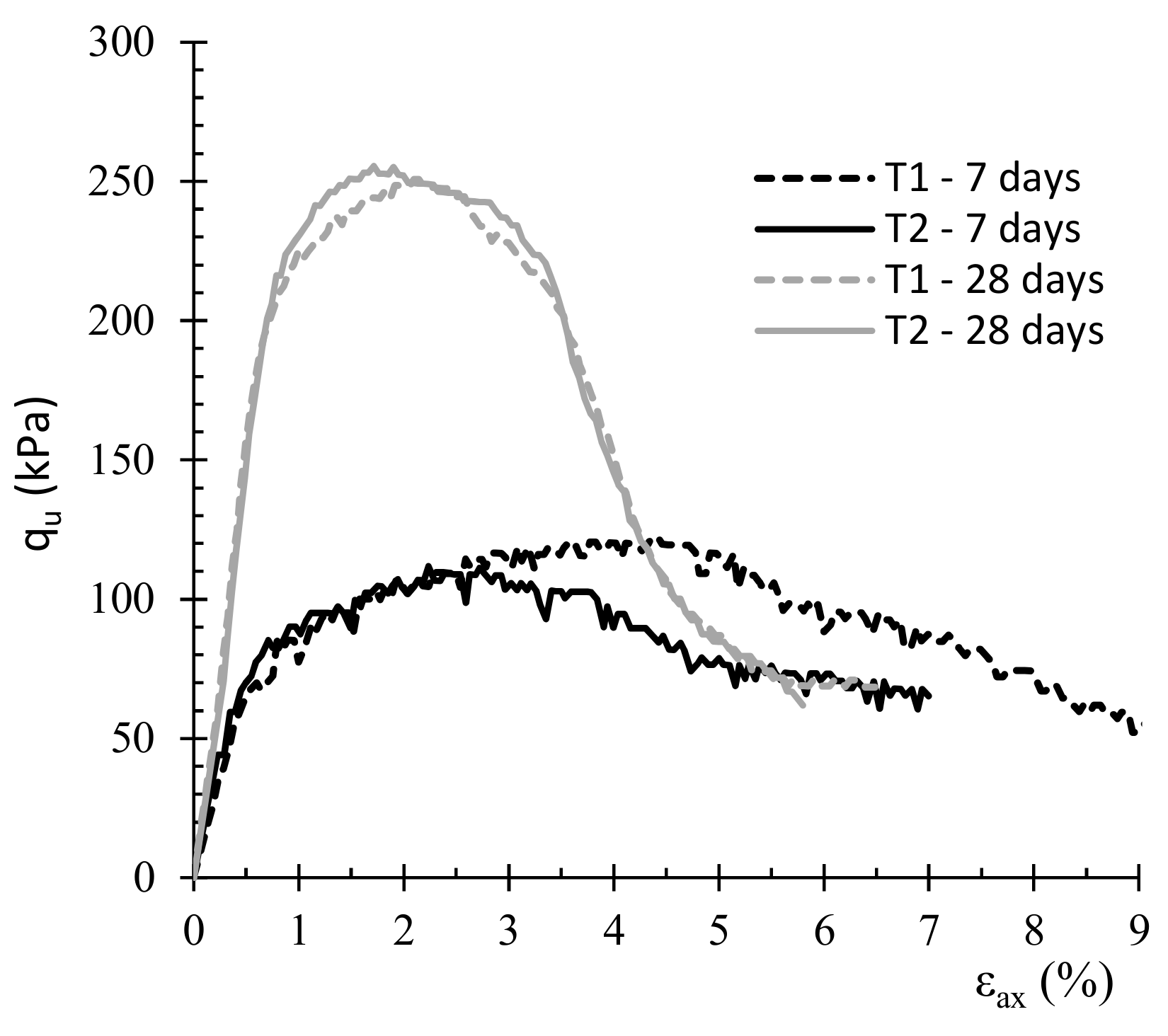
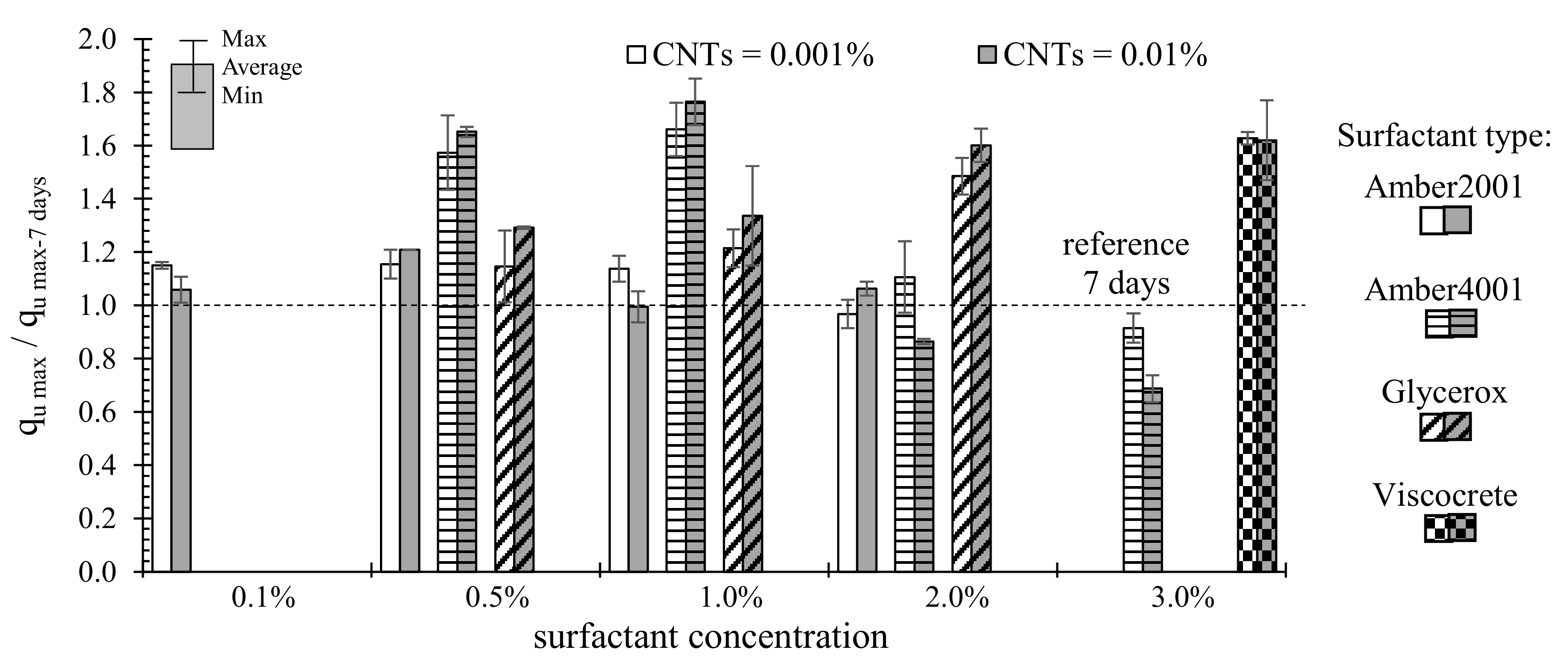
3. Results and Discussion
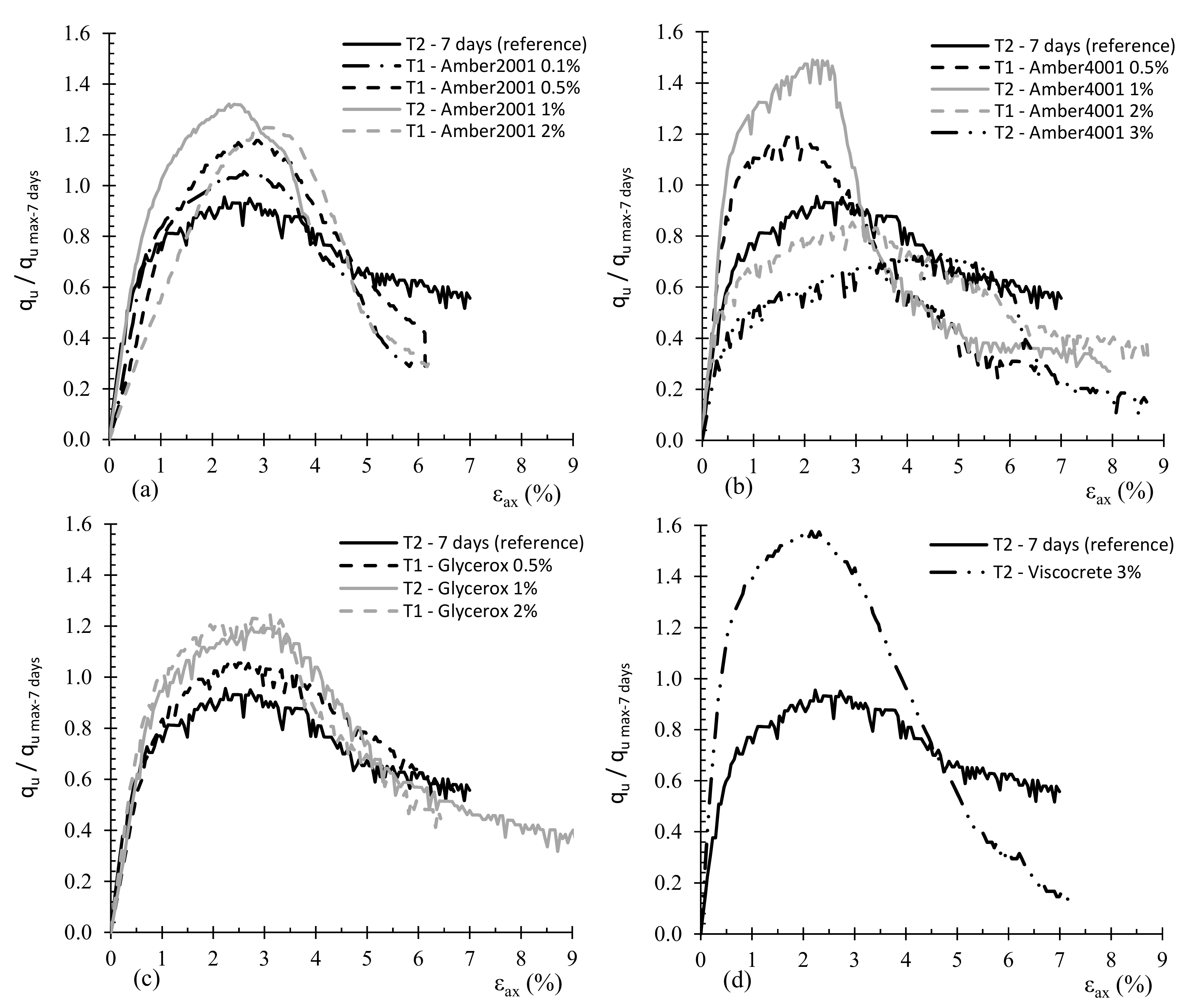
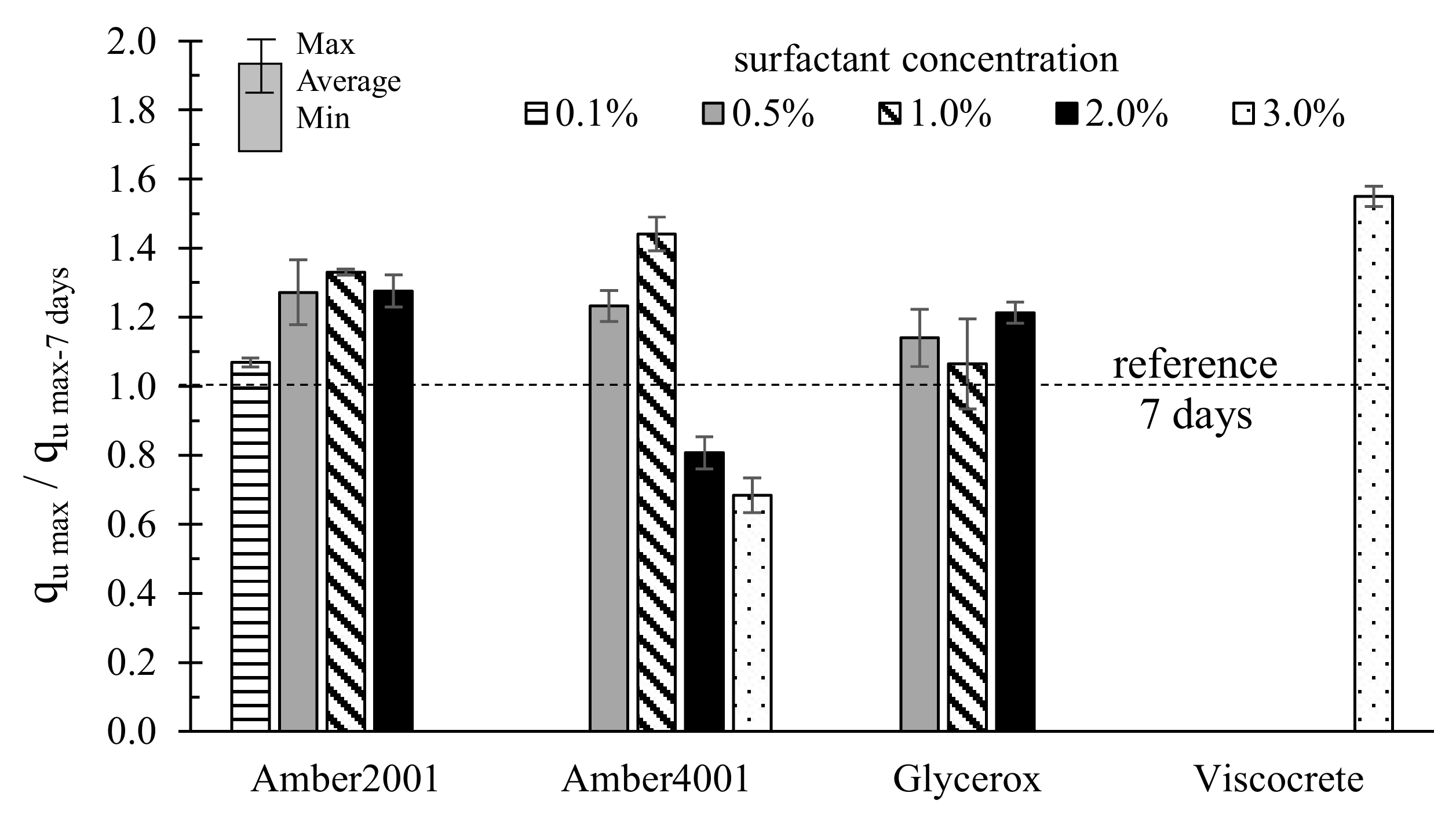
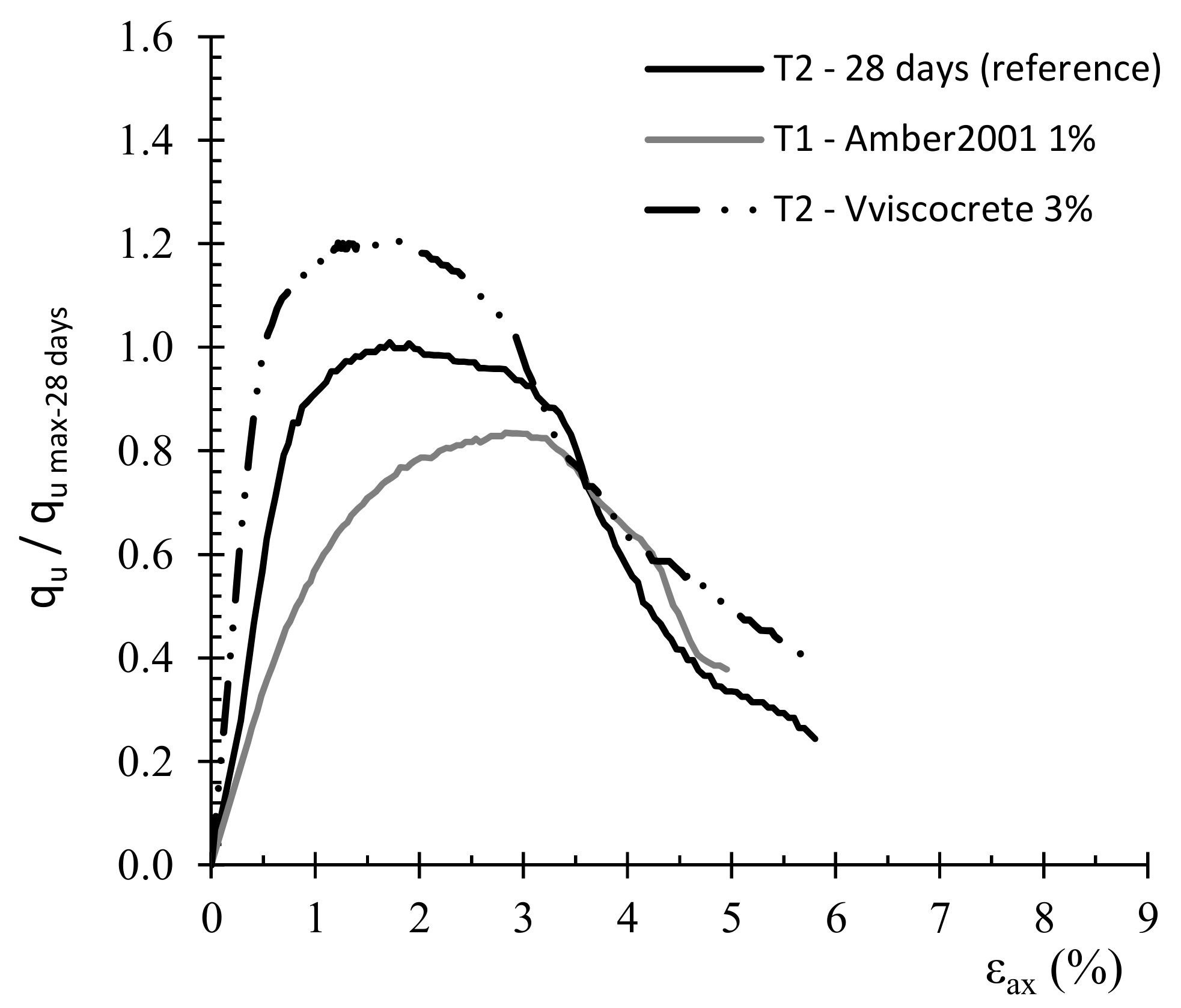
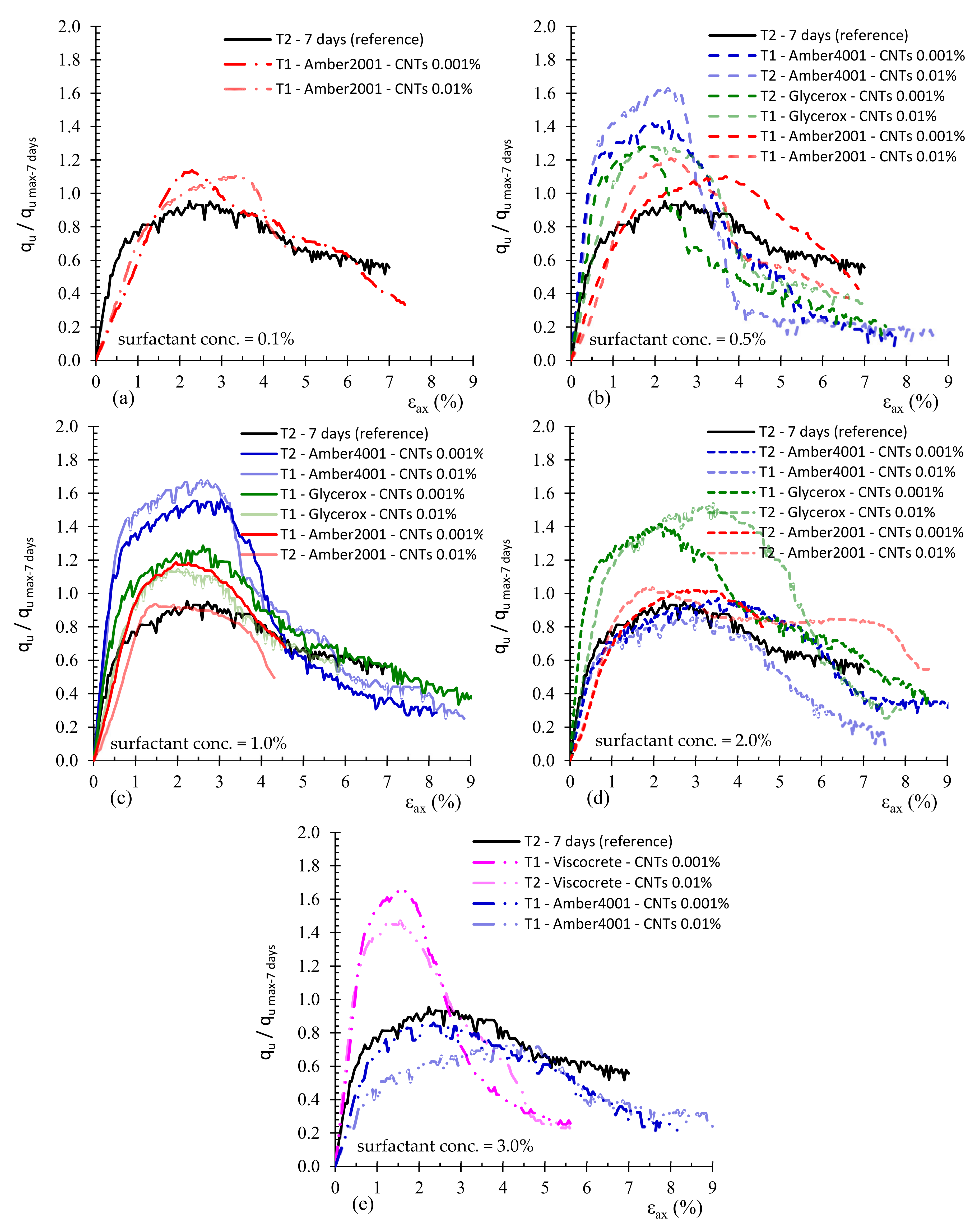
3.1. Effect of Surfactant Type
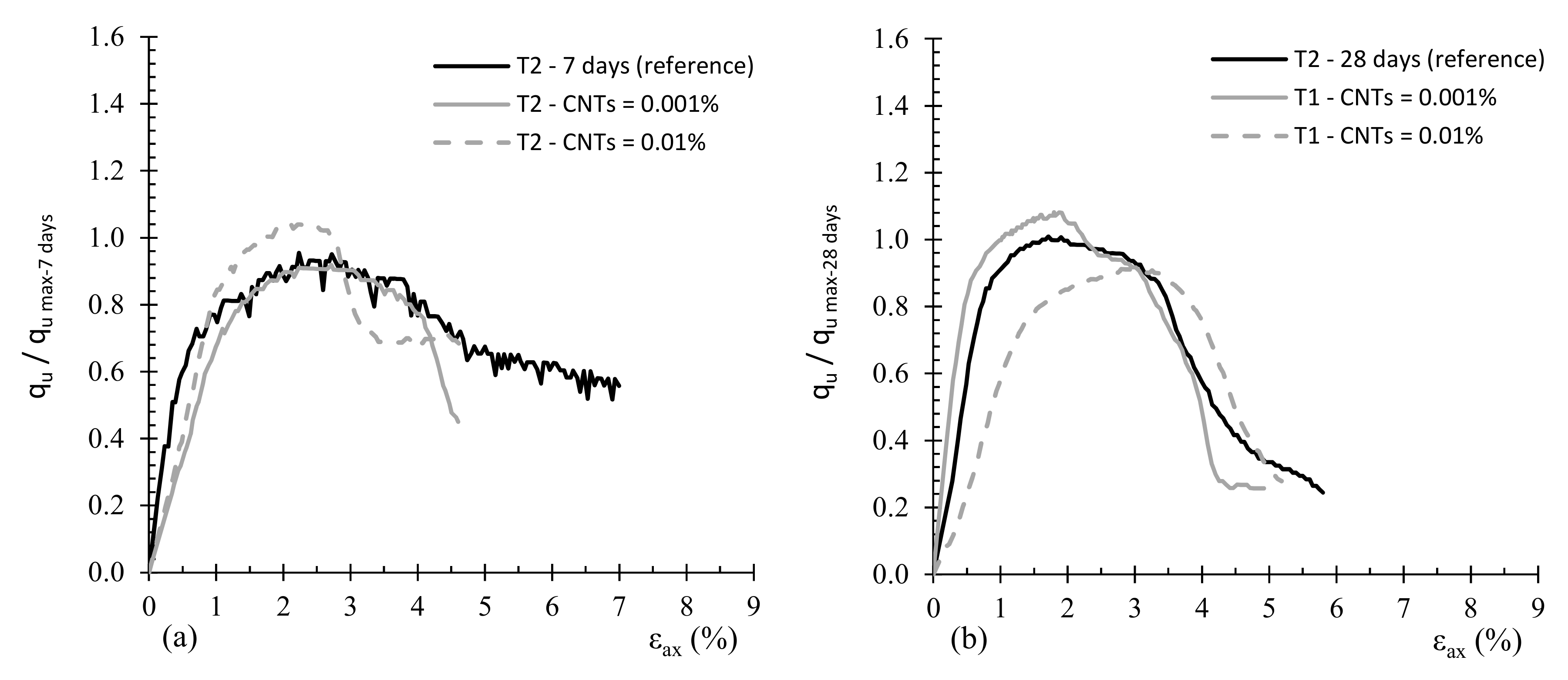
3.2. Effect of MWCNTs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bolt, G.H. Composition and Physical Properties of Soils. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 1983; pp. 1–36. [Google Scholar]
- Conklin, A.R., Jr. Introduction to Soil Chemistry: Analysis and Instrumentation; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chu, J.; Varaksin, S.; Menge, U.K.P. Construction Processes—State of the Art Report. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Alexandria, Egypt, 5–9 October 2009; pp. 3006–3135. [Google Scholar]
- Venda Oliveira, P.J.; Correia, A.A.S.; Lopes, T.J.S. Effect of organic matter content and binder quantity on the uniaxial creep behavior of an artificially stabilized soil. J. Geotech. Geoenviron. Eng. 2014, 140, 04014053. [Google Scholar] [CrossRef]
- Venda Oliveira, P.J.; Correia, A.A.S.; Garcia, M.R. Effect of Stress Level and Binder Composition on Secondary Compression of an Artificially Stabilized Soil. J. Geotech. Geoenviron. Eng. 2013, 139, 810–820. [Google Scholar] [CrossRef]
