Abstract
Currently, various immunotherapeutic treatments are revolutionizing therapies that treat solid neoplasms. For these treatments, within immunotherapy, immune checkpoint inhibitors (ICIs) are the most widely used drugs. Diverse studies have shown the influence of diet and probiotics on the response to ICIs and consequently on the survival rates associated with different neoplasms. The use of various antibiotics, probiotics, and prebiotics has been associated with changes in the gut microbiota, and this, in turn, with resistance to immunotherapy. Together with the above, a lower intake of red meat and greater consumption of a Mediterranean, vegetarian, or vegan diet have led to a new way of understanding the mechanisms of resistance to ICIs. Omega-3 and polyphenol supplements are also powerful regulators of the microbiome whose influence on the immune system. Therefore, this review covers the influence of diet and probiotics on the response to immunotherapy in patients who have solid tumours.
1. Background
Many studies have investigated the relationship of the microbiota’s composition to its functions in human diseases [1,2]. The reasoning behind the hypothesis of a relationship between microbiota and various diseases derives from studies that use animal models in which the prevalence of disease was observed to depend on the specific microbial community of the animals. The term gut microbiota, or intestinal flora, refers to the set of microorganisms that normally occur in the digestive tract of the human organism. The human gastrointestinal tract holds, on average, 1014 microorganisms/mL of luminal content, including about 5000 species of bacteria, with 150–200 bacterial species being frequent and the rest unusual [3]. Bacteria make up much of the gut microbiota; however, archaebacteria, viruses, and protozoa are also present [4].
The gut microbiota is dynamic and evolves throughout life. In the foetus, there is an absence of bacteria (although some authors have proposed the existence of non-pathogenic commensal bacteria in the placenta) [5]; this evolves into a diverse microbiota in the adult. In the adult patient, high interindividual variability increases over time. It has been suggested that in adult life, the microbiota is the result of both positive and negative selection. Some of these mechanisms may be due to host- or bacteria-specific factors; however, the most important of them all is diet [6]. Many studies have observed how the diversity of the microbiota is influenced not only by the intake of macronutrients but also by caloric intake [7].
In recent decades, industrialized countries have undergone a profound change in their dietary habits, abandoning the abundant intake of cereals, tubers, legumes, fruits, and vegetables in favour of a more western diet that features the frequent consumption of refined foods, meats, and other products of animal origins [8]. This change in dietary habits and other lifestyle factors, such as a sedentary lifestyle or a higher level of anxiety, are the main causes of the changes in the gut microbiota [9]. In their study on gnotobiotic mice into which human faecal microbiota had been transplanted, the authors found that the replacement of a diet low in fat and rich in polysaccharides by one with a high fat and sugar content induced a rapid change in the gut microbiota, with an increase in the bacterial groups belonging to the genus Filobacillus and a decrease in those belonging to Bacteroidetes (Turnbaugh et al., 2009) [10]. These results have been confirmed in other studies (Filippo et al., 2010) [11].
All these changes in the intestinal flora lead to alterations in the immune system that in turn lead to pathologies or responses to drugs in the treatment of various diseases. The interaction in the intestinal epithelium between the superficial mucus layer, the immunoglobulin A (IgA)-producing cells (B lymphocytes), antimicrobial peptides, and the complex adhesion and binding system of the epithelial cells makes this epithelium the immune system’s first barrier of defence and regulation [12]. Because of this, dietary supplements, such as probiotics, and diet are fundamental not only to the genesis of cancer but also to the response to cancer treatments, in particular to immunotherapy [13]. That is why the objective of this review is to assess the influence of dietary supplements and diet on the immunotherapeutic treatment of solid tumours.
2. The Immunity of the Intestinal Mucosa
The mucous membranes of the human gastrointestinal tract have a surface area of 400 m2 and are covered by a thin layer of cells that make up the intestinal absorptive epithelium, structured as crypts and villi [14]. The special architecture of the intestinal epithelium consists of highly specific molecules on the surface of enterocytes, which allows substrates to be absorbed while keeping the barrier intact against antigenic impacts. The barrier function of the epithelium is paramount, with the narrow intercellular junctions being crucial to the maintenance of the homeostatic symbiosis between the gut microbiota and the host [15].
