Effects of UV and Heating on the Stability of Fucoxanthin, Total Phlorotannin and Total Antioxidant Capacities in Saccharina japonica Ethanol Extract and Solvent Fractions
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Kelp Sample Preparation
2.3. Analysis of Fucoxanthin
2.4. Total Phlorotannin Contents
2.5. ABTS Assay
2.6. FRAP Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Fraction Yield
3.2. Fucoxanthin Content and Stability by UVA and Heat Exposure
3.3. Change of Total Phlorotannin Contents through UVA and Heat Exposure
3.4. Change in the Total Antioxidant Capacity through UVA and Heat Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, E.K.; Park, C.S. Seaweed cultivation and utilization of Korea. ALGAE 2020, 35, 107–121. [Google Scholar] [CrossRef]
- An, S.-R.; Koo, J.-G. Properties of Porphyran and Hemicellulose extracted with Different extract Solutions and Enzymatic Pretreatments from Porphyra. J. FISHRIES Mar. Sci. Educ. 2017, 29, 108–117. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2017, 58, 1342–1358. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Boulom, S.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Seasonal changes in lipid, fatty acid, alpha-tocopherol and phytosterol contents of seaweed, Undaria pinnatifida, in the Marlborough Sounds, New Zealand. Food Chem. 2014, 161, 261–269. [Google Scholar] [CrossRef]
- Saini, R.K.; Mahomoodally, M.F.; Sadeer, N.B.; Keum, Y.-S.; Rengasamy, K.R. Characterization of nutritionally important lipophilic constituents from brown kelp Ecklonia radiata (C. Ag.) J. Agardh. Food Chem. 2020, 340, 127897. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Kawee-ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and antioxidant activities of fucoxanthin purified from the mi-croalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1934578X1300801010. [Google Scholar]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Airanthi, M.W.-A.; Hosokawa, M.; Miyashita, K. Comparative Antioxidant Activity of Edible Japanese Brown Seaweeds. J. Food Sci. 2010, 76, C104–C111. [Google Scholar] [CrossRef]
