1. Introduction
Myocardial infarction is one of the most common coronary heart diseases. After myocardial infarction, inflammatory cells infiltrate the infarct area, and secrete matrix metalloproteinases and other active degradation substances that degrade the extracellular matrix, leading to replacement of the infarct area by scar fibrous tissues. The loss of extracellular matrix support in the infarct border induces necrosis or apoptosis of the cardiomyocytes, which further leads to continuous physiological changes, such as infarct area expansion, and ultimately causes final heart failure and death. Current treatments for myocardial infarction, including drug therapy and myocardial reperfusion, does not repair the myocardium. Biomaterials provide new options for the treatment of myocardial injuries after myocardial infarction. These materials mainly include synthetic [
1,
2,
3], natural [
4,
5,
6,
7,
8], and biological materials [
9,
10,
11]. Biological tissue-derived decellularized matrices have excellent cell affinity due to their large content in bioactive substances, such as collagen, glycosaminoglycans (GAG), proteoglycans, various growth factors, and other bioactive components, all promoting specific cell growth, differentiation, endogenous tissue regeneration, and repair. Thus, in recent years, researchers in the field have put a special focus on tissue-derived decellularized matrices [
12]. Porcine decellularized small intestinal submucosa matrix (pDSIS), porcine decellularized myocardial matrix (pDMYO), and human decellularized amniotic membrane/human decellularized placenta (HDAM/HDP) matrices have been used to repair myocardial infarction [
9,
10,
11,
13]. Two of them, pDSIS myocardial patch (CorMatrix
TM) and pDMYO injectable hydrogel (Ventrigel
TM), have already been commercialized.
There are unique advantages in clinical application for injectable hydrogels since they can be directly injected into the injured site through minimally invasive surgery and form hydrogel in situ. Several studies demonstrated in different animal models that decellularized matrix hydrogels derived from diverse tissues can effectively repair myocardial injuries. Boyd et al. [
9] prepared pDSIS hydrogels to repair porcine myocardial infarction and found that, compared with untreated animals, the cardiac functions of treated pigs, including ejection fraction and end systolic volume, were significantly improved. Francis et al. [
10] used HDP hydrogel to repair acute myocardial infarction in rats. Compared with that of control animals injected with a saline solution, the scar tissue of myocardial infarction area in treated animals was significantly reduced, and the surviving tissue maintained normal electrophysiological activity. Singelyn et al. [
11] prepared pDMYO hydrogels to repair myocardial infarction in rats. This hydrogel recruited endogenous cardiomyocytes and improved cardiac contractions.
Although decellularized matrix hydrogels from different tissues display some repairing effects in myocardial injury, whether and how exactly their material source affects their repair effectiveness remains unclear. It is likely there is a correlation based on previous research showing that extracellular matrix materials have tissue specificity. Decellularized matrix hydrogels derived from peripheral nerves (DNM-G) can promote myelination of axons [
14] but are not conducive to synapse formation. Meanwhile, hydrogels derived from spinal cord decellularized matrix facilitate the survival, proliferation, and migration of neural stem/progenitor cells (NSPCs), and promote NSPCs neuronal differentiation [
15]; nucleus pulposus decellularized matrix hydrogels derived from intervertebral discs are beneficial to the differentiation of mesenchymal stem cells (MSCs) into nucleus pulposus-like cells; and decellularized matrix hydrogels from annulus fibrosus facilitate the differentiation of MSCs into annulus fibrosus-like cells [
16]. Ungerleider et al. [
17] also found that skeletal muscle and human umbilical cord-derived decellularized hydrogels can repair peripheral arterial disease in a rat hind limb ischemia model, and the former reconstructed muscle tissue was closer to natural healthy skeletal muscle.
