Abstract
Euglena gracilis (E. gracilis) accumulates paramylon, an immune-functional beta-glucan that can be used as a functional food. Paramylon production is strongly affected by the organic carbon source and the initial pH conditions. Food processing byproducts have attracted attention for microalgal cultivation because of their low cost and abundance of nutrients, including carbon and nitrogen. We investigated the optimal carbon source and its concentration for efficient paramylon production. A spent tomato byproduct (STB) generated from a tomato processing plant was applied for biomass and paramylon production from E. gracilis with respect to the initial pH condition. The highest paramylon concentration (1.2 g L−1) and content (58.2%) were observed with 15 g L−1 glucose. The biomass production increased when STB was used as compared with that when a synthetic medium was used (1.6-fold higher at pH 3 and 2-fold higher at pH 8). The optimal initial pH was determined according to the maximum production of biomass and paramylon. Upcycling the food processing byproduct, STB, can contribute not only to cost reduction of the biorefinery process using E. gracilis but also to environmental remediation by removing organic carbon and nitrogen from the byproducts.
1. Introduction
Microalgae have attracted attention as a promising source for carbon dioxide (CO2) utilization and, recently, as raw materials for functional foods. E. gracilis, a fast-growing microalgal species, can produce value-added materials, including immune-functional paramylon (β-1,3-glucan). Higher amounts of paramylon can accumulate under heterotrophic and mixotrophic conditions in the presence of organic carbon than under photoautotrophic conditions [1]. However, the use of the carbon source for cultivation is expensive and may account for 50% of the total medium cost [2].
Upcycling of food processing byproducts can be a compelling option to address the cost problem of organic carbon sources. Several nutrients in food processing byproducts can be potentially useful for microbial cultivation [3]. The use of food processing byproducts as low-cost substrates to produce value-added materials from microalgae may simultaneously enhance productivities and reduce costs [4,5,6]. To date, potato liquor [7], corn steep solid [8], corn steep liquor, and brewer’s spent grain [9] have been used to produce paramylon efficiently and economically from E. gracilis.
Spent tomato byproducts (STBs), such as pomace and peels, are generated from the tomato processing industry, and their amounts are expected to be 20–50 g kg−1 of tomato. STB contains high moisture and thus requires an additional drying process in order to be utilized in a powdered form [10]. In general, STB is mixed for use in animal feed [10] or used as a resource for extracting value-added chemicals, such as carotenoids and lycopene [11,12,13]. Peels or seeds of tomatoes contain 27% to 31% carbohydrates [14], which are preferred substrates for E. gracilis growth.
E. gracilis can utilize various carbon sources, such as polysaccharides, fatty acids, and alcohols [15]. However, knowledge on how E. gracilis consumes such carbon sources from food processing byproducts is still limited. The growth of E. gracilis is more competent under mixotrophic cultivation conditions than under other conditions. Cultivation factors related to organic carbon (e.g., carbon source type and concentration) have an impact on paramylon accumulation. Therefore, it is imperative to investigate the optimal carbon sources for paramylon production.
Cultivation conditions related to pH are also important factors affecting the growth and biomass production of E. gracilis. While the optimal pH for the cultivation of E. gracilis has been reported to be 3.5, a pH range of 7.4 to 8 has been shown to enhance the activity of paramylon synthase [16]. However, the impact of pH on the biomass and paramylon productivity of E. gracilis using food processing byproducts has not yet been reported.
The objectives of this study were (1) to investigate the optimal conditions of the carbon source type and concentration based on the biomass and paramylon production, and (2) to develop a strategy for the application of STB depending on the initial pH condition. Glucose, ethanol, lactate, and acetate were tested to determine the optimal carbon source. The concentration range of the optimal carbon source was 5–60 g L−1. The organic carbon concentration of the STB was adjusted to the optimal conditions before its application for the cultivation experiments. The impact of initial pH conditions on biomass and paramylon production from E. gracilis was also investigated.
