Abstract
Changes in nutritional and lighting conditions to obtain compounds of interest and biomass via microalgal cultures are among the main foci of studies in algal biotechnology. Growth medium supplementation using organic compounds, such as glycerol, is a promising approach for increasing biomass productivity and the viability of microalgal cultivation and adding value to byproducts of the biodiesel industry. In this study, the influence of crude glycerol on Spirulina sp. LEB 18 was investigated via culturing using different photoperiods, and its effect on biomass composition and cell growth was evaluated. The microalgae were subjected to three photoperiods (continuous light, 24:0; 12 h light and 12 h dark, 12:12; and no illumination, 0:24) and crude glycerol supplementation (2.5 g L−1); better productivity and biomass concentrations were obtained in cultures with a 12:12 photoperiod (28.36 mg L−1 h−1 and 1.24 g L−1, respectively). Under this condition, the highest protein yield was achieved (647.3 mg L−1, 52.2% w w−1), and the obtained biomass showed favorable characteristics for applications in animal feed enrichment.
1. Introduction
Microalgae are photosynthetic microorganisms capable of producing compounds such as proteins, carbohydrates, lipids, fatty acids, biopeptides, and pigments that can be used to develop foods, drugs, cosmetics, biofuels, and animal feed [1]. The cellular growth of microalgae varies according to a combination of parameters, such as nutrient composition, temperature, and light [2].
Luminosity is one of the most important factors that, in addition to modifying the composition of the biomass, influence the economic efficiency of the process. This factor can vary in intensity and frequency of microorganisms’ exposure. Several studies have demonstrated the interference of photoperiod alterations on the content of proteins, carbohydrates, and fatty acids in microalgal cultures [3,4]. In addition, changes in light intensity and photoperiod are the main factors that determine the growth rate of microalgae cultures, as they mainly interfere with the mechanism of obtaining energy from cells [5].
Alterations to photoperiods can have significant effects, especially when combined with other factors that influence cell growth and photosynthesis, such as the use of organic compounds such as glycerol to supplement the culture medium [3,6]. Glycerol is a byproduct of the transesterification reaction of oils during biodiesel production. Purified glycerol has several applications; however, in its raw form, its commercial value is much lower [7]. In addition to the high cost of the purification process, especially for small industries, the market demand for this product is lower than the amount generated by the biodiesel industry [8].
Glycerol has positively influenced the cell growth of microalgal cultures [9,10,11] and biomass productivity. For the profitability of high-value add products through microalgae, it is essential to obtain a balance between the compounds of interest and biomass productivity, as some applied conditions can reduce its yield. Spirulina cultured in glycerol has already shown remarkable growth and protein production, reaching 384.15 mg L−1 in only 36 h of culture [9]. However, results regarding the association of glycerol with Spirulina cultures with different photoperiods have not yet been reported.
Spirulina strain is an important commercial microalga certified with GRAS (generally recognized as safe by the Food and Drug Administration, USA) since 2002 and is on the European Novel Food list (EU, 2017/2470). The produced biomass can be applied to proteins and unsaturated fatty acids [9,10,11]. These compounds can be used to improve the nutritional and functional value of foods [12], as a supplement [13], as animal feed [14], and as biofuel [15,16,17]. In this context, this study aimed to evaluate the influence of organic carbon source addition in the form of glycerol on a Spirulina sp. LEB 18 culture subjected to different photoperiods and its effect on biomass composition and cell growth.
2. Materials and Methods
2.1. Culture Conditions
A Spirulina sp. LEB 18 strain from the collection of the Biochemical Engineering Laboratory of the Federal University of Rio Grande (FURG, Brazil) [18] was cultivated in a Zarrouk medium supplemented with 2.5 g L−1 glycerol [9] obtained from the transesterification of castor oil for biodiesel production [19]. Different photoperiods were used: continuous light (24:0), 12 h light and 12 h dark (12:12), and no light (0:24). Control cultures that did not have glycerol added were studied for photoperiods. The light for the cultures was provided by LED lamps (High Flux LED Bulb HO OSRAM, München, Germany—40 W, radiation spectrum 400–700 nm) with white light at 60 µmolfótons.m−2 s−1; the luminosity was monitored using a digital radiometer (WALZ ULM-500, Effeltrich, Germany) at 30 °C. The cultures were performed in triplicate in Erlenmeyer flasks (800 mL working volume), with an initial concentration of 0.2 g L−1, and aeration was provided by pneumatic pumps (airflow 0.5 L min−1). The experiments were carried out for 36 h, as this period was previously reported to be the period of maximum cell growth for Spirulina sp LEB 18 cultures with 2.5 g L−1 glycerol [9].