The gastrointestinal tract hosts a rich microbiota, whose density increases from the stomach to the distal colon and comprises more than 500 bacterial species [16]. The first bacteria to colonize the large bowel are strains of Escherichia coli and Enterococcus, along with strict anaerobes [17]. Subsequently, depending on diet, there are changes in the gut microbiota, with a predominance of the species Bifidobacterium in children who have been breastfed or of Bacteroides in neonates fed with infant formula. These bacteria change throughout adult life and interact with the secretory immunoglobulin A (S-IgA) produced by the plasma cells located in the Peyer’s patches and the lamina propria [18]. These complexes are driven to the subepithelial area by a specialized receptor in cells that have microfolds (M cells), which leads to the production of interleukin-10 (IL-10), which contributes to a change in the class of IgA [19]. This process is fundamental for the establishment of a stable microbiome, ensuring effective and non-pathological communication between the gut microbiota and the immune system, thus creating an environment of tolerance.
Dendritic cells are also central to immune tolerance and the regulation of intestinal immunity. Depending on the bacterial strain, these cells are stimulated to produce either a Th1-type response and the secretion of IL-12 or a Th2-type response and the secretion of IL-10 [20]. These responses are unique and very specific. This demonstrates how complex and specialized this system is, capable of discriminating between a pathogenic and a tolerogenic response [21].
These mechanisms explain the beneficial interactions between the gut microbiota and the health status of the host. They are why the different changes in the gut microbiota that occur in childhood and in adult life have consequences both for the development of the different neoplasms and for their treatment. This opens a new path in the treatment of neoplasms: modulating the immune system of patients who are undergoing immunotherapeutic treatment for tumours. Therefore, from now on, it will be crucial to keep in mind the possible role that diet and probiotics can play in the survival and quality of life of these patients [22].
3. The Regulation of the Microbiota by Prebiotics and Probiotics
Foods of plant origin induce an important positive modification of the gut microbiota. Fibre is one of the most frequently used dietary components of food and is present in vegetables and fruits. Its use was already recommended by Hippocrates when he recommended consuming bread for “its beneficial effects on the intestines” [23]. The define of prebiotics: they are non-digestible food ingredients that stimulate the growth of bifidogenic bacteria and lactic acid in the gastrointestinal tract. Typically, prebiotics consist of dietary fibre and oligosaccharides, although not all dietary fibres are classified as prebiotic. Nonetheless, most prebiotics can be classified as containing dietary fibre.
Additionally, probiotics are living microorganisms (mostly bacteria, such as Pediococcus, Lactobacillus, or Weissella) that are beneficial for maintaining digestive tract homeostasis [24]. Currently, probiotics are available to consumers, mainly in the form of dietary supplements. The introduction into the diet of probiotics (such as Bifidobacterium bifidum) or prebiotics (such as fructooligosaccharides) promotes the growth and activity of certain bacterial species and is the mechanism currently used to manipulate the intestinal microbiota. Currently, milk of animal origin contains a considerable number of beneficial bacteria, such as Lactobacillus, Enterococcus, and Pediococcus [25]. Other milk products, such as kefir or traditional Greek dairy foods, also contain many species of bacteria beneficial to the intestinal immune system [26,27]. Enterococcus species are found in high numbers in sea oysters and in some meat products, and their intake is possibly beneficial in the prevention of colorectal cancer [28]. All these foods can have an effect on the response to immunotherapy by altering the gut microbiota and positively influencing T lymphocytes, with an increase in the antitumor response of lymphocytes [29].
4. Diet and the Gut Microbiota
In recent years, the importance of diet in the development of different digestive pathologies (not only of cancer), such as chronic inflammatory diseases, has been shown [30]. Western diets bring an increased risk of cancer at different organs, especially the risk of colorectal cancer, for which the relationship has been clearly established [31]. Therefore, knowing the relationships between diet, the microbiome, and the digestive, immune system is important for later establishing their links to oncological therapies for solid tumours.