2. Materials and Methods
2.1. Microalgal Strain and Medium
E. gracilis LIMS-1351 was obtained from the Library of Marine Samples (Geoje-si, Korea) and subcultivated under photoautotrophic cultivation conditions in 75 cm2 cell culture flasks (SPL Life Sciences, Pocheon-si, Korea). The subcultivations were carried out using an initial inoculum concentration of 5 × 104 cells mL−1 at a temperature of 27 °C with hand shaking on a daily basis. The light sources were fluorescent lamps with an intensity of 80 umol g m−2 s−1. Light was continuously provided without a dark period. Modified Hutner medium (HUT) or modified Cramer–Myers medium (CM) were used as the synthetic medium. The chemical composition of the HUT was based on a previous study [9]. The chemical composition of the CM in 1 L was as follows: 1 g KH2PO4, 1 g (NH4)2SO4, 0.2 g MgSO4∙7H2O, 0.2 g CaCl2∙2H2O, 0.758 g EDTA-2Na∙2H2O, 3 mg Fe(SO4)2∙6H2O, 1.8 mg MnCl2∙4H2O, 1.5 mg CoSO4∙7H2O, 0.4 mg ZnSO4∙7H2O, 0.2 mg Na2MoO4∙2H2O, 0.02 mg CuSO4∙5H2O, 100 µg vitamin B1, and 0.5 µg vitamin B12. Stock solutions were autoclaved at 121 °C for 15 min and stored at 4 °C until use. The pH of the medium from the mixed stock solutions was adjusted to pH 3.5 using 2 M hydrochloric acid (HCl). The medium was filtered through a vacuum filtration unit of 0.22 μm pore size (Guangzhou Jet Bio-Filtration Co., Ltd., Guangzhou, China).
2.2. Cultivation Experiments with Different Carbon Source Types and Concentrations
2.2.1. Experiment Using Different Carbon Sources
The primary carbon sources used for the assessment of the optimal carbon source were glucose, ethanol, lactate, and acetate. The HUT was supplemented with 15 g L−1 carbon source and 5 g L−1 monosodium glutamate. Glucose was used as the carbon source during the precultivation step. The precultivation experiments were carried out under mixotrophic cultivation conditions with an initial inoculum concentration of 1 × 105 cells mL−1. The initial pH was set to 3.5. The temperature was maintained at 27 °C. Erlenmeyer flasks were used as cultivation reactors and placed in a shaking incubator for 3 days at a speed of 120 rpm. From the precultivation step, the E. gracilis culture that entered the exponential phase was used as inoculum for the next step. The concentration of the carbon source applied was 15 g L−1. The temperature, initial inoculum concentration, light source and intensity, and initial pH were the same as those applied in the precultivation step. The cultivation reactors were incubated for 5 days. Based on cell growth, biomass, and paramylon production, the optimal carbon source was determined and applied for the subsequent experiments.
2.2.2. Experiment Using Different Carbon Concentrations
The carbon source concentrations used in this experiment were 5, 15, 30, and 60 g L−1 (represented as GL5, GL15, GL30, and GL60). As a control, the HUT supplemented with 15 g L−1 glucose and 5 g L−1 monosodium glutamate was used. The cultivation conditions (initial inoculum concentration, temperature, initial pH, shaking speed, light source, light–dark cycle, and light intensity) were the same as those described in Section 2.2.1.
2.3. Composition Analysis of STB
The STB used in this study was obtained from a tomato processing plant and stored at −20 °C before use. The STB samples were centrifuged and filtered for compositional analyses. Chemical oxygen demand (COD) and total nitrogen (TN) were analyzed using a Water Quality Kit (Humas, Daejeon, Korea) with a spectrophotometer. A portable pH meter (Compact PH Meter 33, Horiba Scientific, Kyoto, Japan) was used to measure the pH of the samples. The concentrations of organic acids were analyzed by high-performance liquid chromatography (HPLC; UltiMate 3000 HPLC System coupled with a UV detector (Dionex, CA, USA) and a YMC-Triart C18 column with a particle size of 5 μm, pore size of 120 Å, length of 250 mm, and internal diameter of 4.6 mm) (YMC Co., Ltd., Kyoto, Japan). The column temperature was set to 27 °C. The eluent for analysis was 20 mM phosphoric acid in HPLC-grade water. The flow rate of the mobile phase was 1 mL min−1.
The raw STB sample had an acidic pH (4.2) and contained high amounts of fructose and glucose (Table 1). The COD concentration was 32,000 ± 950 mg L−1, and the TN concentration was 703 ± 22 mg L−1; thus, the C/N ratio was calculated to be 45. The concentration of fructose was the highest, followed by glucose, citric acid, glutamic acid, ascorbic acid, malic acid, and ascorbic acid. The fructose concentration was determined to be 14,640 ± 100 mg L−1, and the glucose concentration was 13,120 ± 130 mg L−1 (39.9% and 35.7%, respectively, based on total organic concentration).

Table 1.