- Mazzola, P.; Lopes, A.; A Hasmann, F.; Jozala, A.F.; Penna, T.C.; Magalhães, P.D.O.; Rangel-Yagui, C.; Jr, A.P. Liquid–liquid extraction of biomolecules: An overview and update of the main techniques. J. Chem. Technol. Biotechnol. 2008, 83, 143–157. [Google Scholar] [CrossRef]
- Medina Perez, E.B. Fucoxanthin from marine microalga Isochrysis galbana: Optimization of extraction methods with organic solvents. Dyna 2019, 86, 174–178. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.-H.; Kang, S.-E.; Cho, J.-Y.; Kim, A.-R.; Park, S.-M.; Hong, Y.-K.; Ahn, D.-H. The Antioxidant Properties of Brown Seaweed (Sargassum siliquastrum) Extracts. J. Med. Food 2007, 10, 479–485. [Google Scholar] [CrossRef]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1, 1-diphenyl-2-picrylhydrazyl (DPPH). IUBMB Life 1997, 42, 361–370. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, S.-M.; Pan, C.-H.; Chung, D. Effects of heating, aerial exposure and illumination on stability of fucoxanthin in canola oil. Food Chem. 2014, 145, 505–513. [Google Scholar] [CrossRef]
- Daood, H.G.; Kapitány, J.; Biacs, P.; Albrecht, K. Drying temperature, endogenous antioxidants and capsaicinoids affect carotenoid stability in paprika (red pepper spice). J. Sci. Food Agric. 2006, 86, 2450–2457. [Google Scholar] [CrossRef]
- Piovan, A.; Seraglia, R.; Bresin, B.; Caniato, R.; Filippini, R. Fucoxanthin from Undaria pinnatifida: Photostability and Coextractive Effects. Molecules 2013, 18, 6298–6310. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef] [Green Version]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- Gómez, I.; Huovinen, P. Induction of Phlorotannins During UV Exposure Mitigates Inhibition of Photosynthesis and DNA Damage in the Kelp Lessonia nigrescens. Photochem. Photobiol. 2010, 86, 1056–1063. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. Environ. Boil. Fishes 1999, 11, 559–566. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Wijesekara, I.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process. Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Cabane, B.; Bautista-Ortín, A.