- Mithcell, J.K. Soil Improvement—State-of-the-Art Report. In Proceedings of the 10th International Conference on Soil Mechanics and Foundation Engineering, Stockholm, Sweden, 15–19 June 1981; pp. 509–565. [Google Scholar]
- Correia, A.A.S.; Oliveira, P.J.V.; Lemos, L.J.L. Strength assessment of chemically stabilised soft soils. Proc. Inst. Civil Eng. Geotech. Eng. 2019, 172, 218–227. [Google Scholar] [CrossRef]
- Figueiredo, D.T.R.; Correia, A.A.S.; Hunkeler, D.; Rasteiro, M.G.B.V. Surfactants for dispersion of carbon nanotubes applied in soil stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, G.A.; Bergado, D.T. Fundamental Parameters of Cement-Admixed Clay—New Approach. J. Geotech. Geoenviron. Eng. 2004, 130, 1042–1050. [Google Scholar] [CrossRef]
- Correia, A.A.S.; Oliveira, P.J.V.; Custódio, D.G. Effect of polypropylene fibres on the compressive and tensile strength of a soft soil, artificially stabilised with binders. Geotext. Geomembr. 2015, 43, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Edil, T.; Staab, D. Practitioner’s Guide for Deep-Mixed Stabilization of Organic Soils and Peat. Final Report, The National Deep Mixing Research Program, Project Number NDM302. 2005; p. 60. [Google Scholar]
- Porbaha, A. State of the art in deep mixing technology: Part I. Basic concepts and overview. Proc. Inst. Civil Eng. Ground Improv. 1998, 2, 81–92. [Google Scholar] [CrossRef]