Several studies showed that protein composition found in decellularized matrices derived from specific tissue has specific biological function on the target tissue. As previously shown that DNM-G preserved critical biological factors unique of peripheral nerve which facilitated myelination. Proteins found in DNM-G only, such as collagen IV α1, collagen IV α2, and collagen V α2, show a positive correlation with regulation for myelination [
14]. There are 22 and 16 specific ECM matrisome proteins for the decellularized spinal cord matrix (DSCM) and decellularized peripheral nerve matrix, respectively. The DSCM specific glycoprotein and secreted factor, tenascin family, and fibroblast growth factor 2, are proved to be able to promote the NSPCs proliferation [
15]. For regeneration of complex tissues, a study from Cunniffe et al. [
18] showed that specific proteins were found in scaffolds derived from Growth plate ECM, such as chondroitin sulfate proteoglycan 4 and angiopoietin like 2, which are believed to play a role in angiogenesis; and C-Type Lectin Domain Containing 11A, Matrix Metallopeptidase 13 and S100 Calcium-Binding Protein A10, which can promote osteogenesis. In contrast, specific proteins were found in scaffolds derived from articular cartilage ECM, such as Gremlin 1, Frizzled Related Protein, and Transforming Growth Factor Beta 1, which can inhibit hypertrophy and promote chondrogenesis.
Therefore, the comparison of pDSIS-gel to pDMYO-gels in order to determine tissue-specific advantages for myocardial injury repair in animals, the relationship between the protein composition in pDMYO and potential tissue-specific biological functions needs to be defined. To this end, this study investigated the micromorphology, mechanical strength, composition, effect on cardiac-related cell behavior in vitro, and cardiac function in rat acute myocardial infarction models in vivo of these two gels. The ultimate aim was to correlate the differential effects exerted by these two gels on cell.
2. Materials and Methods
2.1. Materials and Reagents
Male landrace pigs (8-month-old, ≈48 kg) were supplied by The Original Pig Farm, South China Agricultural University, China. Sprague-Dawley neonatal rats and adult male rats were supplied by the Laboratory Animal Center of Sun Yat-sen University, China. Collagen I (#354249) was purchased from Corning Incorporated, Corning, NY, USA. DNA extraction kit, DNA detection kit, PageRuler pre-staining protein marker, colorimetric peptide assay kit, silver plating staining kit, liquid chromatography column, and live/dead cell staining solution were purchased from Thermo Fisher Scientific, Inc., Waltham, MA, USA. A commercially available kit containing a 1,9-dimethylmethylene blue (DMMB) dye-binding assay was purchased from Genmed Scientifics Inc., Arlington, MA, USA. The hydroxyproline test kit was purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Cell Counting Kit-8 was purchased from Dojindo, Kumamoto, Japan. Trypsin was purchased from Promega, Fitchburg, WI, USA. Pepsin, collagenase, DMEM/F12 basic medium, Complete medium/DMEM/F12, fetal bovine serum, horse serum, and penicillin, were purchased from GIBCO, Grand Island, NY, USA. Anti-cTn1 (#SAB4500539), -Cx43 (#SAB4301326) antibodies, and DAPI were purchased from Abcam, Cambridge, UK. Human umbilical vein endothelial cells (HUVECs) were purchased from ScienCell, Carlsbad, CA, USA. Triton X-100, sodium deoxycholate, tetramethylethylenediamine (TEMED), trimethylol aminomethane (Tris), urea (UA), iodoacetamide (IAA), dithiothreitol (DTT), formic acid (FA), triethylammonium bicarbonate (TEAB), trifluoroacetic acid, 5-bromodeoxyuridine (BrdU), and paraformaldehyde, were purchased from Sigma Aldrich, St. Louis, MO, USA. Dichloromethane and other reagents were purchased from Guangzhou Chemical Reagent Factory, Guangzhou, China.
2.2. Preparation of Decellularized Matrix and Hydrogel
The inner and outer membranes, fat, nerves, and blood vessels were dissected from the porcine heart and discarded, and the porcine myocardial tissues were washed and cut into pieces about 2-mm thick. The porcine small intestine was first washed with sterilized water, and then the submucosa was separated from the small intestine, scraped, and cut into pieces. The pieces were washed with sterilized water, soaked in 3% Triton X-100 for 24 h, and then soaked in 4% sodium deoxycholate on a shaker for 24 h. The small intestinal submucosa was degreased in dichloromethane/ethanol (v/v = 2/1) for 24 h. Finally, the decellularized matrix was soaked 4–6 times for 12 h in sterilized water on a shaker, and finally lyophilized.