-B.; Carrillo, S.; Fulcrand, H.; Pérez, J.; Vernhet, A. Tannin Oxidation: Intra- versus Intermolecular Reactions. Biomacromolecules 2010, 11, 2376–2386. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yong, H.I.; Lee, B.W.; Park, S.; Baek, K.H.; Kim, T.H.; Jo, C. Plasma-Polymerized Phlorotannins and Their Enhanced Biological Activities. J. Agric. Food Chem. 2020, 68, 2357–2365. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Norra, I.; Aminah, A.; Suri, R.; Arif Zaidi, J. Effect of drying temperature on the content of fucoxanthin, phenolic and antioxidant activity of Malaysian brown seaweed, Sargassum sp. J. Trop. Agric. food Sci. 2017, 45, 25–36. [Google Scholar]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and pH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Generalic Mekinic, I. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. Environ. Boil. Fishes 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Vo Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]

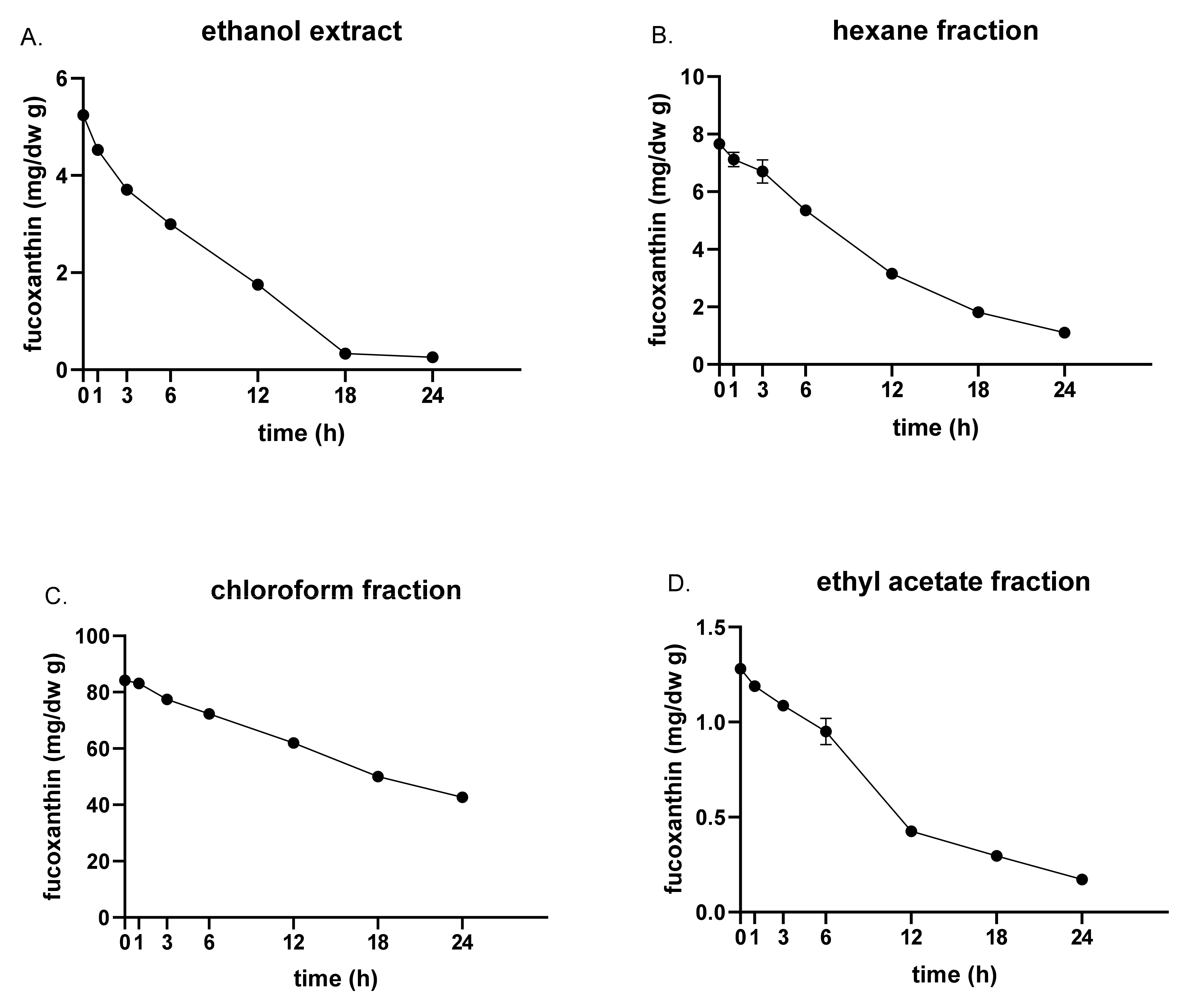

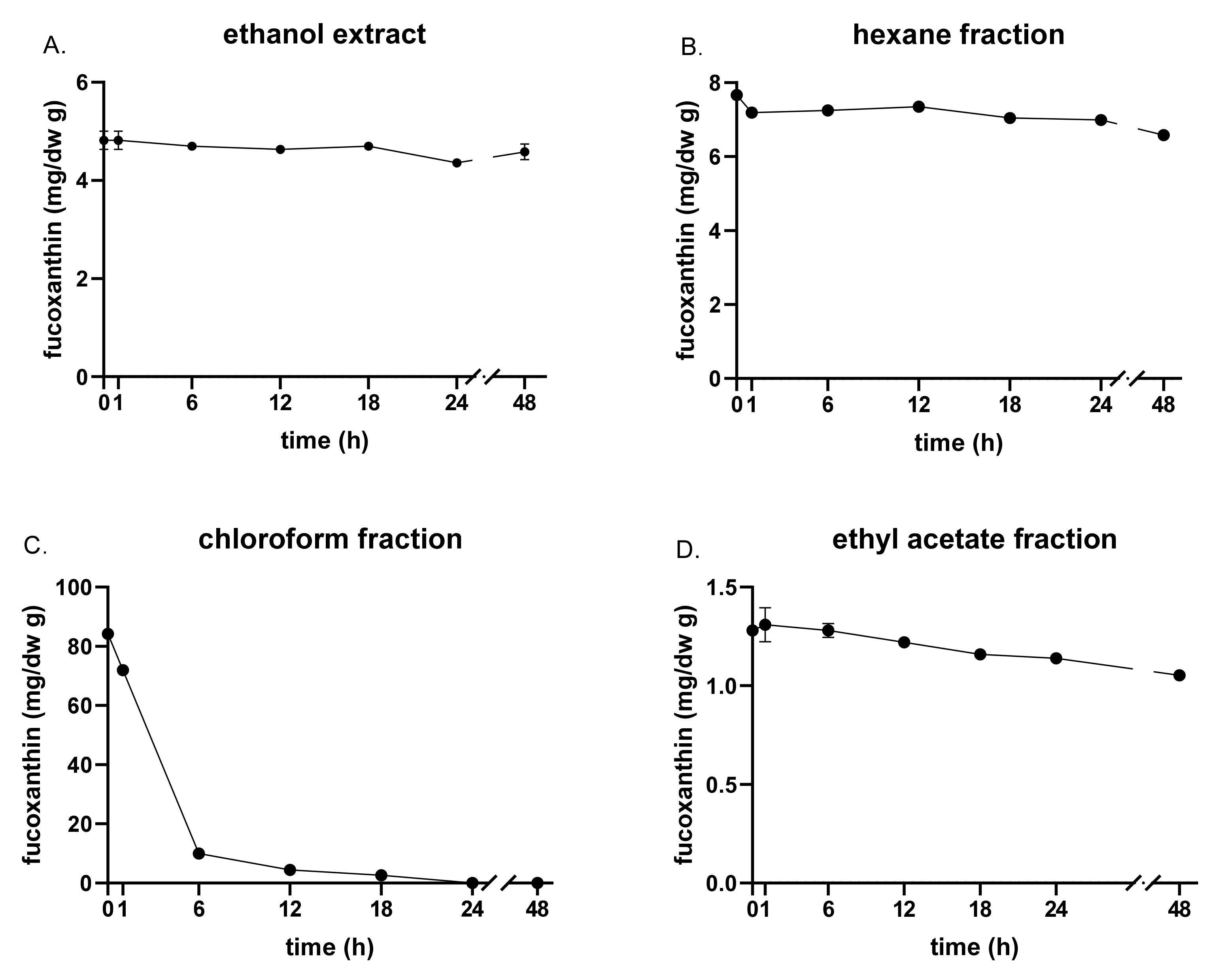
| Solvent | Yield (%) 1 | Fucoxanthin (mg/g) | TPC (mg PGE/g dw) | ABTS (mg VCE/g dw) | FRAP (mM FeSO4/g dw) |
|---|---|---|---|---|---|
| Ethanol extract | 5.25 ± 0.02 c | 5.05 ± 0.00 d | 2.96 ± 0.56 c | 0.04 ± 0.00 d | |
| Hexane fraction | 14.39 | 7.66 ± 0.02 b | 11.60 ± 0.26 c | 4.19 ± 0.37 b,c | 0.10 ± 0.00 b |
| Chloroform fraction | 1.23 | 84.24 ± 0.11 a | 58.85 ± 1.29 a | 20.43 ± 0.81 a | 0.22 ± 0.00 a |
| Ethyl acetate fraction | 0.32 | 1.28 ± 0.02 d | 20.42 ± 0.92 b | 10.75 ± 0.47 b | 0.07 ± 0.00 c |
| Butanol fraction | 8.62 | ND 3 | ND | ND | ND |
| Aqueous fraction | 73.78 | ND | ND | ND | ND |
| Time (h) | Ethanol Extract | Hexane Fraction | Chloroform Fraction | Ethyl Acetate Fraction | |
|---|---|---|---|---|---|
| UVA | 0 | 100.00 b | 100.00 b | 100.00 b | 100.00 b |
| 1 | 234.55 ± 14.41 a | 255.38 ± 6.17 a | 237.07 ± 10.82 a | 254.43 ± 10.32 a | |
| 3 | 237.69 ± 10.89 a | 240.56 ± 12.12 a | 243.47 ± 13.21 a | 258.24 ± 10.57 a | |
| 6 | 231.40 ± 16.34 a | 248.64 ± 12.12 a | 236.01 ± 7.25 a | 262.05 ± 14.29 a | |
| 12 | 234.55 ± 23.74 a | 244.60 ± 7.00 a | 235.47 ± 2.88 a | 265.10 ± 5.28 a | |