- Kitazume, M.; Terashi, M. The Deep Mixing Method; CRC Press: Leiden, The Netherlands, 2013. [Google Scholar]
- Janz, M.; Johansson, S.-E. The Function of Different Binding Agents in Deep Satbilization; Swedish Deep Stabilization Research Centre: Linköping, Sweden, 1992; p. 47. [Google Scholar]
- Ghasabkolaei, N.; Choobbasti, A.J.; Roshan, N.; Ghasemi, S.E. Geotechnical properties of the soils modified with nanomaterials: A comprehensive review. Arch. Civ. Mech. Eng. 2017, 17, 639–650. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L. Experimental studies on nanomaterials for soil improvement: A review. Environ. Earth Sci. 2016, 75, 10. [Google Scholar] [CrossRef]
- Venda Oliveira, P.J.; Neves, J.P.G. Effect of Organic Matter Content on Enzymatic Biocementation Process Applied to Coarse-Grained Soils. J. Mater. Civ. Eng. 2019, 31, 04019121. [Google Scholar] [CrossRef]
- DeJong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Paassen, L.A.V.; Qabany, A.A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Ramdas, V.M.; Mandree, P.; Mgangira, M.; Mukaratirwa, S.; Lalloo, R.; Ramchuran, S. Review of current and future bio-based stabilisation products (enzymatic and polymeric) for road construction materials. Transp. Geotech. 2021, 27, 100458. [Google Scholar] [CrossRef]
- Axelsson, K.; Johansson, S.-E.; Anderson, R. Stabilization of Organic Soils by Cement and Puzzolanic Reactions—Feasibility Study; Swedish Deep Stabilization Research Centre: Linköping, Sweden, 2002. [Google Scholar]
