Computer Assisted Surgery and 3D Printing in Orthopaedic Oncology: A Lesson Learned by Cranio-Maxillo-Facial Surgery
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gerrand, C.; Athanasou, N.; Brennan, B. UK guidelines for the management of bone sarcomas. Clin. Sarcoma Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Li, J.; Li, Q.; Li, G.; Cai, Z. Clinical effectiveness of hemipelvic reconstruction using computer-aided custom-made prostheses after resection of malignant pelvic tumors. J. Arthroplast. 2011, 26, 1508–1513. [Google Scholar] [CrossRef]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef]
- Merema, B.J.; Kraeima, J.; Ten Duis, K.; Wendt, K.W.; Warta, R.; Vos, E. The design, production and clinical application of 3D patient-specific implants with drilling guides for acetabular surgery. Injury 2017, 48, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Ronsivalle, V.; Grippaudo, C.; Lucchese, A.; Muraglie, S.; Lagravère, M.; Isola, G. One step before printing-evaluation of imaging software accuracy for 3-dimensional analysis of the mandible: A comparative study using a surface-to-surface matching technique. Materials 2020, 13, 2798. [Google Scholar] [CrossRef]
- Cartiaux, O.; Paul, L.; Francq, B.G.; Banse, X.; Docquier, P.L. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery. Ann. Biomed. Eng. 2014, 42, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J. Surg. Oncol. 2015, 4, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollazzo, V.; Pezzetti, F.; Massari, L.; Palmieri, A.; Brunelli, G.; Zollino, I.; Lucchese, A.; Caruso, G.; Carinci, F. Evaluation of gene expression in MG63 human osteoblastlike cells exposed to tantalum powder by microarray technology. Int. J. Periodontics Restor. Dent. 2011, 31, e17–e28. [Google Scholar]
- Rodriguez y Baena, R.; Pastorino, R.; Gherlone, E.F.; Perillo, L.; Lupi, S.M.; Lucchese, A. Histomorphometric evaluation of two different bone substitutes in sinus augmentation procedures: A randomized controlled trial in humans. Int. J. Oral. Maxillofac. Implant. 2017, 32, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.H.; Zhang, Y.Z.; Zhang, L.C.; He, C.Q.; Wang, Y.; Tang, P.F. Custom-made locked plating for acetabular fracture: A pilot study in 24 consecutive cases. Orthopedics 2014, 37, e660–e670. [Google Scholar]
- Imatani, J.; Ogura, T.; Morito, Y.; Hashizume, H.; Inoue, H. Custom AO small T plate for transcondylar fractures of the distal humerus in the elderly. J. Shoulder Elb. Surg. 2005, 14, 611–615. [Google Scholar] [CrossRef]