2.2. Evaluation of Growth Parameters
Samples were collected every 4 h and evaluated for biomass concentration using a standard curve that related dry weight and optical density at 670 nm [20]. From the concentration data, the maximum productivity was calculated (Pmax) with Equation (1), where X (g L−1) is the concentration at time t (h), and X0 (g L−1) is the initial concentration at time t0 (h).
Pmax = (X − X0)/(t − t0)
The maximum specific growth rate (µmax, h−1) was calculated using exponential regression applied to the logarithmic phase of growth from Equation (2):
μmax = 1/X. dX/dt
The generation time (tg, h) was calculated using Equation (3):
tg = ln2/μmax
2.3. Biomass Harvest
The biomass was harvested via centrifugation at 15,000× g for 10 min. Next, it was washed using distilled water and centrifuged again under the same conditions to improve salt removal from the Zarrouk medium. Finally, it was frozen at −80 °C, lyophilized (LABCONCO, Kansas City, MO, USA), and refrigerated at −4 °C until analyses were performed.
2.4. Biomass Composition
For protein and carbohydrate determination, 5 mg of lyophilized biomass was added to 10 mL distilled water and subjected to ultrasonic treatment (Cole Parmer, CPX 130, Merrillville, IN, USA) for 10 min in 59 s cycles. From this extract, proteins were determined using a standard curve for bovine serum albumin based on Lowry et al. [21], and carbohydrates were determined using a glucose standard curve based on Dubois et al. [22]. Lipid analysis was performed directly on the biomass using a method proposed by Marsh and Weinstein [23], in which lipids were quantified via comparison with a tripalmitin standard curve. To determine fatty acids, lipids were extracted using a method from Folch et al. [24] based on the extraction of polar and nonpolar lipids through a mixture of organic solvents. The lipids were subjected to esterification to transform the fatty acids into their corresponding methyl esters. For this, saponification was carried out using NaOH in methanol, followed by methylation using a BF3 catalyst (12% in methanol). The separation of fatty acid methyl esters was performed via gas chromatography using a Shimadzu 2010 Plus instrument equipped with an ionizing flame detector (FID) and a Zebron ZB-FAME column (20 m × 0.18 mm × 0.15 µm). Helium was used as the carrier gas at a flow rate of 1 mL min−1. The identification of fatty acids was performed by comparing the retention times of FAME peaks with a standard of 37 fatty acids (Supelco 37 Component FAME Mix). The fatty acid content was expressed in milligrams per gram of lipids, and the analysis was performed using an internal standard with a known concentration (C23:0 Sigma-Aldrich, St. Louis, MO, USA). Moisture content was determined via thermogravimetric analysis (Shimadzu DTG-60, Kyoto, Japan).
2.5. Glycerol Consumption
The evaluation of glycerol consumption by the microalgae was carried out in the culture supernatant using a method proposed by Bondioli e Bella [25], in which glycerol oxidation occurs followed by the formation of formaldehyde via the Hantzsch reaction. This process generates color in the medium, which was detected by a spectrophotometer at 410 nm (QUIMIS Q998U, Candiota, Brazil). The results were compared with a previously plotted calibration curve for purified glycerol (Merck, Rahway, NJ, USA—88%) as the standard.
2.6. Determination of Chlorophylls
Chlorophyll was extracted and quantified using a method proposed by Lichtenhaler [26]. Every 12 h, a 1-mL sample was centrifuged at 2000× g for 5 min, and the formed pellet was added to 1 mL 99.8% methanol (v v−1) and incubated at 4 °C for 24 h in the dark. The pigment concentration (Ca+b) was calculated using Equation (4) by reading the optical density at the wavelengths of 652.4 and 665.2 nm.
Ca+b = 1.44 A665.2 + 29.93 A652.4
2.7. Statistical Analysis
The cell growth and biomass composition results were evaluated via analysis of variance (ANOVA) and Tukey’s test (95% confidence).