- Correia, A.A.S.; Casaleiro, P.D.F.; Rasteiro, M.G.B.V. Applying multiwall carbon nanotubes for soil stabilization. Procedia Eng. 2015, 102, 1766–1775. [Google Scholar] [CrossRef] [Green Version]
- Licht, S. Co-production of cement and carbon nanotubes with a carbon negative footprint. J. CO2 Util. 2017, 18, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Manzur, T.; Yazdani, N. Optimum mix ratio for carbon nanotubes in cement mortar. KSCE J. Civ. Eng. 2015, 19, 1405–1412. [Google Scholar] [CrossRef]
- Taylor, H. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Makar, J. The Effect of SWCNT and Other Nanomaterials on Cement Hydration and Reinforcement. In Nanotechnology in Civil Infrastructure; Gopalakrishnan, K., Birgisson, B., Taylor, P., Attoh-Okine, N.O., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Luo, J.; Duan, Z.; Xian, G.; Li, Q.; Zhao, T. Damping Performances of Carbon Nanotube Reinforced Cement Composite. Mech. Adv. Mater. Struct. 2015, 22, 224–232. [Google Scholar] [CrossRef]
- Šmilauer, V.; Hlavácek, P.; Padevet, P. Micromechanical Analysis of Cement Paste with Carbon Nanotubes. Acta Polytech. 2012, 52, 22–28. [Google Scholar] [CrossRef]
- Wang, B.; Pang, B. Properties improvement of multiwall carbon nanotubes-reinforced cement-based composites. J. Compos. Mater. 2019, 54, 2379–2387. [Google Scholar] [CrossRef]