- Qiao, F.; Li, D.; Jin, Z.; Gao, Y.; Zhou, T.; He, J.; Cheng, L. Application of 3D printed customized external fixator in fracture reduction. Injury 2015, 46, 1150–1155. [Google Scholar] [CrossRef]
- Qiao, F.; Li, D.; Jin, Z. A novel combination of computer-assisted reduction technique and three dimensional printed patient-specific external fixator for treatment of tibial fractures. Int. Orthop. 2016, 40, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.B.; Lee, S.; Kim, H. Three-Dimensional Printing: Basic Principles and Applications in Medicine and Radiology. Korean J. Radiol. 2016, 17, 182–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGurk, M.; Amis, A.A.; Potamianos, P.; Goodger, N.M. Rapid prototyping techniques for anatomical modelling in medicine. Ann. R. Coll. Surg. Engl. 1997, 79, 169–174. [Google Scholar] [PubMed]
- Malik, H.H.; Darwood, A.R.; Shaunak, S. Three dimensional printing in surgery: A review of current surgical applications. J. Surg. Res. 2015, 199, 512–522. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Chelule, K.L.; Seedhom, B. Accuracy of MRI vs CT imaging with particular reference to patient specific templates for total knee replacement surgery. Int. J. Med. Robot 2008, 4, 224–231. [Google Scholar] [CrossRef]
- Kunz, M.; Rudan, J.F.; Wood, G.C.; Ellis, R.E. Registration stability of physical templates in hip surgery. Stud. Health Technol. Inform. 2011, 163, 283–289. [Google Scholar]
- Schkommodau, E.; Decker, N.; Klapper, U.; Birnbaum, S.; Staudte, H.W.; Radermacher, K. Pedicle Screw Implantation Using the DISOS Template System. In Navigation and Robotics in Total Joint and Spine Surgery; Stiehl, J.B., Konermann, W.H., Haaker, R.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 501–505. [Google Scholar]
- Dobbe, J.G.; Pre, K.J.; Kloen, P.; Blankevoort, L.; Streekstra, G.J. Computer-assisted and patient-specific 3-D planning and evaluation of a single-cut rotational osteotomy for complex long-bone deformities. Med. Biol. Eng. Comput. 2011, 49, 1363–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birnbaum, K.; Schkommodau, E.; Decker, N.; Prescher, A.; Klapper, U.; Radermacher, K. Computer assisted orthopedic surgery with individual templates and comparison to conventional operation method. Spine 2001, 26, 365–370. [Google Scholar] [CrossRef]