3. Results and Discussion
In the different photoperiods studied, Spirulina sp. LEB 18 cultures that grew in a medium supplemented with glycerol attained higher biomass concentrations than cultures that did not contain this source of organic carbon; the glycerol-added cultures showed 2.3, 3.9, and 4.6 times higher concentrations than the control cultures (24:0, 12:12, and 0:24, respectively). In all assays supplemented with glycerol, from 0 to 24 h, a lag phase occurred, where there was an adaptation of the cells to the culture medium. After 24 h, an exponential growth phase was observed. The control experiments did not show exponential growth phases (Figure 1a). According to Mitra et al. [27], high cell densities can be obtained during cultivation using combinations of organic and inorganic carbon sources due to the availability of large quantities of energy, which promotes a combination of aerobic respiration and catabolism of the glycerol present in the medium during photosynthesis.
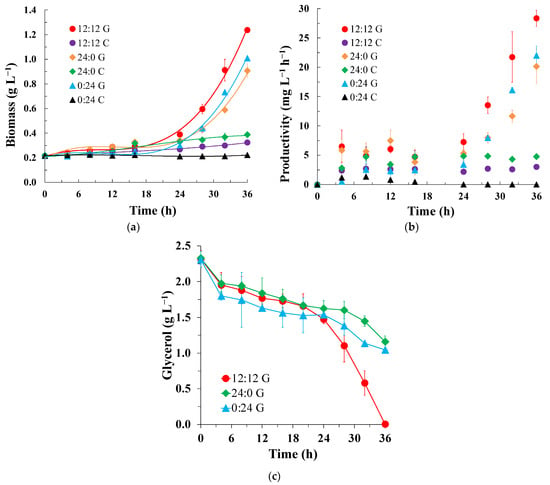
Figure 1.
Growth curves for (a) cell productivity, (b) glycerol consumption, and (c) Spirulina sp. LEB 18 cultured using glycerol supplementation (G) and control (C) under different photoperiods: continuous light (24:0), 12 h light/12 h dark (12:12), and no light (0:24).
Among the experiments supplemented with glycerol, the one with the 12:12 photoperiod showed the highest biomass concentration (1.24 g L−1, p > 0.05) and maximum productivity (28.36 mg L−1 h−1, p >0.05), with a generation time of 7 h (Table 1). The specific growth rate under this condition was 0.10 h−1, with no significant differences from the other photoperiods using glycerol supplementation studied. This condition also resulted in 100% glycerol consumption at 36 h. The other photoperiods resulted in consumption levels of 50.2% and 54.5% (24:0 and 0:24, respectively, Figure 1c). Experiments 24:0 and 0:24 did not show significant differences (p > 0.05) in biomass concentration (0.91 g L−1 and 1.01 g L−1, respectively) or productivity (20.16 mg L−1 h−1 and 22.02 mg L−1 h−1, respectively). For all cultures grown using the organic carbon source, the acceleration in productivity coincided with the beginning of the exponential phase of cell growth at 24 h (Figure 1b).

Table 1.
Maximum biomass concentration (Xmax), maximum productivity (Pmax), maximum specific growth rate (µmax), generation time (tg), protein, carbohydrate, and lipid contents for Spirulina sp. LEB 18 cultured using glycerol supplementation under different photoperiods: continuous light (24:0), 12 h light/12 h dark (12:12), and no light (0:24). Average ± standard deviation. The same superscript in the same column indicates that the averages do not differ significantly at the 95% confidence level (p > 0.05).
Heterotrophic growth and photosynthesis can occur simultaneously and independently in mixotrophic cultures, and the presence of an organic carbon source alters both photosynthetic and heterotrophic metabolism, enhancing cell growth and biomass productivity. This behavior was reported by Chojnacka and Noworyta for the microalgae Spirulina sp. grown in a medium supplemented with glucose in a mixotrophic culture [28]. This may explain the higher biomass concentration and productivity of Spirulina sp LEB 18 cultures grown in the 12:12 photoperiod in a medium supplemented with glycerol compared with the other investigated photoperiods (Table 1). Morais et al. [9] cultured Spirulina sp. LEB 18 using media supplemented with glycerol (2.5 g L−1) and reported a similar maximum cell concentration of 1.08 g L−1 at 36 h for a culture grown in a photoperiod of 12:12.