- Sikora, P.; Elrahman, M.A.; Chung, S.Y.; Cendrowski, K.; Mijowska, E.; Stephan, D. Mechanical and microstructural properties of cement pastes containing carbon nanotubes and carbon nanotube-silica core-shell structures, exposed to elevated temperature. Cem. Concr. Compos. 2019, 95, 193–204. [Google Scholar] [CrossRef]
- Zhou, C.; Li, F.; Hu, J.; Ren, M.; Wei, J.; Yu, Q. Enhanced mechanical properties of cement paste by hybrid graphene oxide/carbon nanotubes. Constr. Build. Mater. 2017, 134, 336–345. [Google Scholar] [CrossRef]
- Taha, M.R.; Alsharef, J.M.A. Performance of Soil Stabilized with Carbon Nanomaterials. Chem. Eng. Trans. 2018, 63, 757–762. [Google Scholar]
- Taha, M.R.; Ying, T. Effects of Carbon Nanotube on Kaolinite: Basic Geotechnical Behavior. In Proceedings of the ICCE 18—18th International Conference on Composites/Nano-engineering, Anchorage, AK, USA, 4–10 July 2010. [Google Scholar]
- Arabani, M.; Haghi, A.; Moradi, Y. Evaluation of Mechanical Properties Improvement of Clayey Sand by Using Carbon Nanotubes. 2012. [Google Scholar]
- Correia, A.A.S.; Rasteiro, M.G. Nanotechnology Applied to Chemical Soil Stabilization. Procedia Eng. 2016, 143, 1252–1259. [Google Scholar] [CrossRef] [Green Version]
- ASTM. ASTM D2487, Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM International: West Conshohocken, PA, USA, 1998. [Google Scholar]