Chemical composition of raw sample of the spent tomato byproducts (STB). All analyses were performed in triplicate.
2.4. Cultivation Experiments under Different Substrate and pH Conditions
The CM supplemented with 15 g L−1 glucose and 5 g L−1 sodium glutamate was used as the control group to investigate the impact of different substrates on biomass production. The COD concentration of the STB treatment group was adjusted to the same level of that of the level observed in the control group. The initial pH conditions were 3 and 8. The pH of the medium was adjusted with HCl or sodium hydroxide (NaOH). The precultivation conditions of the inoculum source are described in Section 2.2.2. CO2 gas mixed with air (3%, v/v) was provided at 0.5 vvm using a mixed flow controller (Sehwa Hightech Ltd., Bucheon, Korea). All reactors were set in duplicate on a magnetic stirrer at a speed of 200 rpm. The light–dark period was 24:0.
2.5. Microalgal Growth and Chemical Analyses
A hemocytometer (C-Chip DHC-N01, INCYTO, Cheonan-si, Korea) and phase contrast microscope (Axioskop 2 plus, ZEISS, Jena, Germany) were used to observe cell growth and calculate cell density (CD). Biomass production was quantified by the gravimetric method measuring dry cell weight (DCW). The sample was filtered through a weighed glass fiber filter (GF/C, pore size 1.2 μm, 47 mm, Whatman®, Maidstone, UK) using a vacuum pump and heated at 105 °C for 2 h in a dry oven. Paramylon (PM) production was quantified using the gravimetric method [9]. The lyophilized biomass was suspended in acetone and sonicated for cell disruption. After centrifugation, the pellet was resuspended in 1%(w/v) SDS, heated at 100 °C, and cooled to room temperature. The suspension was centrifuged and washed twice with distilled water. The final pellet was dried overnight at 60 °C prior to weighing. Based on the measurements, the biomass productivity, paramylon productivity, specific growth rate (SGR), doubling time, and CO2 biofixation rate were calculated using the following equations:
Biomass productivity (unit: g L−1 d−1) = DCWt/t,
Paramylon productivity (unit: g L−1 d−1) = PMt/t,
Specific growth rate (unit: d−1) = ln (CDt2 − CDt1)/(t2 − t1),
Doubling time (unit: d] = ln 2/SGR,
CO2 biofixation rate (unit: g L−1 d−1) = 1.88 × biomass productivity,
2.6. Statistical Analysis
All experiments were performed in duplicate. The results are presented as the mean ± standard deviation. Statistical analysis was performed using R software (ver. 4.0.3) [17]. Analysis of variance (ANOVA) was applied to the data on the production of biomass and paramylon from different carbon sources and under different concentration conditions. For post hoc analysis, either the Durbin–Watson test or the Tukey HSD test was used depending on the autocorrelation. The two-sample t-test was used to determine the significance of the differences between the medium types or among the initial pH conditions. Homogeneity of the variance test for each variable was performed after normality analysis using the Shapiro test.
3. Results
3.1. Biomass Production Based on Carbon Source and Concentration
Glucose was the most effective carbon source for the production of E. gracilis biomass and paramylon (Figure 1a). The highest biomass concentration (2.0 g L−1) and paramylon concentration (1.2 g L−1) and content (58.3%) were achieved with glucose on day 3. The biomass and paramylon concentrations observed using lactate as the carbon source were lower than those observed using glucose, but the paramylon contents were similar between the two groups. The SGR increased in the order of glucose, lactate, and ethanol, thus showing a trend similar to that observed with biomass and paramylon production (Table 2).
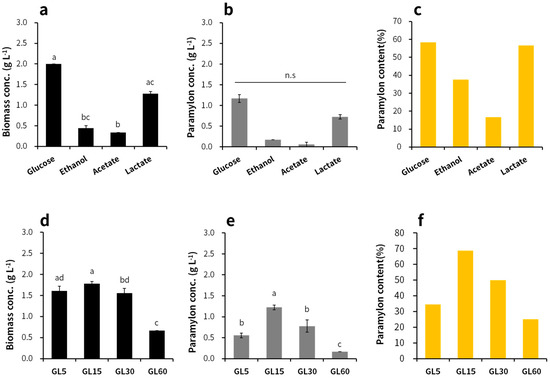
Figure 1.
Biomass and paramylon production and paramylon content on day 3 in the presence of different carbon source types (glucose, ethanol, acetate, and lactate) and different concentrations (glucose 5, 15, 30, and 60 g L−1): (a,d) biomass production, (b,e) paramylon production, and (c,f) paramylon contents. All analyses were performed in duplicate.