According to Li et al. [29], the presence of organic carbon can reduce the production of proteins involved in photosystem II, and ribulose-1,5-bisphosphate carboxylase/oxygenase in the Calvin cycle can repress photoautotrophic growth in algae. A synergistic effect between light and organic substrates on microalgal growth was previously reported by Cheirsilp et al. [30]. The authors found that under continuous illumination (24:0) in a medium supplemented with glucose, Nanochloropis gagitana reached the stationary phase earlier with lower productivity (29 mg L−1 day−1) than mixotrophic cultures. Holdman et al. [31] evaluated the growth of Chlorella sorokiniana under different light intervals and showed that the presence of dark stages results in better productivity compared with continuous lighting. According to Matos et al. [3], the absence of light is necessary during the synthesis of essential metabolic molecules for microalgal growth from ATP and NADPH.
In the 0:24 culture, only the organic carbon source was used, as the inorganic source could not be metabolized during the dark stage. This was confirmed by the fact that the dark control showed no change in biomass concentration and was probably maintained by the energy produced before the start of cultivation. According to Li et al. [32], during mixotrophic growth, organic carbon is used to produce biomass and energy via a heterotrophic metabolism, which generates CO2 that can be assimilated during the light-driven photoautotrophic stage; this does not occur during cultivation in the absence of light and results in lower productivity.
According to Wahidin et al. [4], photoperiod modification causes changes in chemical composition, photosynthetic activity, and pigment concentration in microalgal cultures. In the present study, proteins were the predominant macromolecules in Spirulina biomass grown under different photoperiods with and without glycerol supplementation, followed by carbohydrates and lipids, respectively.
With respect to the influence of photoperiods on biomass composition, the 24:0 culture showed an increase in the concentration of carbohydrates (23.8% w w−1). On the other hand, the 0:24 culture showed positive influences on the concentration of proteins (58.1% w w−1, p > 0.05). According to Holdmann et al. [31], under constant lighting, an increase in productivity and biomass production may occur during cultivation with organic carbon due to CO2 conversion into carbohydrates upon consumption of the substrate. The 12:12 culture showed no variation in biomass composition compared with its control; however, due to high biomass productivity, the lipid and protein yields (99.45 mg L−1 and 647.28 mg L−1, respectively) were higher for this photoperiod relative to the others. For the concentration of carbohydrates, the 24:0 culture showed the highest yield (216.19 mg L−1).
For chlorophylls evaluated over the cultivation time, all cultures supplemented with glycerol showed a reduction in pigment concentration after 8 h of cultivation (Figure 2). Despite the different light supply times, the pigments were influenced by the presence of glycerol in the medium. According to Morais et al. [10], the use of an organic carbon source in cultures results in a reduction in pigments in the biomass due to the preference of algae to consume the organic carbon source, as it is easier for cells to obtain energy in this way than through the process of photosynthesis.
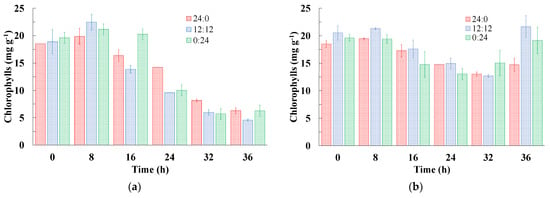
Figure 2.
Variation in chlorophyll content over time for cultivation of Spirulina sp. LEB 18 with different photoperiods in (a) cultures supplemented with glycerol and (b) controls.
The addition of glycerol to the culture medium modified the fatty acid composition of the Spirulina sp. LEB 18 biomass by increasing the unsaturated acid content in all studied photoperiods. In the dark, neither the control nor the culture with the organic carbon source showed higher proportions of unsaturated fatty acids. Palmitic acid (16:0) and oleic (C18:1w9c) were predominant in the Spirulina sp. LEB 18 biomass during the different photoperiods with glycerol supplementation. These results were in accordance with those of Morais et al. [10] and Narayan et al. [33], who cultured Spirulina species microalgae in medium supplemented with glycerol and observed an increase in unsaturated fatty acids with a predominance of C16:0 and C18:1w9c (Table 2).

Table 2.