- Casaleiro, P.D.F. Chemical Stabilization of the Soft Soil of Baixo Mondego by Nanomaterials. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2014. [Google Scholar]
- Figueiredo, D.T.R. Characterization of Carbon Nano-Tubes Dispersions for Application in Soil Stabilization. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2014. [Google Scholar]
- Moura, M.S.M.R. Improvement of Carbon Nanotube Dispersions for Application in Soil Chemical Stabilization. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2015. [Google Scholar]
- Correia, A.A.S. Applicability of Deep Mixing Technique to the Soft Soil of Baixo Mondego. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2011. [Google Scholar]
- BSI. BSI 1377-7: Methods of Test for Soils for Civil Engineering Purposes. Shear Strength Tests (Total Stress); British Standards Institution: London, UK, 1990. [Google Scholar]
- ASTM. ASTM D2166: Standard Test Method for Unconfined Compressive Strength of Cohesive Soil; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Venda Oliveira, P.J.; Correia, A.A.S.; Garcia, M.R. Effect of Organic Matter Content and Curing Conditions on the Creep Behavior of an Artificially Stabilized Soil. J. Mater. Civ. Eng. 2012, 24, 868–875. [Google Scholar] [CrossRef]
- Horpibulsuk, S. Analysis and Assessment of Engineering Behavior of Cement Stabilized Clays. Ph.D. Thesis, Saga University, Saga, Japan, 2001. [Google Scholar]