Table 2.
Effect of carbon source type and concentrations on E. gracilis biomass and paramylon production. Specific growth and doubling time were calculated from the average values. All the reactors were set in duplicate, and the sample analyses were performed in duplicate.
The most effective concentration of glucose was 15 g L−1, which showed the highest paramylon content (1.2 g L−1) (Figure 1b). Biomass productions were similar among the GL5, GL15, and GL30 groups (1.6–1.8 g L−1). The lowest biomass production was observed in the GL60 reactor. Paramylon concentration and content were highest in the GL15 group (1.2 g L−1 and 68.8%, respectively), followed by the GL30, GL5, and GL60 groups. The COD consumption result showed a trend similar to that observed in biomass and paramylon production (Figure 2). The higher was the glucose concentration, the greater was the amount of TN consumed and the lower was the SGR (Table 2). The maximum biomass concentration and productivity were shown in GL15, followed by GL5, GL30, and GL60. The maximum paramylon concentration and productivity were also shown in GL15, followed by GL30, GL5, and GL60.
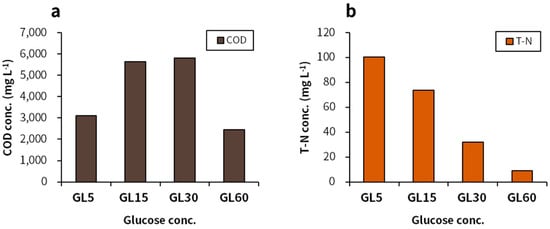
Figure 2.
Nutrient consumption throughout the cultivation period depending on glucose concentration: (a) COD and (b) T-N. The amount of consumed nutrients was calculated from the average values regarding the beginning and the end of the cultivation.
3.2. Mixotrophic Cultivation Using the Synthetic Medium and STB at pH 3
The biomass production after cultivation at an initial pH of 3 was 1.6-fold higher using the STB than with CM (Figure 3a). E. gracilis cultures entered the exponential growth phase on day 2. The culture in the STB reactor entered the stationary phase on day 3, whereas the culture in the CM reactor entered the phase a day later. Paramylon production was 0.54 ± 0.14 g L−1 using STB on day 6. COD and TN consumptions were higher in STB cultures than in CM cultures regardless of the initial pH condition (Figure 3b,c). Approximately 59% of the nitrogen source was consumed in STB cultures. As shown in Figure S1, the green color in the STB reactor was darker than that in the CM reactor. A higher SGR (more than 2-fold) and CO2 biofixation rate (more than 1.5-fold) were observed in STB cultures than in CM cultures (Table 3). Further, the CO2 biofixation rate was more than 1.9-fold higher in the reactors providing CO2 mixed gas (1.5 g L−1 d−1) than in those without the gas flow (0.81 g L−1 d−1).
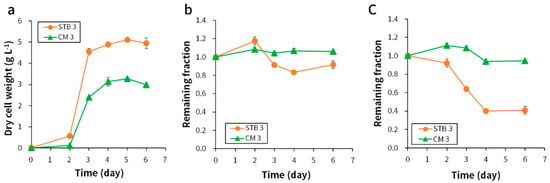
Figure 3.
(a) Biomass production, (b) COD consumption, and (c) T-N consumption using STB and CM medium at an initial pH 3. Sample analyses were performed in duplicate, and the data were represented as average ± standard error (n = 4).

Table 3.
Specific growth rate, doubling time, and CO2 biofixation rate using STB and CM using different initial pH conditions.
3.3. Mixotrophic Cultivation Using Synthetic Medium and STB at pH 8
Similar to the results observed with cultures at an initial pH 3, the biomass production was 1.6-fold higher with STB than with CM at an initial pH 8 (Figure 4a). E. gracilis cultures from all conditions entered the exponential growth phase on day 2. The cultures in the STB reactor entered the stationary phase on day 5, and those in the CM reactor entered the stationary phase on day 3. Paramylon production was 0.89 ± 0.14 g L−1 in STB cultures on day 6, which is higher than that observed with cultures at an initial pH 3. Higher nutrient consumption was shown at an initial pH 8. COD consumption was 39%, which was 3.8-fold higher than that observed at an initial pH 3. TN consumption was 67% and was 1.1-fold higher than that reported at an initial pH 3. The CM reactors with an initial pH 8 lost the green color until day 7 (Figure S1). A higher SGR (more than 1.6-fold) and CO2 biofixation rate (more than 1.6-fold) were shown in STB reactors than in CM reactors (Table 3).