Fatty acid profile (AG, mg g−1) for Spirulina sp. LEB 18 cultured in medium supplemented with glycerol and the control under different photoperiods: continuous light (24:0), 12 h light and 12 h dark (12:12), and dark (0:24).
4. Conclusions
The culture with crude glycerol supplementation and a 12:12 photoperiod showed the highest cell concentration (1.24 g L−1), biomass productivity (28.36 g L−1 h−1), and protein yield (647.3 mg L−1, 52.2% w w−1). Continuous light (24:0) positively influenced carbohydrate production (216.19 mg L−1, 23.8% w w−1) in cultures supplemented with the organic source. The application of crude glycerol to microalgal cultures can add value to biodiesel industry byproducts by generating biomass rich in compounds that are applicable to biofuel and animal feed production.
Author Contributions
Conceptualization, E.G.d.M., M.G.d.M., A.P.C.d.R. and J.A.V.C.; methodology, E.G.d.M., J.d.A.C., A.P.C.d.R. and J.I.D.; formal analysis, E.G.d.M. and J.d.A.C.; investigation, E.G.d.M.; resources, J.A.V.C., J.I.D. and I.L.N.; data curation, E.G.d.M., J.d.A.C., A.P.C.d.R. and M.G.d.M.; writing—original draft preparation, E.G.d.M.; writing—review and editing, M.G.d.M. and A.P.C.d.R.; supervision, J.A.V.C., A.P.C.d.R. and J.I.D.; project administration, J.A.V.C.; funding acquisition, J.A.V.C., M.G.d.M., J.I.D. and I.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)-Finance Code 001.
Acknowledgments
The authors thank the Ministry of Science, Technology, Innovation, and Communications and the Program to Support the Publication of Academic Production/PROPESP/FURG/2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morais, M.G.; da Silva Vaz, B.; Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, Y.; Xiao, L.; Masuda, A.; Ozawa, T.; Tsuda, M.; Omasa, K. Effects of temperature, photosynthetic photon flux density, photoperiod and O2 and CO2 concentrations on growth rates of the symbiotic dinoflagellate, Amphidinium sp. J. Appl. Phycol. 2009, 20, 287–292. [Google Scholar] [CrossRef]
- Matos, Â.P.; Cavanholi, M.G.; Moecke, E.H.S.; Sant’Anna, E.S. Effects of different photoperiod and trophic conditions on biomass, protein and lipid production by the marine alga Nannochloropsis gaditana at optimal concentration of desalination concentrate. Bioresour. Technol. 2017, 224, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour. Technol. 2013, 129, 7–11. [Google Scholar] [CrossRef]
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef]
- Deng, X.; Chen, B.; Xue, C.; Li, D.; Hu, X.; Gao, K. Biomass production and biochemical profiles of a freshwater microalga Chlorella kessleri in mixotrophic culture: Effects of light intensity and photoperiodicity. Bioresour. Technol. 2018, 273, 358–367. [Google Scholar] [CrossRef]
- Abad, S.; Turon, X. Valorization of biodiesel derived glycerol as a carbon source to obtain added-value metabolites: Focus on polyunsaturated fatty acids. Biotechnol. Adv. 2012, 30, 733–741. [Google Scholar] [CrossRef]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef]
- Morais, E.G.; Druzian, J.I.; Nunes, I.L.; Morais, M.G.; Costa, J.A.V. Glycerol increases growth, protein production and alters the fatty acids profile of Spirulina (Arthrospira) sp. LEB 18. Process Biochem. 2018, 76, 40–45. [Google Scholar] [CrossRef]
- Morais, E.G.; Nunes, I.L.; Druzian, J.I.; de Morais, M.G.; da Rosa, A.P.C.; Costa, J.A.V. Increasing the cell productivity of mixotrophic growth of Spirulina sp. LEB 18 with crude glycerol. Biomass Convers. Biorefinery 2022, 1, 3. [Google Scholar] [CrossRef]
- Morais, E.G.; Nunes, I.L.; Druzian, J.I.; Morais, M.G.; da Rosa, A.P.C.; Costa, J.A.V. Increase in Biomass Productivi-ty and Protein Content of Spirulina sp. LEB 18 (Arthrospira) Cultivated with Crude Glycerol. Biomass Convers. Biorefinery 2020, 12, 597–605. [Google Scholar] [CrossRef]