| 18 | 243.98 ± 14.41 a | 240.56 ± 14.57 a | 233.87 ± 12.31 a | 262.82 ± 10.81 a | |
| 24 | 234.55 ± 14.41 a | 243.25 ± 20.74 a | 235.74 ± 12.65 a | 268.15 ± 7.35 s | |
| 55 °C | 0 | 100.00 f | 100.00 d | 100.00 c | 100.00 c |
| 1 | 109.63 ± 7.15 e,f | 111.18 ± 2.74 c,d | 107.4 ± 0.20 a,b | 113.15 ± 3.28 b,c | |
| 6 | 128.89 ± 6.30 c,d | 120.75 ± 3.74 b,c | 109.41 ± 0.41 a | 116.55 ± 9.14 b,c | |
| 12 | 117.88 ± 4.13 d,e | 121.35 ± 7.18 b,c | 104.45 ± 1.75 a,b,c | 119.27 ± 1.56 a,b | |
| 18 | 139.89 ± 6.30 b,c | 128.54 ± 3.11 b | 102.09 ± 1.67 b,c | 121.99 ± 3.06 a,b | |
| 24 | 144.02 ± 4.76 b | 130.93 ± 6.31 b | 100.67 ± 3.19 c | 119.61 ± 5.89 a,b | |
| 48 | 203.16 ± 4.76 a | 163.26 ± 3.74 a | 101.73 ± 3.48 c | 131.86 ± 5.80 a | |
| 75 °C | 0 | 100.00 d | 100.00 g | 100.00 b | 100.00 d |
| 1 | 96.56 ± 4.76 d | 110.94 ± 5.49 f | 110.46 ± 2.99 a | 114.37 ± 2.04 c | |
| 6 | 118.11 ± 2.10 d | 124.71 ± 1.04 e | 97.31 ± 2.66 b,c | 118.12 ± 2.12 c | |
| 12 | 142.41 ± 6.35 c | 136.08 ± 4.52 d | 95.19 ± 0.84 b,c | 119.82 ± 3.12 c | |
| 18 | 156.63 ± 13.91 c | 147.46 ± 0.00 c | 96.17 ± 1.79 c | 120.16 ± 4.25 b,c | |
| 24 | 184.59 ± 4.13 b | 158.83 ± 4.52 b | 94.05 ± 2.15 c | 129.00 ± 5.62 a,b | |
| 48 | 228.15 ± 15.82 a | 175.59 ± 5.18 a | 94.12 ± 0.79 c | 133.42 ± 2.36 a |
| Time (h) | Ethanol Extract | Hexane Fraction | Chloroform Fraction | Ethyl Acetate Fraction | |
|---|---|---|---|---|---|
| UVA | 0 | 100.00 c | 100.00 d | 100.00 e | 100.00 b |
| 1 | 184.36 ± 16.59 a,b | 225.67 ± 10.07 c | 109.79 ± 2.26 d,e | 171.18 ± 7.97 a | |
| 3 | 166.78 ± 3.30 b | 241.18 ± 6.72 b | 119.34 ± 7.95 c,d | 161.73 ± 8.18 a,b | |
| 6 | 190.95 ± 16.59 a,b | 246.99 ± 14.64 b | 124.72 ± 3.88 c,d | 172.69 ± 8.51 a | |
| 12 | 195.34 ± 6.86 a,b | 275.10 ± 14.54 b,c | 130.29 ± 5.98 b,c | 165.89 ± 3.65 a | |
| 18 | 201.93 ± 13.72 a | 315.82 ± 17.69 a | 138.25 ± 5.10 a,b | 178.36 ± 5.71 a | |
| 24 | 200.83 ± 3.81 a | 320.66 ± 14.35 a | 149.59 ± 2.60 a | 179.88 ± 4.94 a | |
| 55 °C | 0 | 100.00 d | 100.00 f | 100.00 c | 100.00 e |
| 1 | 93.57 ± 11.79 d | 107.18 ± 3.97 e,f | 113.1 ± 2.86 a,b | 108.45 ± 0.00 f | |