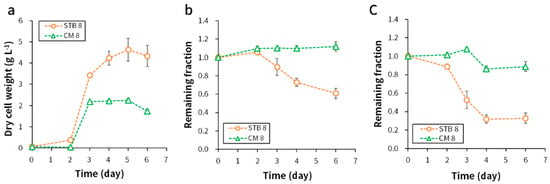
Figure 4.
(a) Biomass production, (b) COD consumption, and (c) T-N consumption using STB and CM medium at an initial pH 8. Sample analyses were performed in duplicate, and the data were represented as average ± standard error (n = 4).
4. Discussion
4.1. Impact of Carbon Source Type and Concentration
For biomass and paramylon production from the mixotrophic cultivation of E. gracilis, glucose was determined to be the most effective carbon source as compared with ethanol, lactate, and acetate. Biomass production using glucose or lactate significantly differed from that achieved using ethanol or acetate (p < 0.05) (Figure 1). It has been widely reported that glucose can be used as an effective carbon source for biomass production from E. gracilis under both mixotrophic and heterotrophic cultivation conditions. Ethanol has also been reported as an efficient carbon source under heterotrophic cultivation [18,19]. Fujita et al. (2008) reported a higher SGR with ethanol than with glucose [20]. Ethanol, as an organic carbon source under the heterotrophic cultivation of E. gracilis, participates in respiration, glycolysis, gluconeogenesis, and paramylon synthesis, and is rapidly oxidized to acetate [21]. Under the mixotrophic cultivation of E. gracilis, ethanol increased the production of β-carotene, chlorophyll, and tocopherol [22]. Thus, the preferred carbon source for E. gracilis can vary depending on the trophic mode. In accordance with other studies, the higher is the number of carbon atoms in the carbon sources, the higher is the biomass and paramylon production.
The highest biomass and paramylon production was observed under the GL15 condition (p < 0.05). There were significant differences in both biomass and paramylon production between GL5 and GL15 conditions. It has been reported that low glucose concentrations result in low SGR, consequently leading to lipid accumulation. Jeong et al. (2016) demonstrated a significant decrease in E. gracilis at C/N ratio 10 [23]. In this study, the C/N ratio of GL60 was 12, and the lowest biomass and paramylon production was observed under these conditions. Although glucose is widely preferred for many microalgal species, including Chlorella sp. and Euglena sp., the optimal C/N ratio is varied. While the most preferred carbon source for Chlorella sp. HS2 was glucose compared with sucrose, sodium acetate, and lactic acid, the optimal C/N ratio was 21.8 [24]. Barsanti et al. (2021) reported the correlation between biomass productivity and nutrient consumption (BOD5, COD, NH3-N, and PO43--P), where nutrient concentrations decreased with an increase in biomass production [5]. In this study, the greater was the consumption of COD and TN, the higher was the production of biomass and paramylon (except for GL5).
4.2. Application of Spent Tomato Byproduct as an Alternative Medium
The STB used as a substrate for E. gracilis cultivation was acidic (pH 4.2) and contained a large amount of various nutrients. The carbon and nitrogen source concentrations in the STB were higher than the optimal concentrations set in the previous experiment (Section 3.2). In particular, the STB mainly comprised fructose and glucose, which are reported as efficient organic carbon sources for E. gracilis cultivation [8]. Accordingly, STB was assumed to be an effective carbon source for biomass and paramylon production. Wang et al. (2010) reported that the growth rate of Chlorella sp. was affected when the turbidity of the media was beyond 100 NTU [25]. Although the turbidity of the STB reactor was presumed to inhibit growth for photosynthesis (Figure S1), no such affection occurred due to results from the enhanced productivities. Since there would be differences between microalgal species, further study on the impact of turbidity on E. gracilis growth is necessary.