The lyophilized decellularized matrix was milled into powders using Mini-Mill (Thomas Wiley), and sieved with an 80-mesh sieve. A definite mass (M) of powder was digested with pepsin (m) (M:m = 10:1). A 1 mg/mL pepsin solution was prepared in 0.01 M HCl. The digestion was stirred at room temperature for 24 h and stored at 4 °C. The pH of the digestion was adjusted to 8.0–8.5 with 1 M NaOH, and then 1 M HCl was used to adjust the pH value to 7. A 10× phosphate-buffered saline (PBS) solution was used for isosmotic equilibrium to obtain a pre-hydrogel at 4 °C. A hydrogel was obtained by increasing temperature of pre-hydrogel from 4 °C to 37 °C. The two different hydrogels prepared from the small intestine and heart were abbreviated pDSIS-gel and pDMYO-gel, respectively. Collagen type I hydrogel was prepared according to the instruction manual provided by Corning Incorporated and abbreviated COL1-gel. The above procedures were carried out under aseptic conditions. The concentration of each hydrogel was 5 mg/mL.
2.3. Characterization of Decellularized Matrices and Hydrogels
Freshly isolated tissues and decellularized matrices were fixed in 4% paraformaldehyde, washed three times with distilled water, embedded, frozen, sectioned at low temperature, and stained with hematoxylin and eosin (H&E). The stained sections were photographed with a Nikon eclipse E100 microscope.
The DNA from freshly isolated tissues and decellularized matrices was extracted using a DNA extraction kit (Thermo Fisher Scientific, USA), and analyzed with Quant-iT™ PicoGreen™ dsDNA Assay Kit (Thermo Fisher Scientific, USA) according to the instruction manual provided by the manufacturer, using 485 nm as the excitation wavelength and 528 nm as the emission wavelength on a Synergy HTX microplate reader (Biotek, Santa Barbara, CA, USA), with triplicates for each group.
Total glycosaminoglycan (GAG) content was isolated from freshly isolated tissues and decellularized matrices by high salt extraction, and quantified with a kit (Genmed Scientifics Inc., USA) based on a dimethylmethylene blue colorimetric assay, according to the manufacturer’s instructions.
Collagen content was determined indirectly by measuring hydroxyproline concentration, based on the fact that hydroxyproline represents 13.4% of the total amino acids contained in collagen. Freshly isolated tissues and decellularized matrix samples were hydrolyzed by alkali, hydroxyproline oxide, and dimethylaminobenzaldehyde, to produce a purplish red compound quantified by colorimetry.
The pepsin-digested porcine decellularized myocardial and intestinal submucosa matrices and the collagen solutions were analyzed and compared by SDS-PAGE, in 12% polyacrylamide separation gel and 5% polyacrylamide concentration gel. The electrophoresis was performed in a XCell SureLock Mini-Cell electrophoresis system (Thermo Fisher Scientific) and the proteins were detected by silver staining.
A scanning electron microscope (SEM, HITACHI S-4800, Japan) was used to analyze the hydrogel scaffolds. pDMYO-gel, pDSIS-gel, and COL1-gel at a concentration of 5 mg/mL were fixed in 2.5 wt% glutaraldehyde solution for 6 h. The samples were washed three times with deionized water for 30 min. Then, they were dehydrated successively in 30%, 50%, 75%, and 100% ethanol solutions for 30 min at each step. The samples were soaked in deionized water for 6 h, with the deionized water being changed every 2 h. The final hydrogel scaffolds were lyophilized. The samples were coated by platinum and observed by SEM. The SEM images were analyzed in the software ImageJ (version No. 1.53c). Three samples per experimental group were selected, and two SEM images/sample were analyzed. Ten fibers were randomly selected from each SEM image for diameter measurement and statistics.
The elastic storage modulus of the hydrogel scaffolds was tested in a Rotational Rheometer (Haake MARS, Thermo Fisher). The concentration of each tested sample was 5 mg/mL. The operation steps were as follows: 0.2 mL of hydrogel solution was placed on the parallel sample stand. Analysis was performed in flow mode and in conjunction with parallel steel plate geometry (20 mm diameter) with a gap of 0.5 mm. The temperature of the sample table was set at 4 °C. The test parameters were set at 1% strain and 1 Hz frequency. The temperature of the sample table rapidly raised from 4 °C to 37 °C (the heating rate was 200 °C per min) and maintained at 37 °C for 6 min. The elastic storage modulus (g) of each group was recorded.