4.1. The Mediterranean Diet and Nutritional Supplements
The relationship between the Mediterranean diet and health has been known since the mid-twentieth century. A Mediterranean diet features a high concentration of antioxidants (fruits and vegetables), foods low in cholesterol, and unsaturated fatty acids (present in fish and olive oil) [32]. Several studies have shown that high consumption of products belonging to the Mediterranean diet is inversely correlated with the risks of breast, colorectal, gastric, prostate, liver, head and neck, pancreatic, and lung cancer [33]. This protective effect is due to, amongst other causes, the anti-inflammatory effect of this diet, the positive regulation of the microbiome, and the greater presence of the species Filobacillus firmicutes.
In addition to the above, nutritional supplements that contain arginine, omega-3 fatty acids, or nucleotides increase the action of the immune system and the consequent destruction of tumour cells in oncological pathologies [34]. The activation of the immune system is dependent on innate immunity, which is carried out by activating key enzymatic processes in the antitumor function of these cells [35]. It has been observed that administering these supplements to cancer patients reduces their infections after surgery and reduces their hospital stay [36]. It is important to consider these factors since their involvement in the alterations of the immune system can be a modulable factor that influences the patient’s response to immunotherapy.
4.2. Ketogenic and Very Low-Calorie Diets
These types of diets theoretically lead to lower levels of glycemia in plasma and consequently to a decrease in insulin growth factor (IGF), a factor that facilitates tumourigenesis. These diets increase apoptosis through the Akt pathway, decrease proinflammatory signals, and increase lipid synthesis in the cytosol [37]. All this results in less production of reactive oxygen species, which positively regulates the effects of the immune system on the homeostasis of the digestive tract [38]. Therefore, although these diets have not been proven to increase the response to chemotherapeutic or immunotherapeutic treatments, they should be considered for research since their theoretical implications could be major.
4.3. Diets Rich in Animal Fats and Proteins
Several studies have validated the influence of diet on the composition of the microbiome [39]. This relationship arose through a complex evolutionary process in which the polysaccharide-rich diet of our ancestors forced the evolution towards a microbiota dominated by saccharolytic bacteria, mainly by Filobacillus firmicutes, capable of extracting additional energy from food. Wu et al., 2013, found that enterotypes were strongly associated with long-term eating habits: although diets rich in protein and saturated fats were associated with a Bacteroides enterotype, carbohydrate-rich and vegetarian diets were associated with a Prevotella enterotype [40].
In general, the western diet features a decrease in beneficial bacteria that positively regulate the immune system, such as Bifidobacteria and Eubacteria. In contrast, harmful species yield a proinflammatory state, such as Bacteroides and Clostridia, increase [41] (Table 1). A diet high in saturated fatty acids increases anaerobic and bacteroid enterotypes [42]. A diet low in saturated fats should be considered, not only to improve cancer patients’ response to treatment but also to prevent a second neoplasm.

Table 1.
Changes in the species of bacteria of the gut microbiota depending on the diet carried out. The different bacteria are shown in two columns, the ones on the left being those beneficial for the regulation of the immune system and those on the right being non-beneficial.
5. Principles of Immunotherapy
The first works linking the immune system to antitumour activity can be traced back to the late nineteenth century. It was William Coley, in 1891, who first injected bacteria into a tumour, thereby reducing the tumour size in a patient affected by sarcoma [43]. The histological analysis of human tumours has revealed the presence of very heterogeneous immune infiltrates across different tumours and patients. These infiltrates include different subpopulations of T cells and cells of innate immunity. Their existence has enabled the development of oncological therapies that are based on the modulation of the immune system [44].