This is the first study to investigate upcycling tomato processing byproducts as substrates for E. gracilis. Enhanced cell growth, biomass production, and paramylon production demonstrated the strong potential of STB. Although various food processing byproducts have been applied as substrates for other microalgal species, no study has reported the use of E. gracilis. The alternative medium with kinnow peels demonstrated the biomass and lipid productivity of Tetraselmis indica, which was comparable to that of synthetic medium [4]. Chlorella sp. would be the most studied microalgae to date in the valorization of food waste hydrolysate. Out of 67 kinds of fruit waste hydrolysates, 59 kinds were proven to be effective for biomass and chlorophyll production from Chlorella pyrenoidosa [26]. Using a pH-adjusted waste papaya fruit as a sole substrate, Chlorella protothecoides showed robust cell growth and oil production [27]. Biomass and lipid yield were enhanced by using cassava bagasse hydrolysates for the cultivation of C. pyrenoidosa when mixed with yeast Rhodotorula glutinis [28]. Additionally, food waste hydrolysate and vegetable waste hydrolysate have been reported to enhance the biomass production of Chlorella vulgaris [29]. Pratap et al. (2017) reported that the dilution ratio of waste hydrolysates affects the cell growth rate [29]. The byproducts from Citrus limetta used as an alternative medium for C. culgaris enhanced biomass and lipid productivity by more than 2-fold [3]. Wang et al. (2020) reported enhanced biomass, lipid, and lutein production from Chlorella sp. using food waste hydrolysate, probably because glucose was a major organic component in the source [30].
In this study, a maximum of 5.2 g L−1 of E. gracilis biomass was shown using STB, which is more than 1.6-fold compared with the results using the synthetic medium. Therefore, STB can be utilized as an effective alternative medium for E. gracilis because it has high contents of glucose. Higher biomass and paramylon production was observed using STB as an alternative medium over synthetic medium (1.6-fold and 1.5-fold, respectively). Statistical analysis showed that biomass production between STB and CM was significantly different (t-test, p < 0.05). Using the STB, paramylon production was significantly different between cultures with initial pH 3 and 8 (t-test, p < 0.05).
The valuable materials to be applied for biofuel can be produced from E. gracilis even with nonedible industrial byproducts, such as wastewater, wood materials, and food waste. Zhu and Wakisaka (2018) reported that ferulic acid extracted from rice bran promoted 1.4-fold higher growth of E. gracilis under phototrophic conditions than the control group [31]. However, high-value materials that can be applied to functional foods or pharmaceuticals can be produced with byproducts generated from highly sterilized processes treating foods. Accordingly, STB as an alternative medium for E. gracilis can allow the production of high-value immune-functional paramylon.
4.3. The Impact of Initial pH for Value-Added Production from E. gracilis Using STB
The initial pH is an important factor for E. gracilis cultivation. The photosynthetic efficiency of E. gracilis might decrease under unfavorable pH conditions, such as extreme acidic or alkaline conditions [32]. The activities of enzymes such as laminaribiose phosphorylase in E. gracilis cells can be strongly affected by not only the trophic mode but also the pH [33]. Higher paramylon production and content were observed at pH 5 and 7 than at pH 2 and 3.5, while biomass production showed opposite results [9]. This result is in accordance with that observed in this study, wherein paramylon production at pH 8 was higher than that at pH 3. The optimal pH for cell growth and value-added material production is strongly dependent on the microalgal species [34]. The optimal pH conditions differed according to the maximum titer of biomass and paramylon, probably owing to the change in the dominant species of CO2 (CO32−, HCO3−) in the medium depending on the pH; thus, the type of substances (biomass, protein, polysaccharides, pigments, etc.) accumulated in the cell changed [34].
Based on the results, the material stream input and output on biomass and paramylon production from E. gracilis using STB are depicted in Figure 5. Preparing hydrolysates by adding 560 L of STB into 440 L of distilled water can make an alternative medium containing 18 g L−1 of organic carbon source. Setting the initial pH of the process to 3 can produce either 3 kg of biomass or 0.5 kg of paramylon. On the other hand, setting the initial pH of the process to 8 can yield either 2 kg of biomass or 1 kg of paramylon. In summary, it is advantageous to set the initial pH 3 for the efficient production of biomass or the initial pH 8 for the efficient production of paramylon. Since enhanced productivity can be expected from scaled-up cultivation reactors under different operation modes (e.g., semicontinuous) [30], further investigation on E. gracilis cultivation using STB is required.
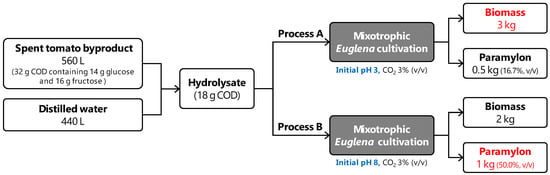
Figure 5.
Biomass and paramylon production using STB at initial pH 3 and 8.