2.4. Isolation of Neonatal Rat Cardiomyocytes (NRCMs)
Neonatal (1–3-day old) Sprague Dawley rats were anesthetized with ether, and their hearts were harvested. The hearts were washed in sterile phosphate buffer saline (PBS) containing 0.9 mM CaCl2 and 0.5 mM MgCl2 to induce cardiac contraction, and the corpuscles were removed. The hearts were then transferred to PBS buffer without CaCl2 and MgCl2, and the ventricles were separated from the atrium and cut into 1-mm3 pieces with thin scissors. The ventricular tissues were incubated in PBS containing 0.25% of collagenase at 37 °C for 30 min for digestion. After repeating the digestion procedure twice, the cell suspension was transferred to cell culture medium supplemented with 10% fetal bovine serum and 100 U/mL penicillin, and the cells were filtered through a 40 μm-diameter nylon mesh. The samples were centrifuged at room temperature at 180× g for 5 min. The cells were resuspended in cell culture medium and incubated for 60 min. The non-adherent cells were centrifuged, resuspended in cell culture medium containing BrdU, and transferred to a new culture dish.
2.5. Culture of NRCMs and Human Umbilical Vein Endothelial Cells (HUVECs) on Hydrogels
Seven microliters of the pre-hydrogels from
Section 2.2 were added to the wells of a 48-well plate at 4 °C, respectively. Then the hydrogels were formed by increasing temperature of the pre-hydrogels from 4 °C to 37 °C. The extracted NRCMs were seeded on the formed hydrogels at a concentration of 8 × 10
4 cells per well, and cultured in complete medium/DMEM/F12. After passage, HUVECs were seeded on the formed hydrogels at 2 × 10