Within the variety of treatments for solid tumours, the most important mechanism of modulation is the negative co-stimulation pathway of the tumour microenvironment. The inhibition of T cells by tumour cells is performed through the two main inhibitory pathways best described for T cells: the cytotoxic T-lymphocyte antigen 4 (CTLA-4) protein receptor and programmed cell death protein 1 (PD-1) [45,46]. These two are the pathways through which immune checkpoint inhibitors (ICIs) act by negatively regulating these receptors and consequently stimulating the T lymphocytes.
Various factors are known as predictors or modifiers of the response to immunotherapy or, more accurately, to ICIs in solid tumours. Amongst the best known are the PD-L1 marker, the performance status, the mutational load or the tumour mutation burden, and tumour-infiltrating lymphocytes (TILs). However, these biomarkers are far from perfect, and biomarkers with greater sensitivity and specificity in response prediction are currently under investigation. Because of this, the microbiome is currently one of the main points under study that influences the response to ICIs [47].
The use of antibiotics during or before treatment by ICIs could compromise its outcome. The administration of antibiotics leads to a decrease in Bacteroid and Burkholderian species, which shows that antibiotics weaken the effect of anti-CTLA4 drugs in animal models [48]. The reestablishment of the microbiome in these cases may increase the response to ICIs (especially to anti-CTLA4). In the same way, it has been observed in mice that the restoration of the intestinal flora by faecal transplantation or by the administration of probiotics of the species Bifidobacterium produces a stronger response to anti-PD1 treatments [49].
The effect of gut microbiota in the response to immunotherapy is currently under investigation. It is vital to take this role into account not only as a predictor of the response but also as a modifiable factor during or prior to treatment with ICIs that could increase the survival rate of patients who do not respond to treatment or who initially have characteristics that predict a poor response to treatment. The main immunotherapy drugs currently in the treatment of solid tumours are summarized in Table 2.

Table 2.
ICIs currently approved by the European Medicines Agency (EMA) in the treatment of solid tumours. Different examples of your current indications are shown in the right column.
6. The Influence of Diet and Probiotics on the Response to Immunotherapy
Various studies are currently being conducted to evaluate the efficacy of dietary interventions or microbiome modification in patients who have received immunotherapeutic treatment [50]. Most clinical trials in this domain have involved faecal microbiota transplantation, with smaller trials using probiotics or diets rich in fibre and low in fat. Especially relevant is the study NCT03700437, which evaluated the efficacy of a dietary treatment 72 h before and 24 h after the administration of chemo- and immunotherapy (carboplatin plus pemetrexed plus pembrolizumab) in patients who had a lung adenocarcinoma. Another relevant study is NCT03950635, on patients who had a history of melanoma and who did not have tumour disease. In these patients, the effects of a dietary intervention were evaluated for two groups: the first group was on a diet rich in fibre and the second group on a ketogenic diet [51]. These studies allow us to evaluate the direct effects of a dietary intervention both in diseased patients undergoing an immunotherapeutic treatment and in patients who have highly immunogenic tumours (e.g., melanoma) without tumour disease. The main studies are found in Table 3.

Table 3.
Summary of the main studies evaluating the effects of dietary interventions in solid tumours.
Studies have shown how a dietary intervention led to changes in the microbiome in the first five days of the diet [52]. Diets that include fibre, as previously stated, produce beneficial changes in the gut microbiota for the maintenance of homeostasis. A recent study showed that 46 patients who were treated with anti-PD1, underwent a dietary intervention, and received probiotics (a high-fibre diet) were five times more likely to respond to immunotherapeutic treatment than those on low-fibre diets [50]. Diets that contain a large amount of fibre and few calories increase the number of species of bacteria that are responsible for digesting and secreting mucin in the intestinal epithelium (Akkermansia municiphila, Bifidobacterium longum, or Faecalibacterium prausnitzii), which are responsible for maintaining the epithelial barrier and therefore for increasing the effectiveness of ICIs. According to several studies, the abundance of these bacteria in the microbiome correlates to the consumption of a high-fibre diet [53]. Likewise, the increase in the number of bacteria of the species Bacteroides fragilis that occurs by following a diet rich in fibre can induce the expression of IL-10, which prevents the expansion of Th17 lymphocytes, the damage to the intestinal mucosa that this would cause, and, in turn, the deregulation of the immune system and the least response to ICIs [54]. The influence of diet on the response to immunotherapy is summarized in Figure 1.