- Donati, D.; Di Bella, C.; Frisoni, T.; Cevolani, L.; DeGroot, H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clin. Orthop. Relat. Res. 2011, 469, 1450–1458. [Google Scholar] [CrossRef] [Green Version]
- De Paolis, M.; Biazzo, A.; Romagnoli, C.; Alì, N.; Giannini, S.; Donati, D.M. The use of iliac stem prosthesis for acetabular defects following resections for periacetabular tumors. Sci. World J. 2013, 22, 717031. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Dunham, W.K. Resection and reconstruction for primary neoplasms involving the innominate bone. J. Bone Jt. Surg. Am. 1978, 60, 731–746. [Google Scholar] [CrossRef]
- Smolle, M.A.; Andreou, D.; Tunn, P.U.; Leithner, A. Advances in tumour endoprostheses: A systematic review. EFORT Open Rev. 2019, 4, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, P.; Mavrogenis, A.F.; Bianchi, G.; Sakellariou, V.I.; Mercuri, M.; Papagelopoulos, P.J. Outcome of the intramedullary diaphyseal segmental defect fixation system for bone tumors. J. Surg. Oncol. 2011, 104, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Traub, F. Biological reconstruction following the resection of malignant bone tumors of the pelvis. Sarcoma 2013, 2013, 745360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, T.; Hillmann, A.; Bettin, D.; Wuisman, P.; Winkelmann, W. High complication rates with pelvic allografts. Experience of 22 sarcoma resections. Acta Orthop. Scand. 1996, 67, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Hillmann, A. Tumors of the pelvis: Complications after reconstruction. Arch. Orthop. Trauma Surg. 2003, 123, 340–344. [Google Scholar] [CrossRef]
- Liang, H.; Ji, T.; Zhang, Y.; Wang, Y.; Guo, W. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Jt. J. 2017, 99, 267–275. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.T.; Wang, X.Q.; Wang, J.J. Functional outcome of en bloc excision and custom prosthetic replacement for giant cell tumor of the distal radius. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2015, 20, 1090–1097. [Google Scholar] [CrossRef]
- Zhang, Y.D. Effect of 3D printing technology on pelvic fractures: A Meta-analysis. J. Orthop. Traumatol. 2018, 31, 465–471. [Google Scholar]
- Hung, C.C. Conventional plate fixation method versus pre-operative virtual simulation and three-dimensional printing-assisted contoured plate fixation method in the treatment of anterior pelvic ring fracture. Int. Orthop. 2019, 43, 425–431. [Google Scholar] [CrossRef]