4 cells per well, respectively, and cultured in complete medium/DMEM/F12.
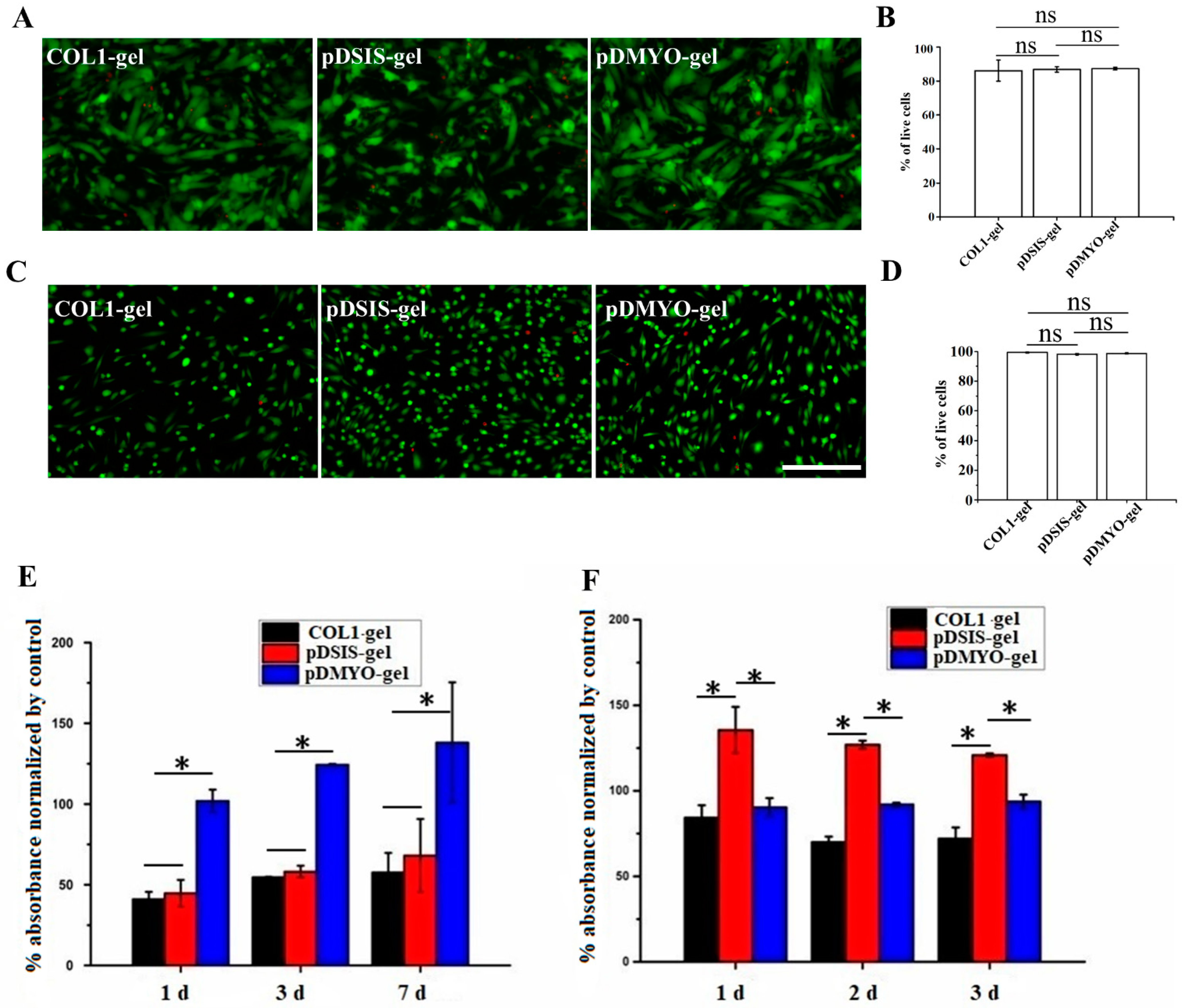
2.6. Monitoring of Myocardial Cell Survival on Gel Materials by Live/Dead Staining
After cultured for three days, NRCMs and HUVECs were stained with live/dead dye and photographed. The culture medium was removed by aspiration and the cells were washed in PBS 1–2 times. The following steps were performed in dark condition. One milliliter of live/dead dye solution was prepared in PBS by mixing 2 μM live green and 4 μM dead red dyes, and 100 μL of this solution was added per culture well. The cells were completely immerged and incubated at room temperature for 10 min. Finally, the dye was removed by aspiration, the cells were washed three times in PBS solution, and the samples were observed and photographed under a Leica SP8 confocal microscope (Wetzlar, Germany).
2.7. Detection of NRCMs and HUVECs Viability on Gel Materials
After one, three, and seven days in culture, the viability of the NRCMs was assessed with the Cell Counting Kit-8 assay (CCK8) colorimetric method. The HUVECs were assessed with the same method after one, two, and three days of culture. Briefly, the CCK-8 solution (10 μL) was added to each culture well, the plates were cultured at 37 °C for 4 h. The absorbance was read in a microplate reader (Thermo Scientific, Waltham, MA, USA) at 450 nm.
2.8. Immunofluorescence Staining
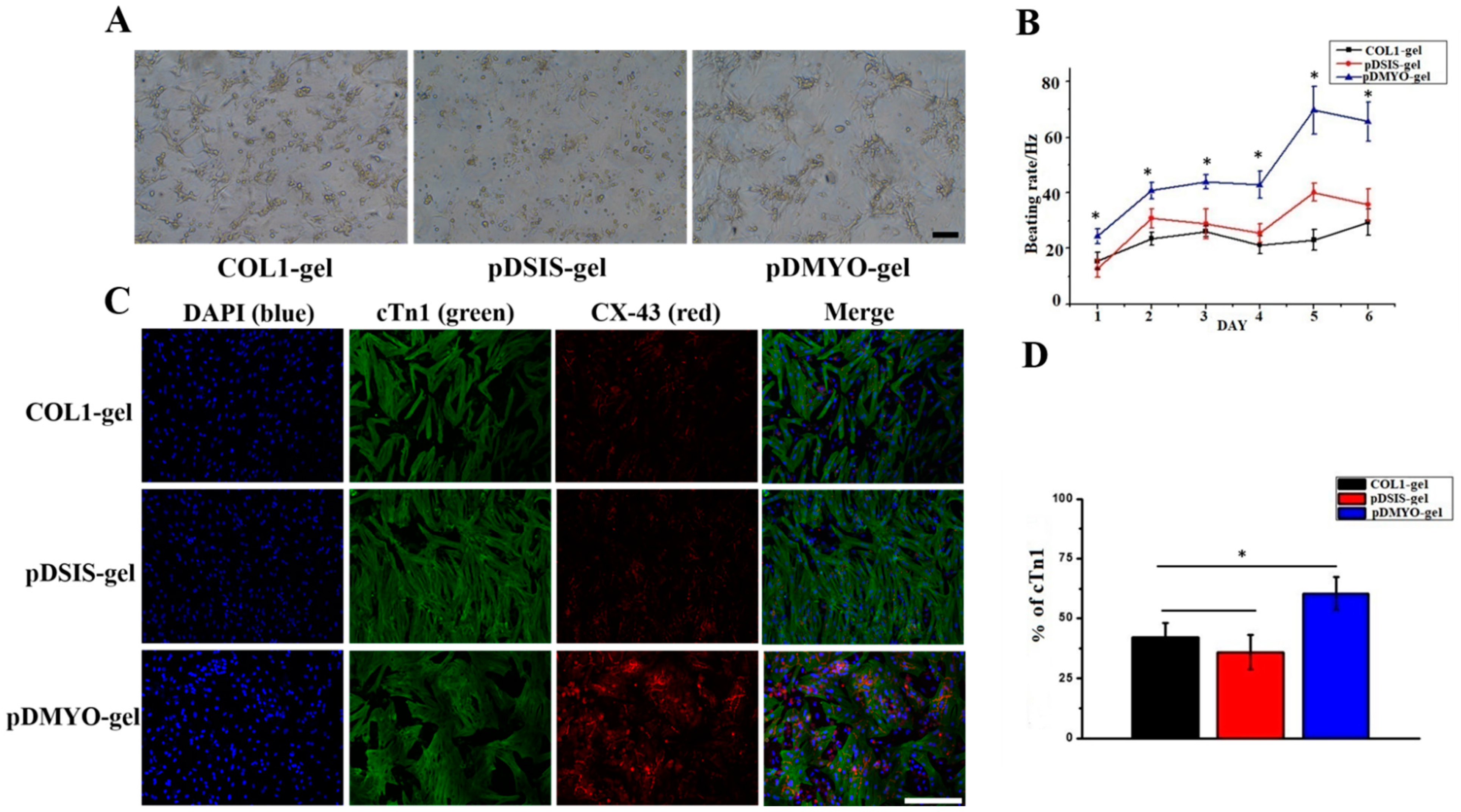