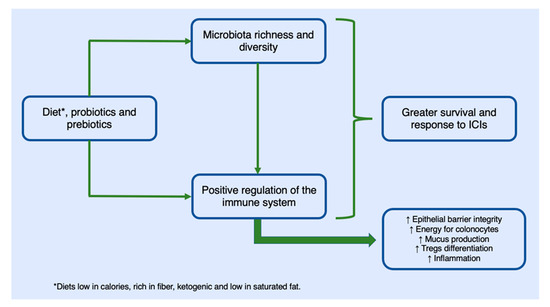
Figure 1.
Mechanism of action of diets and food supplements in their positive influence on the response and increased survival of cancer patients undergoing immunotherapy treatment. The regulation of the immune system occurs through an increase in inflammatory processes that increase the activity of T lymphocytes with antitumor function. This increase in inflammatory processes does not translate into an alteration of homeostasis given the role of regulatory T cells.
7. Immunotoxicity and the Gut Microbiota
Currently, immunotherapies, especially ICIs, have a better toxicity profile than does conventional chemotherapy. However, despite this, some patients experience toxic effects from ICIs in the form of inflammation of various organs (especially in the large intestine, lung, heart, liver, thyroid, and kidney) [55]. This inflammation is observed in patients with an elevated immune response against the tumour, which can lead to immune alterations in different organs [56]. The regulation of the microbiome through diet low-calorie and high-fibre diets, probiotics, or prebiotics could in future prevent or regulate the toxicity associated with ICIs [57]. This field, which is currently in the theoretical phase, should be key to eliminating the toxicity associated with immunotherapy, especially in cases of immunomediated colitis. The increase in the digestive tract of the species of bacteria that regulate the activity of the immune system could constitute a treatment of the first choice in these cases. Since a dietary change is safe and cheap, it could in the not too distant future take precedence over corticosteroid or immunosuppressive treatments, whose effectiveness in some cases is poor and whose long-term side effects are a problem.
An example of this theoretical exposure would be, for example, the use of the species Bacteroidetes by faecal transplantation into patients who experience toxicity due to Ipilimumab (anti-CTLA4) [58]. An association has been observed between the abundance of this species and a decrease in the colitis that Ipilimumab induces by the immunomodulation of T lymphocytes in the colon [59]. It will therefore be interesting in future to use the microbiome to control the toxicity of ICIs, especially in the stomach and the intestine.
8. Future Perspectives
The use of diet and probiotics as regulators of the response to immunotherapy and therefore as contributors to the survival of cancer patients is currently a concept rather than a practice. The current perspective envisages the use of these tools only 10 or 15 years from now. However, the moment when it is known more exactly how immunotherapy functions and the patients who ought to respond well can be identified, it will be possible to manipulate their responses in order to achieve higher survival rates, especially when manipulating the responses of patients who have negative factors for their predicted response to ICIs.
The microbiome currently opens a door to the modification of the immune system and its influence on solid tumours. High-fibre, low-calorie, and, probably, ketogenic diets are a path not only to improving the response to ICIs but also to preventing cancer and increasing the survival of patients who have any type of treatment—not just immunotherapy. Therefore, the introduction of these dietary measures in cancer patients should be very valuable, given their potential benefits, safety, low cost, and accessibility.