| 6 | 124.45 ± 5.90 c,d | 128.37 ± 12.14 d,e | 108.47 ± 5.88 a,b | 106.9 ± 4.65 e,f | |
| 12 | 130.89 ± 7.73 c | 148.24 ± 9.18 c,d | 108.74 ± 2.62 b | 117.23 ± 2.37 d | |
| 18 | 157.91 ± 23.49 b,c | 157.52 ± 4.59 b,c | 108.74 ± 1.70 b | 119.30 ± 1.55 c,d | |
| 24 | 173.36 ± 6.68 a,b | 178.71 ± 6.88 a,b | 113.64 ± 1.70 a,b | 127.05 ± 1.55 b | |
| 48 | 233.20 ± 24.56 a | 244.95 ± 12.78 a | 119.35 ± 0.47 a | 142.55 ± 3.10 a | |
| 75 °C | 0 | 100.00 f | 100.00 e | 100.00 a,b | 100.00 g |
| 1 | 98.71 ± 5.58 f | 97.90 ± 6.88 f,g | 93.52 ± 0.00 b,c | 102.77 ± 4.74 f,g | |
| 6 | 174.65 ± 7.80 e | 141.62 ± 7.95 e | 91.89 ± 0.82 c | 112.58 ± 3.10 e | |
| 12 | 182.36 ± 11.15 d,e | 182.69 ± 6.07 d | 90.53 ± 3.77 c | 122.92 ± 0.89 d | |
| 18 | 220.97 ± 11.14 c | 219.78 ± 8.27 c | 92.43 ± 2.05 c | 131.70 ± 2.37 c | |
| 24 | 246.73 ± 0.00 b,c | 250.25 ± 10.00 b | 95.42 ± 1.25 b,c | 142.03 ± 1.55 b | |
| 48 | 318.15 ± 18.39 a | 305.89 ± 12.14 a | 104.94 ± 1.63 a | 154.43 ± 1.55 a |
| Time (h) | Ethanol Extract | Hexane Fraction | Chloroform Fraction | Ethyl Acetate Fraction | |
|---|---|---|---|---|---|
| UVA | 0 | 100 c | 100 a | 100 b | 100 b |
| 1 | 124.39 ± 4.15 a | 91.79 ± 2.22 b,c | 128.17 ± 4.12 a | 176.64 ± 6.94 a | |
| 3 | 127.26 ± 5.49 a | 88.97 ± 2.04 b,c | 129.16 ± 4.57 a | 174.54 ± 4.05 a | |
| 6 | 114.57 ± 4.98 a,b | 86.92 ± 2.70 b,c | 126.46 ± 1.28 a | 185.04 ± 10.00 a | |
| 12 | 104.75 ± 4.21 b,c | 85.13 ± 4.28 c | 123.32 ± 5.10 a | 185.88 ± 7.60 a | |
| 18 | 113.85 ± 5.70 a,b | 88.46 ± 1.78 b,c | 119.33 ± 4.71 a | 182.52 ± 6.67 a | |
| 24 | 115.53 ± 6.52 a,b | 93.59 ± 2.04 a,b | 122.32 ± 1.73 a | 182.94 ± 5.09 a | |
| 55 °C | 0 | 100 b,c | 100 b | 100 a,b,c | 100 b,c |
| 1 | 84.8 ± 4.69 d | 72.17 ± 1.70 e | 88.46 ± 4.91 c | 92.79 ± 1.67 c | |
| 6 | 85.71 ± 5.63 c,d | 80.71 ± 1.70 d,e | 97.89 ± 4.26 b,c | 104.33 ± 5.46 b | |
| 12 | 97.43 ± 6.25 b,c,d | 87.01 ± 2.23 c,d | 105.85 ± 5.62 a,b,c | 102.88 ± 6.50 b | |
| 18 | 92.02 ± 4.13 b,c,d | 92.95 ± 4.63 b,c | 97.56 ± 1.29 b,c | 102.88 ± 2.20 b | |
| 24 | 102.85 ± 4.13 b | 101.11 ± 4.50 b | 96.59 ± 5.36 b,c | 107.69 ± 2.20 b | |