- Paternina, L.P.R.; Moraes, L.; Santos, T.D.; Morais, M.G.; Costa, J.A.V. Spirulina and Açai as Innovative Ingredients in the Development of Gummy Candies. J. Food Process. Preserv. 2022, 1, e17261. [Google Scholar] [CrossRef]
- Veiga, M.C.; Fontoura, M.M.; de Oliveira, M.G.; Costa, J.A.V.; Santos, L.O. Magnetic Fields: Biomass Potential of Spirulina Sp. for Food Supplement. Bioprocess Biosyst. Eng. 2020, 43, 1231–1240. [Google Scholar] [CrossRef]
- Benemann, J. Microalgae for Biofuels and Animal Feeds. Energies 2013, 6, 5869–5886. [Google Scholar] [CrossRef]
- Paranjape, K.; Leite, G.B.; Hallenbeck, P.C. Effect of Nitrogen Regime on Microalgal Lipid Production during Mixo-trophic Growth with Glycerol. Bioresour. Technol. 2016, 214, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, K.; Leite, G.B.; Hallenbeck, P.C. Strain variation in microalgal lipid production during mixotrophic growth with glycerol. Bioresour. Technol. 2016, 204, 80–88. [Google Scholar] [CrossRef][Green Version]
- Baldisserotto, C.; Sabia, A.; Guerrini, A.; Demaria, S.; Maglie, M.; Ferroni, L.; Pancaldi, S. Mixotrophic cultivation of Thalassiosira pseudonana with pure and crude glycerol: Impact on lipid profile. Algal Res. 2021, 54, 102194. [Google Scholar] [CrossRef]
- Morais, M.G.; Reichert, C.D.C.; Dalcanton, F.; Durante, A.J.; Marins, L.F.; Costa, J.V. Isolation and Characterization of a New Arthrospira Strain. Z. Fur Nat. Sect. C J. Biosci. 2008, 63, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.L.L.; da Silva, A.C.M.S.; Filho, J.A.M.; Druzian, J.I. Impact of different by-products from the biodiesel industry and bacterial strains on the production, composition, and properties of novel polyhydroxyalkanoates containing achiral building blocks. Ind. Crops Prod. 2015, 69, 212–223. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Colla, L.M.; Filho, P.D.; Kabke, K.; Weber, A.; Vieira Costa, J.A.; Colla, L.M.; Filho, P.D.; Kabke, K.; We-ber, A.; et al. Modelling of Spirulina platensis growth in fresh water using response surface methodology. World J. Microbiol. Biotechnol. 2002, 18, 603–607. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Bondioli, P.; Della Bella, L. An alternative spectrophotometric method for the determination of free glycerol in biodiesel. Eur. J. Lipid Sci. Technol. 2005, 107, 153–157. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Mitra, D.; van Leeuwen, J.; Lamsal, B. Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res. 2012, 1, 40–48. [Google Scholar] [CrossRef]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- Li, T.; Kirchhoff, H.; Gargouri, M.; Feng, J.; Cousins, A.B.; Pienkos, P.T.; Gang, D.R.; Chen, S. Assessment of photosynthesis regulation in mixotrophically cultured microalga Chlorella sorokiniana. Algal Res. 2016, 19, 30–38. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Suwannarat, W.; Niyomdecha, R. Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. New Biotechnol. 2011, 28, 362–368. [Google Scholar] [CrossRef]
- Holdmann, C.; Schmid-Staiger, U.; Hornstein, H.; Hirth, T. Keeping the light energy constant—Cultivation of Chlorella sorokiniana at different specific light availabilities and different photoperiods. Algal Res. 2018, 29, 61–70. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Han, F.; Mu, J.; Chen, D.; Feng, B.; Zeng, H. Regulation of lipid metabolism in the green microalga Chlorella protothecoides by heterotrophy–photoinduction cultivation regime. Bioresour. Technol. 2014, 192, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.S.; Manoj, G.P.; Vatchravelu, K.; Bhagyalakshmi, N.; Mahadevaswamy, M. Utilization of Glycerol as Carbon Source on the Growth, Pigment and Lipid Production in Spirulina platensis. Int. J. Food Sci. Nutr. 2005, 56, 521–528. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).