Author Contributions
Conceptualization, A.O.-H. and J.P.M.-G. Methodology, A.O.-H. and L.F.-P. Validation, A.O.-H., D.L.-J., R.G.-S., J.J.C.-H., J.P.M.-G. Writing—original draft preparation, A.O.-H., L.F.-P. and J.P.M.-G. Investigation, A.O.-H., L.F.-P. and J.P.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef]
- Vandana, U.K.; Barlaskar, N.H.; Gulzar, A.B.; Laskar, I.H.; Kumar, D.; Paul, P.; Pandey, P.; Mazumder, P.B. Linking gut microbiota with the human diseases. Bioinformation 2020, 16, 196–208. [Google Scholar] [CrossRef]
- Levin, D.; Raab, N.; Pinto, Y.; Rothschild, D.; Zanir, G.; Godneva, A.; Mellul, N.; Futorian, D.; Gal, D.; Leviatan, S.; et al. Diversity and functional landscapes in the microbiota of animals in the wild. Science 2021, 372, eabb5352. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, A.B.; Delaney, M.L.; DuBois, A.M.; Allred, E.N.; Leviton, A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am. J. Obstet. Gynecol. 2008, 198, 110.e1–110.e7. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Shi, Z. Gut Microbiota: An Important Link between Western Diet and Chronic Diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A meta-genomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. Faculty Opinions recommendation of Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Nannini, G.; Dinu, M.; Pagliai, G.; Sofi, F.; Amedei, A. Exploring the food-gut axis in immunotherapy response of cancer patients. World J. Gastroenterol. 2020, 26, 4919–4932. [Google Scholar] [CrossRef] [PubMed]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- van Spaendonk, H.; Ceuleers, H.; Witters, L.; Patteet, E.; Joossens, J.; Augustyns, K.; Lambeir, A.M.; De Meester, I.; De Man, J.G.; De Winter, B.Y. Regulation of intestinal permeability: The role of proteases. World J. Gastroenterol. 2017, 23, 2106–2123. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Gut Bacteria in Health and Disease. Gastroenterol. Hepatol. 2013, 9, 560–569. [Google Scholar]
- Wang, S.; Hibberd, M.L.; Pettersson, S.; Lee, Y.K. Enterococcus faecalis from Healthy Infants Modulates Inflammation through MAPK Signaling Pathways. PLoS ONE 2014, 9, e97523. [Google Scholar] [CrossRef]
- Gutzeit, C.; Magri, G.; Cerutti, A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014, 260, 76–85. [Google Scholar] [CrossRef]
- Hummelshoj, L.; Ryder, L.P.; Poulsen, L.K. The Role of the interleukin-10 Subfamily Members in Immunoglobulin Production by Human B Cells. Scand. J. Immunol. 2006, 64, 40–47. [Google Scholar] [CrossRef]
- Lee, N.; Kim, W.U. Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med. 2017, 49, e340. [Google Scholar] [CrossRef]
- Qian, L.J.; Kang, S.M.; Xie, J.L.; Huang, L.; Wen, Q.; Fan, Y.Y.; Lu, L.J.; Jiang, L. Early-life gut microbial colonization shapes Th1/Th2 balance in asthma model in BALB/c mice. BMC Microbiol. 2017, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Limeta, A.; Ji, B.; Levin, M.; Gatto, F.; Nielsen, J. Meta-analysis of the gut microbiota in predicting response to cancer immuno-therapy in metastatic melanoma. JCI Insight. 2020, 5, e140940. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef]
- Moslemi, M.; Fard, R.M.N.; Hosseini, S.M.; Rad, A.H.; Mortazavian, A.M. Incorporation of Propionibacteria in Fermented Milks as a Probiotic. Crit. Rev. Food Sci. Nutr. 2013, 56, 1290–1312. [Google Scholar] [CrossRef]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir: A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 183. [Google Scholar] [CrossRef]
- Ralston, R.A.; Truby, H.; Palermo, C.; Walker, K.Z. Colorectal Cancer and Nonfermented Milk, Solid Cheese, and Fermented Milk Consumption: A Systematic Review and Meta-Analysis of Prospective Studies. Crit. Rev. Food Sci. Nutr. 2014, 54, 1167–1179. [Google Scholar] [CrossRef]