- Shon, H.C.; Choi, S.; Yang, J.Y. Three-dimensional printing-assisted surgical technique with limited operative exposure for both-column acetabular fractures. J. Trauma Emerg. Surg. 2018, 24, 369–375. [Google Scholar]
- Kim, J.W. Clinical experience with three-dimensional printing techniques in orthopedic trauma. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2018, 23, 383–388. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, C.; Liu, Y.; He, H. Surgical treatment for shepherd’s crook deformity in fibrous dysplasia: THERE IS NO BEST, ONLY BETTER. Int. Orthop. 2019, 43, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. Three dimensional patient-specific printed cutting guides for closing-wedge distal femoral osteotomy. Int. Orthop. 2019, 43, 619–624. [Google Scholar] [CrossRef]
- Cernat, E. Patient Specific Instruments for Complex Tumor Resection-Reconstruction Surgery within the Pelvis: A Series of 4 Cases. Chir. Buchar. Rom. 2016, 111, 439–444. [Google Scholar] [CrossRef]
- Gouin, F.; Paul, L.; Odri, G.A.; Cartiaux, O. Computer-Assisted Planning and Patient-Specific Instruments for Bone Tumor Resection within the Pelvis: A Series of 11 Patients. Sarcoma 2014, 2014, 842709. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Isola, G.; Ramaglia, L.; Dalessandri, D.; Lucchese, A.; Alibrandi, A.; Fabiano, F.; Cordasco, G. Periodontal biotype: Characteristic, prevalence and dimensions related to dental malocclusion. Minerva Stomatol. 2016, 65, 231–238. [Google Scholar]
- Bertossi, D.; Giampaoli, G.; Lucchese, A.; Manuelli, M.; Albanese, M.; Nocini, R.; Nocini, P.F. The skin rejuvenation associated treatment-Fraxel laser, Microbotox, and low G prime hyaluronic acid: Preliminary results. Lasers Med. Sci. 2019, 34, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Sambri, A.; Sebastiani, E.; Caldari, E.; Donati, D. Is unicondylar osteoarticular allograft still a viable option for reconstructions around the knee? Knee 2016, 23, 692–697. [Google Scholar] [CrossRef] [PubMed]
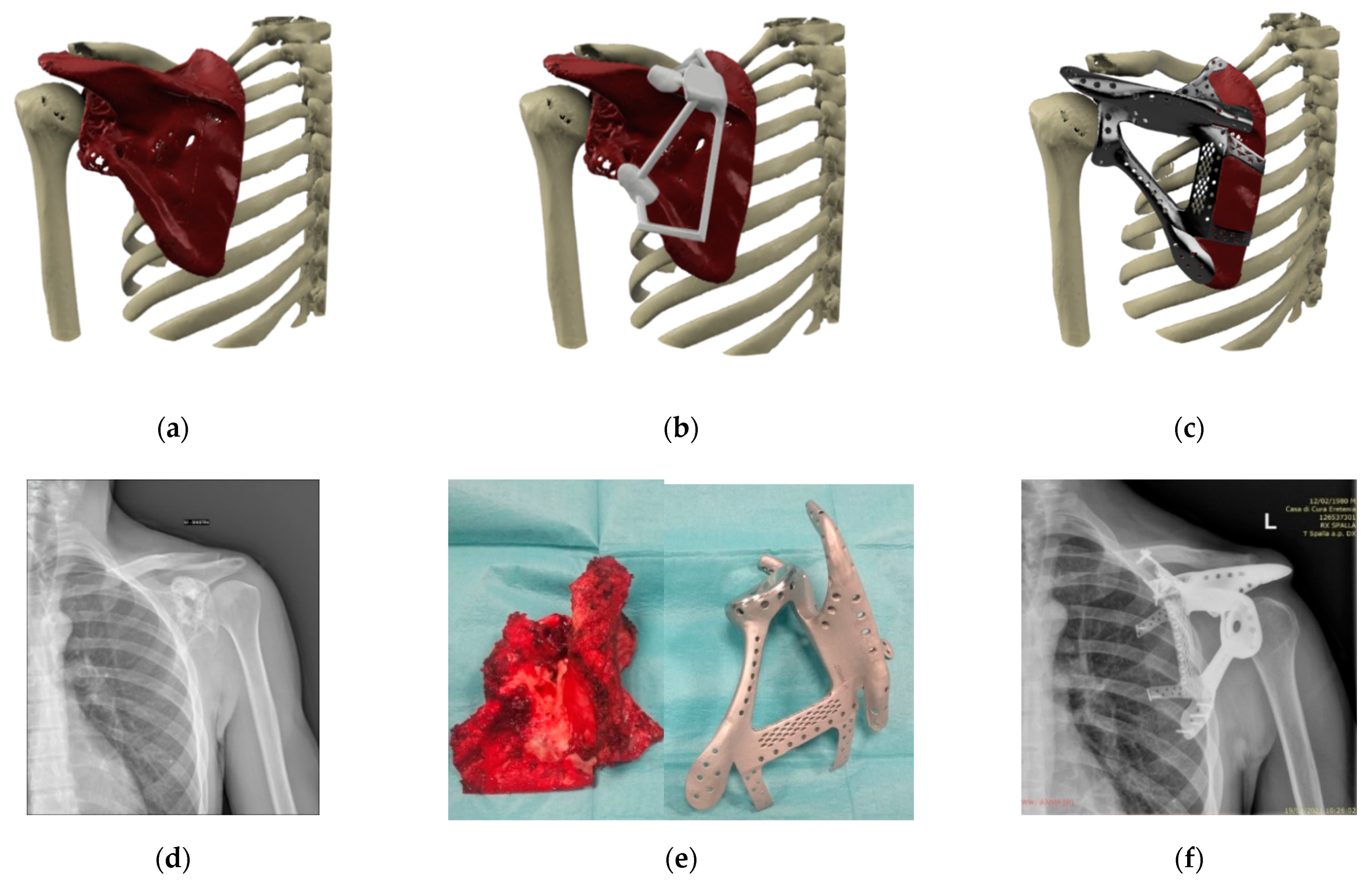
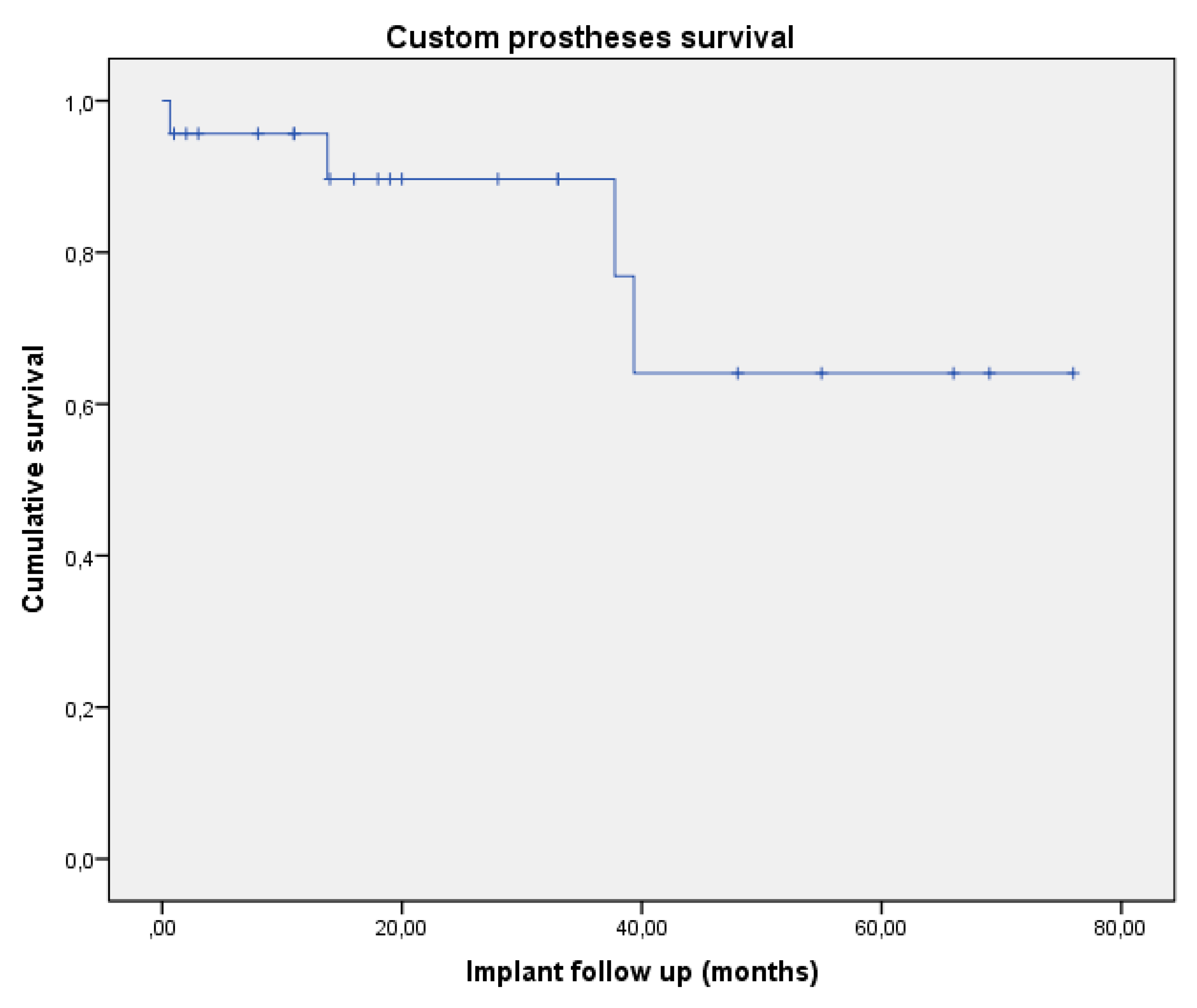
| Patient | Gender | Age | Site | Joint Involvement | Diagnosis | Surgical Margins | Revision Surgery (Months) | Implant Removal (Months) | Patient Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | Tibia | Myxofibrosarcoma | Marginal | Infection (1) | 14 | ||
| 2 | M | 76 | Pelvis | Hip | Chondorsarcoma | Wide | Infection (1) | 2 | |
| 3 | M | 18 | Pelvis | Hip | Ewing Sarcoma | Wide | Infection (1) | 76 | |
| 4 | F | 23 | Pelvis | Hip | Ewing Sarcoma | Wide | 69 | ||