- Correia, A.A.S.; Lopes, L.; Reis, M.S. Advanced predictive modelling applied to the chemical stabilisation of soft soils (Ahead of print). Proc. Inst. Civil Eng. Geotech. Eng. 2021, 1–11. [Google Scholar] [CrossRef]
- Venda Oliveira, P.J.; Correia, A.A.S.; Lemos, L.J.L. Numerical modelling of the effect of curing time on the creep behaviour of a chemically stabilised soft soil. Comput. Geotech. 2017, 91, 117–130. [Google Scholar] [CrossRef]


| Surfactant | CNTs | Time | |
|---|---|---|---|
| Name | Conc. (%) | Conc. (%) | (Days) |
| - | - | - | 7/28 |
| 0.001 | |||
| 0.01 | |||
| Viscocrete | 3 | - | 7/28 |
| 0.001 | |||
| 0.01 | |||
| Glycerox | 0.5/1/2 | - | 7 |
| 0.001 | |||
| 0.01 | |||
| Amber 2001 | 0.1 */0.5/1 */2 | - | 7 * |
| 0.001 | |||
| 0.01 | |||
| Amber 4001 | 0.5/1/2/3 | - | 7 |
| 0.001 | |||
| 0.01 | |||
| CaO (%) | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | MgO (%) | SO3 (%) | Cl− (%) | S (m2/kg) | Z (mV) |
|---|---|---|---|---|---|---|---|---|
| 62.8 | 19.2 | 4.9 | 3.2 | 2.5 | 3.4 | 0.01 | 349 | −2.14 |
| Surfactant | Market Condition | Charge (-) | Z (mV) | Dz (nm) | MW (kDa) |
|---|---|---|---|---|---|
| Viscocrete | commercial | nonionic | −2.8 | 4.65 | 242 |
| Glycerox | commercial | nonionic | ~0 | 41.93 | 4265 |
| Amber 2001 | noncommercial | cationic | 66.7 | 170.84 | 1155 |
| Amber 4001 | noncommercial | cationic | - | 5.65 | 54 |