5. Conclusions
This study demonstrated that the most efficient conditions in terms of carbon source and concentration for E. gracilis cultivation and paramylon production involve the use of 15 g L−1 glucose. STB, which contains abundant glucose, enhanced the production of biomass and paramylon. In comparison with the synthetic medium, the STB increased the biomass production by 1.6-fold at pH 3 and 2-fold at pH 8. Optimal initial pH conditions were determined according to the maximum production results of biomass and paramylon. This study provides a strategy for the efficient production of value-added materials by upcycling food processing byproducts for the cultivation of E. gracilis. Therefore, this strategy can contribute to increasing the profits from enhanced productivity, decreasing the cost of the cultivation medium, and reducing environmental pollution through the removal of organic carbon and nitrogen from industrial byproducts.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11178182/s1: Figure S1: Medium and reactors throughout the cultivation period using STB and CM at different initial pH conditions.
Author Contributions
Conceptualization, S.K. and R.W.; methodology, S.K.; formal analysis, R.W.; data curation, D.L.; writing—original draft preparation, S.K.; writing—review and editing, J.Y.; visualization, D.L.; supervision, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Korea Electric Power Corporation (Grant No: R21XO01-41). Additionally, this work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C2012288).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lewis, A.; Guéguen, C. How growth conditions of Euglena gracilis cells influence cellular composition as evidenced by Fourier transform infrared spectroscopy and direct infusion high-resolution mass spectrometry. J. Appl. Phycol. 2020, 32, 153–163. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Kumar, A.; Bharti, R.K.; Biswas, S.; Pruthi, V. A novel approach using low-cost Citrus limetta waste for mixotrophic cultivation of oleaginous microalgae to augment automotive quality biodiesel production. Enviro. Sci. Pollut. Res. 2019, 26, 16115–16124. [Google Scholar] [CrossRef]
- Ghosh, U.K. Utilization of kinnow peel extract with different wastewaters for cultivation of microalgae for potential biodiesel production. J. Environ. Chem. Eng. 2019, 7, 103135. [Google Scholar]
- Barsanti, L.; Ciurli, A.; Birindelli, L.; Gualtieri, P. Remediation of dairy wastewater by Euglena gracilis WZSL mutant and β-glucan production. J. Appl. Phycol. 2021, 33, 431–441. [Google Scholar] [CrossRef]
- Calixto, C.D.; Santana, J.K.S.; de Lira, E.B.; Sassi, P.G.P.; Rosenhaim, R.; Sassi, C.F.C.; da Conceição, M.M.; Sassi, R. Biochemical compositions and fatty acid profiles in four species of microalgae cultivated on household sewage and agro-industrial residues. Bioresour. Technol. 2016, 221, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Šantek, B.; Felski, M.; Friehs, K.; Lotz, M.; Flaschel, E. Production of paramylon, a β-1, 3-glucan, by heterotrophic cultivation of Euglena gracilis on potato liquor. Eng. Life. Sci. 2010, 10, 165–170. [Google Scholar]
- Ivušić, F.; Šantek, B. Optimization of complex medium composition for heterotrophic cultivation of Euglena gracilis and paramylon production. Bioprocess Biosyst. Eng. 2015, 38, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.; Lim, D.; Lim, S.; Park, S.; Kang, C.; Yu, J.; Lee, T. Paramylon production from heterotrophic cultivation of Euglena gracilis in two different industrial byproducts: Corn steep liquor and brewer’s spent grain. Algal Res. 2020, 47, 101826. [Google Scholar] [CrossRef]
- Knoblich, M.; Anderson, B.; Latshaw, D. Analyses of tomato peel and seed byproducts and their use as a source of carotenoids. J. Sci. Food Agric. 2005, 85, 1166–1170. [Google Scholar] [CrossRef]
- Mehta, P.; Singh, D.; Saxena, R.; Rani, R.; Gupta, R.P.; Puri, S.K.; Mathur, A.S. High-Value Coproducts from Algae—An Innovational Way to Deal with Advance Algal Industry. In Waste to Wealth; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 343–363. [Google Scholar]
- Rozzi, N.; Singh, R.; Vierling, R.; Watkins, B. Supercritical fluid extraction of lycopene from tomato processing byproducts. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Caruso, T.; Iannece, P.; Aragòn, A.; Santini, A. Characterization of high purity lycopene from tomato wastes using a new pressurized extraction approach. J. Agric. Food Chem. 2008, 56, 6227–6231. [Google Scholar] [CrossRef] [PubMed]
- Karthika, D.; Kuriakose, S.; Krishnan, A.; Choudhary, P.; Rawson, A. Utilization of by-product from tomato processing industry for the development of new product. J. Food Sci. Technol. 2016, 7, 608. [Google Scholar]
- Hosotani, K.; Ohkochi, T.; Inui, H.; Yokota, A.; Nakano, Y.; Kitaoka, S. Photoassimilation of fatty acids, fatty alcohols and sugars by Euglena gracilis Z. Microbiology 1988, 134, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Bäumer, D.; Preisfeld, A.; Ruppel, H.G. Isolation and characterization of paramylon synthase from Euglena gracilis (euglenophyceae) 1. J. Phycol. 2001, 37, 38–46. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 12 July 2021).