| 5 | F | 26 | Femur | Osteosarcoma | Wide | 11 | |||
| 6 | M | 22 | Femur | Ewing Sarcoma | Wide | Infection (1) | 33 | ||
| 7 | F | 46 | Astragalus | Ankle | Osteosarcoma | Wide | Arthritis (19) | 19 | |
| 8 | M | 56 | Pelvis | Sacro Iliac | Chondorsarcoma | Wide | Infection (1) | Yes | 39 |
| 9 | F | 47 | Pelvis | Hip | Chondorsarcoma | Wide | Recurrence (39) | Yes | 44 |
| 10 | M | 40 | Scapula | Shoulder | Osteosarcoma ** | Wide | 3 | ||
| 11 | M | 26 | Pelvis | Sacro Iliac | Ewing Sarcoma | Wide | Screw (1) | 11 | |
| 12 | M | 45 | Tibia | Osteosarcoma | Wide | Non union (9) | 18 | ||
| 13 | M | 55 | Pelvis | Hip | Chondorsarcoma | Wide | Infection (1) | 1 | |
| 14 | F | 40 | Pelvis | Hip | Osteoblastoma * | Wide | Recurrence (37) | Yes | 39 |
| 15 | M | 38 | Tibia | Ankle | Chondorsarcoma | Wide | 16 | ||
| 16 | M | 14 | Pelvis | Sacro Iliac | Ewing Sarcoma | Wide | 55 | ||
| 17 | M | 35 | Pelvis | Hip | Chondorsarcoma | Wide | 20 | ||
| 18 | M | 28 | Pelvis | Hip | Osteosarcoma | Marginal | 48 | ||
| 19 | M | 45 | Pelvis | Hip | Chondorsarcoma | Wide | 33 | ||
| 20 | M | 26 | Pelvis | Hip | Ewing Sarcoma | Wide | 66 | ||
| 21 | M | 43 | Pelvis | Hip | Osteosarcoma | Wide | 8 | ||
| 22 | M | 26 | Pelvis | Hip | Giant Cell Tumor | Wide | 28 | ||
| 23 | M | 36 | Pelvis | Hip | Chondorsarcoma | Wide | Recurrence (14) | Yes | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, G.; Frisoni, T.; Spazzoli, B.; Lucchese, A.; Donati, D. Computer Assisted Surgery and 3D Printing in Orthopaedic Oncology: A Lesson Learned by Cranio-Maxillo-Facial Surgery. Appl. Sci. 2021, 11, 8584. https://doi.org/10.3390/app11188584
Bianchi G, Frisoni T, Spazzoli B, Lucchese A, Donati D. Computer Assisted Surgery and 3D Printing in Orthopaedic Oncology: A Lesson Learned by Cranio-Maxillo-Facial Surgery. Applied Sciences. 2021; 11(18):8584. https://doi.org/10.3390/app11188584
Chicago/Turabian StyleBianchi, Giuseppe, Tommaso Frisoni, Benedetta Spazzoli, Alessandra Lucchese, and Davide Donati. 2021. "Computer Assisted Surgery and 3D Printing in Orthopaedic Oncology: A Lesson Learned by Cranio-Maxillo-Facial Surgery" Applied Sciences 11, no. 18: 8584. https://doi.org/10.3390/app11188584
APA StyleBianchi, G., Frisoni, T., Spazzoli, B., Lucchese, A., & Donati, D. (2021). Computer Assisted Surgery and 3D Printing in Orthopaedic Oncology: A Lesson Learned by Cranio-Maxillo-Facial Surgery. Applied Sciences, 11(18), 8584. https://doi.org/10.3390/app11188584