| Surfactant | Dz (for MWCNTs = 0.001%/0.01%) | |
|---|---|---|
| Name | Conc. (%) | (nm) |
| Viscocrete | 3 | 155.1/119.5 |
| Glycerox | 0.5 | -/197.2 |
| 1 | -/167.6 | |
| 3 | -/175.2 | |
| Amber 2001 | 0.1 | -/- |
| 0.5 | 548.0/684.5 | |
| 1 | -/718.2 | |
| 2 | -/954.2 | |
| Amber 4001 | 0.5 | -/521.5 |
| 1 | -/322.9 | |
| 3 | -/316.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.A.S.; Casaleiro, P.D.F.; Figueiredo, D.T.R.; Moura, M.S.M.R.; Rasteiro, M.G. Key-Parameters in Chemical Stabilization of Soils with Multiwall Carbon Nanotubes. Appl. Sci. 2021, 11, 8754. https://doi.org/10.3390/app11188754
Correia AAS, Casaleiro PDF, Figueiredo DTR, Moura MSMR, Rasteiro MG. Key-Parameters in Chemical Stabilization of Soils with Multiwall Carbon Nanotubes. Applied Sciences. 2021; 11(18):8754. https://doi.org/10.3390/app11188754
Chicago/Turabian StyleCorreia, António Alberto S., Pedro D. F. Casaleiro, Diogo T. R. Figueiredo, Marta S. M. R. Moura, and Maria Graça Rasteiro. 2021. "Key-Parameters in Chemical Stabilization of Soils with Multiwall Carbon Nanotubes" Applied Sciences 11, no. 18: 8754. https://doi.org/10.3390/app11188754
APA StyleCorreia, A. A. S., Casaleiro, P. D. F., Figueiredo, D. T. R., Moura, M. S. M. R., & Rasteiro, M. G. (2021). Key-Parameters in Chemical Stabilization of Soils with Multiwall Carbon Nanotubes. Applied Sciences, 11(18), 8754. https://doi.org/10.3390/app11188754