| 48 | 167.8 ± 7.81 a | 162.34 ± 6.33 a | 110.24 ± 3.99 a | 130.29 ± 2.20 a | |
| 75 °C | 0 | 100 d,e | 100 d | 100 a | 100 c,d |
| 1 | 90.04 ± 1.44 e | 59.18 ± 2.95 g | 92.69 ± 3.41 a | 80.77 ± 1.44 e | |
| 6 | 92.53 ± 16.01 e | 71.06 ± 3.40 f | 74.32 ± 3.59 b | 99.04 ± 6.81 d | |
| 12 | 121.58 ± 8.00 c,d | 86.64 ± 3.40 e | 75.46 ± 3.42 b | 105.29 ± 2.88 c,d | |
| 18 | 133.2 ± 6.59 c,d | 116.33 ± 6.13 c | 68.31 ± 3.68 b | 112.02 ± 3.33 b,c | |
| 24 | 163.07 ± 7.47 b | 130.8 ± 6.13 b | 74 ± 7.50 b | 118.75 ± 3.63 b | |
| 48 | 250.21 ± 16.33 a | 148.98 ± 2.32 a | 94.31 ± 3.25 a | 131.25 ± 5.00 a |
| □ | Fucoxanthin Contents | TPC | TAC (ABTS Assay) | TAC (FRAP Assay) |
|---|---|---|---|---|
| Fucoxanthin contents | 1 | 0.834 (p < 0.001) | 0.691 (p < 0.001) | 0.788 (p < 0.001) |
| TPC | 1 | 0.894 (p < 0.001) | 0.899 (p < 0.001) | |
| TAC (ABTS assay) | 1 | 0.926 (p < 0.001) | ||
| TAC (FRAP assay) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.; Cao, L.; Lee, H.; Lee, Y.; Lee, S. Effects of UV and Heating on the Stability of Fucoxanthin, Total Phlorotannin and Total Antioxidant Capacities in Saccharina japonica Ethanol Extract and Solvent Fractions. Appl. Sci. 2021, 11, 7831. https://doi.org/10.3390/app11177831
Baek S, Cao L, Lee H, Lee Y, Lee S. Effects of UV and Heating on the Stability of Fucoxanthin, Total Phlorotannin and Total Antioxidant Capacities in Saccharina japonica Ethanol Extract and Solvent Fractions. Applied Sciences. 2021; 11(17):7831. https://doi.org/10.3390/app11177831
Chicago/Turabian StyleBaek, Suhyeon, Lei Cao, Hyeju Lee, Yoonmi Lee, and Sanggil Lee. 2021. "Effects of UV and Heating on the Stability of Fucoxanthin, Total Phlorotannin and Total Antioxidant Capacities in Saccharina japonica Ethanol Extract and Solvent Fractions" Applied Sciences 11, no. 17: 7831. https://doi.org/10.3390/app11177831
APA StyleBaek, S., Cao, L., Lee, H., Lee, Y., & Lee, S. (2021). Effects of UV and Heating on the Stability of Fucoxanthin, Total Phlorotannin and Total Antioxidant Capacities in Saccharina japonica Ethanol Extract and Solvent Fractions. Applied Sciences, 11(17), 7831. https://doi.org/10.3390/app11177831