After three days in culture, the NRCMs were characterized by immunofluorescence staining with anti-cTn1 and anti-Cx43 antibodies. The protocol was as follows: the culture medium was removed by aspiration, and the cardiomyocyte cultures were washed 1–2 times with PBS. The cells were fixed in 4% paraformaldehyde for 30 min and washed several times with PBS for 5 min. They were permeabilized with 0.3% Triton X-100 for 30 min at room temperature, and after removal of the permeabilization solution, they were blocked with 10% horse serum for 30 min at 37 °C. Then the primary antibody was added. According to the recommended the anti-cTn1 antibody was diluted at 1:200, the anti-Cx43 antibody was diluted at 1:2000, and incubated with the cells overnight at 4 °C. The first antibody was removed by aspiration and the cells were washed three times with PBS for 5 min. The second fluorescent antibody was added according at the dilution indicated by the manufacturer and incubated at room temperature for 2 h in the dark. The samples were maintained in the dark. After removing the fluorescent secondary antibody by aspiration, the cells were washed three times with PBS for 3 min. DAPI diluted at 1:200 was added to stain the nucleus. The cells were incubated in dark for 15 min at room temperature. Finally, the cells were washed three times with PBS for 5 min. The staining images were observed under a Leica SP8 confocal microscope (Wetzlar, Germany).
2.9. Measurement of Cardiomyocyte Beating Frequency
Cardiomyocyte function was assessed by quantifying the beating frequency of the cardiomyocytes maintained in hydrogel culture for eight days. The beating frequency of NRCMs cultured on different materials for one to eight days was monitored, counted under a microscope, and recorded manually twice daily (interval > 3 h, observation window < 15 min).