- Barsanti, L.; Vismara, R.; Passarelli, V.; Gualtieri, P. Paramylon (β-1, 3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. J. Appl. Phycol. 2001, 13, 59–65. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.; Ortiz-Cruz, M.; Mendoza-Hernández, G.; Moreno-Sánchez, R. Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J. Appl. Microbiol. 2010, 109, 2160–2172. [Google Scholar] [CrossRef]
- Fujita, T.; Aoyagi, H.; Ogbonna, J.C.; Tanaka, H. Effect of mixed organic substrate on α-tocopherol production by Euglena gracilis in photoheterotrophic culture. Appl. Microbiol. Biotechnol. 2008, 79, 371–378. [Google Scholar] [CrossRef]
- Garlaschi, F.M.; Garlaschi, A.M.; Lombardi, A.; Forti, G. Effect of ethanol on the metabolism of Euglena gracilis. Plant Sci. Lett. 1974, 2, 29–39. [Google Scholar] [CrossRef]
- Mokrosnop, V.; Polishchuk, A.; Zolotareva, E. Accumulation of α-tocopherol and β-carotene in Euglena gracilis cells under autotrophic and mixotrophic culture conditions. Appl. Biochem. Microbiol. 2016, 52, 216–221. [Google Scholar] [CrossRef]
- Jeong, U.; Choi, J.; Kang, C.; Choi, B.; Kang, S. Effect of growth conditions on the biomass and lipid production of Euglena gracilis cells raised in mixotrophic culture. Korean J. Fish. Aquat. Sci. 2016, 49, 30–37. [Google Scholar]
- Kim, H.S.; Park, W.; Lee, B.; Seon, G.; Suh, W.I.; Moon, M.; Chang, Y.K. Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, X.; Sun, K.; Li, H. Effects of fruit waste hydrolysates on biomass and chlorophyll a fluorescence parameters of Chlorella pyrenoidosa. Int. J. Environ. Bioener 2014, 9, 105–121. [Google Scholar]
- Heller, W.P.; Kissinger, K.R.; Matsumoto, T.K.; Keith, L.M. Utilization of papaya waste and oil production by Chlorella protothecoides. Algal Res. 2015, 12, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, J.; Lim, P.; Wei, D. Enhanced single cell oil production by mixed culture of Chlorella pyrenoidosa and Rhodotorula glutinis using cassava bagasse hydrolysate as carbon source. Bioresour. Technol. 2018, 255, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Kumar, M.; Sibi, G. Fruit and Vegetable Waste Hydrolysates as Growth Medium for Higher Biomass and Lipid Production in Chlorella vulgaris. J. Environ. Manag. 2017, 4, 204–210. [Google Scholar]
- Wang, X.; Zhang, M.; Sun, Z.; Liu, S.; Qin, Z.; Mou, J.; Zhou, Z.; Lin, C.S.K. Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M. Growth promotion of Euglena gracilis by ferulic acid from rice bran. AMB Express 2018, 8, 16. [Google Scholar] [CrossRef]
- Danilov, R.; Ekelund, N. Effects of pH on the growth rate, motility and photosynthesis in Euglena gracilis. Folia Microbiol. 2001, 46, 549–554. [Google Scholar] [CrossRef]
- Abi, A.; Müller, C.; Jördening, H. Improved laminaribiose phosphorylase production by Euglena gracilis in a bioreactor: A comparative study of different cultivation methods. Biotechnol. Bioprocess Eng. 2017, 22, 272–280. [Google Scholar] [CrossRef]
- Khalil, Z.I.; Asker, M.M.; El-Sayed, S.; Kobbia, I.A. Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J. Microbiol. Biotechnol. 2010, 26, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).