- de Almeida, C.V.; Taddei, A.; Amedei, A. The controversial role of Enterococcus faecalis in colorectal cancer. Ther. Adv. Gastroenterol. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Shui, L.; Yang, X.; Li, J.; Yi, C.; Sun, Q.; Zhu, H. Gut Microbiome as a Potential Factor for Modulating Resistance to Cancer Im-munotherapy. Front Immunol. 2020, 10, 2989. [Google Scholar] [CrossRef]
- Galland, L. Diet and inflammation. Nut. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology 2017, 152, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Arós, F.; Estruch, R. Mediterranean Diet and Cardiovascular Prevention. Rev. Española De Cardiol. 2013, 66, 771–774. [Google Scholar] [CrossRef]
- Ariav, Y.; Ch’Ng, J.H.; Christofk, H.R.; Ron-Harel, N.; Erez, A. Targeting nucleotide metabolism as the nexus of viral infections, cancer, and the immune response. Sci. Adv. 2021, 7, eabg6165. [Google Scholar] [CrossRef]
- Sorensen, L.S.; Thorlacius-Ussing, O.; Schmidt, E.B.; Rasmussen, H.H.; Lundbye-Christensen, S.; Calder, P.; Lindorff-Larsen, K. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. BJS 2013, 101, 33–42. [Google Scholar] [CrossRef]
- McDaniel, S.S.; Rensing, N.R.; Thio, L.L.; Yamada, K.A.; Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011, 52, e7–e11. [Google Scholar] [CrossRef]
- Pardo, A.C. Ketogenic diet: A role in immunity? Pediatr. Neurol. Briefs 2020, 34, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frame, L.A.; Costa, E.; Jackson, S.A. Current explorations of nutrition and the gut microbiome: A comprehensive evaluation of the review literatura. Nutr. Rev. 2020, 78, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ren, Z.; Pae, M.; Han, S.N.; Meydani, S.N. Diet-induced obesity has a differential effect on adipose tissue and macrophage inflammatory responses of young and old mice. BioFactors 2013, 39, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe. 2018, 23, 27–40. [Google Scholar] [CrossRef]
- Suskind, D.L.; Lee, D.; Solan, P.; Wahbeh, G.; Hayden, H.; Brittnacher, M.; Nuding, M.; Miller, S. Dietary therapy for clostridium difficile colonization: A case series. Anaerobe 2019, 57, 1–3. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William, B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Inthagard, J.; Edwards, J.; Roseweir, A.K. Immunotherapy: Enhancing the efficacy of this promising therapeutic in multiple can-cers. Clin. Sci. 2019, 133, 181–193. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Uruga, H.; Mino-Kenudson, M. Predictive biomarkers for response to immune checkpoint inhibitors in lung cancer: PD-L1 and beyond. Virchows Arch. 2021, 478, 31–44. [Google Scholar] [CrossRef]
- Miller, P.L.; Carson, T.L. Mechanisms and microbial influences on CTLA-4 and PD-1-based immunotherapy in the treatment of cancer: A narrative review. Gut Pathog. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Zitvogel, L. Fecal microbiota transplantation: Can it circumvent resistance to PD-1 blockade in melanoma? Signal Transduct. Target. Ther. 2021, 6, 1–2. [Google Scholar] [CrossRef]
- Lee, K.A.; Shaw, H.M.; Bataille, V.; Nathan, P.; Spector, T.D. Role of the gut microbiome for cancer patients receiving immunotherapy: Dietary and treatment implications. Eur. J. Cancer 2020, 138, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, S.; Pol, J.G.; Ferrere, G.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Trial watch: Dietary interventions for cancer therapy. OncoImmunology 2019, 8, e1591878. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.W.; Tunsjo, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, S.; Piozarro, T.T. The Treg/Th17 Axis: A Dynamic Balance Regulated by the Gut Microbiome. Front Immunol. 2015, 6, 639. [Google Scholar] [CrossRef]
- Mantia, C.M.; Buchbinder, E.I. Immunotherapy Toxicity. Hematol. Clin. N. Am. 2019, 33, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef]
- Spyrou, N.; Vallianou, N.; Kadillari, J.; Dalamaga, M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin. Cancer Biol. 2021, 73, 356–376. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).