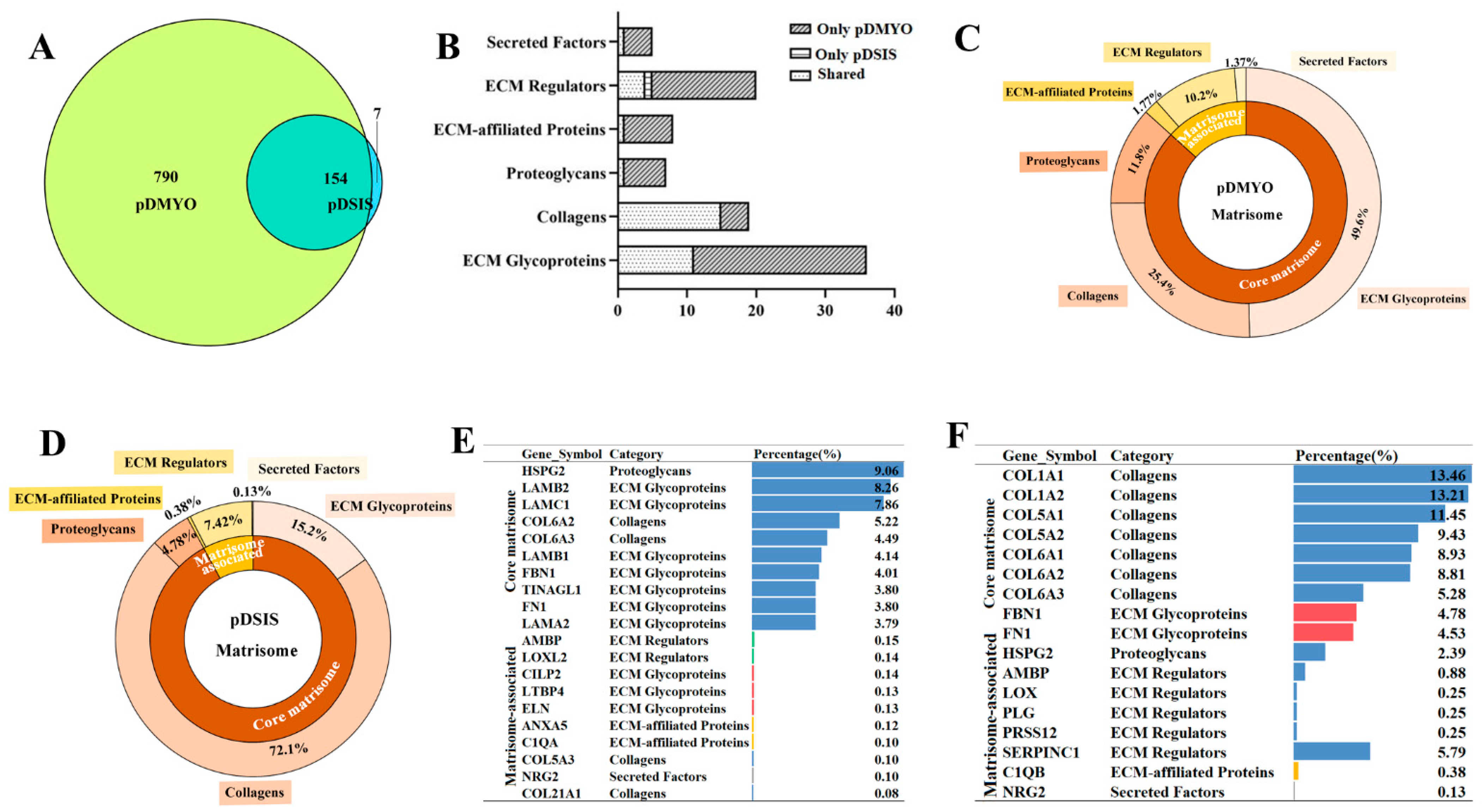
2.10. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS) Analysis
2.10.1. Protein Digestion and Peptide Extraction
Protein digestion and peptide extraction from the decellularized matrices were conducted as reported previously. [
15] Briefly, Ultrafiltration-assisted sample preparation was used for protein digestion. A sample of 300 μL UA solution (8 M urea dissolved in 0.1 M Tris HCl, pH 8.5) was used for diluting samples. A 10-kDa filter was used for centrifuging. A 200-μL UA solution with 50 mM DTT was used for reduction of samples. A 200-μL UA solution with 100 mM IAA was used for sample alkylation. After sample alkylation, a 200-μL UA solution was used for sample rinsing twice and a 200-μL TEAB solution (50 mM) was for rinsing for five times. The sample was centrifugated at 12,000×
g for 12 min at room temperature after each rinsing. A 100-μL TEAB solution containing 2 μg Trypsin Gold (Promega, USA) was used for digestion at 37 °C for 18 h. The resulted peptide was collected centrifugation at 12,000×
g for 15 min at least twice. A 100-μL TEAB solution was added after each centrifugation. The final peptide concentration was tested by a pierce quantitative colorimetric peptide assay (Thermo Fisher Scientific). Lyophilization and desaltation were performed on the peptide mixture. Finally, the peptide samples were lyophilized again and stored at −20 °C until LC-MS analysis.
2.10.2. LC-MS Analysis
LC-MS analysis was conducted as reported previously [
15]. Briefly, a nanoflow high-performance liquid chromatograph (HPLC) system (Easy nLC1200 system, Thermo Fisher Scientific, USA) coupled to an Orbitrap Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific, USA) with a nanoelectrospray ion source (Thermo Fisher, USA) was used. The sample dissolved in buffer A (0.1% formic acid [FA]) was injected into a 2-cm trap column (75-μm inner diameter, Acclaim PepMap 100C18, 3 μm, Thermo Scientific) and separated on a 75-μm-inner-diameter column with a length of 25 cm (Acclaim PepMap 100C18, 2 μm; Thermo Scientific) over a 120-min gradient (buffer A, 0.1% FA in water; buffer B, 0.1% FA in 80% Acetonitrile [ACN]) at a flow rate of 300 nL/min (0–3 min, 3–8% B; 3–99 min, 8–28% B; 99–113 min, 28–33% B; 113–116 min, 33–98% B; and 116–120 min, 98% B). The Q Exactive HF mass spectrometer was operated in positive ion mode at ion transfer tube temperature 320 °C, and the positive ion spray voltage was 3.7 kV. MS scans were acquired at resolution of 60,000, and the maximum injection time was 50 ms. High-energy collisional dissociation fragmentation was carried out at 28% normalized collision energy. MS2 AGC target was set to 1 × 10
5, the maximum injection time was set to 30 ms, and the dynamic exclusion was set to 60 s.
The MS data were searched against the Sus scrofa (pig) UniProt database (taxon identifier 9823, version 2018061348865) using Proteome Discoverer (software version 2.3, Thermo Scientific). The Precursor_Quan_LFQ_Sequest™ HT search engine was used to search the data. The search parameters were set as follows: trypsin was selected as proteolytic enzyme, and two missed cleavage sites were allowed; cysteine carbamylation was used as immobilization modification; oxidation of M and acetylation of N-terminal were used as modification variables; search quality tolerance was set as 10 ppm; false discovery rates of peptide-spectrum matching (PSMs) and protein were set as less than 1%.
2.11. Rat acute Myocardial Infarction Model and Injection of Decelluarized Matrix Hydrogels
Adult male SD rats (450–650 g) were provided by the Laboratory Animal Center of Sun Yat-Sen University. All animals were housed in SFP animal facility. Food and water were available ad libitum.
The rats were randomly divided into four groups: Shame group, PBS group, pDSIS-gel and pDMYO-gel group (n = 8 for 4 weeks each group).
The rats were anaesthetized using 10 wt% chloral hydrate solution (0.33 mL/100 g, i.p.). After anesthesia, the chest and neck were shaved and the skin was cleaned and disinfected. The anaesthetized rats were placed on a heatable operating table in supine position. Intubation was performed with an 18 G intravenous catheter, and the rats were ventilated with a volume-controlled small animal ventilator at 3 mL/100 g tidal volume at 60 breaths per minute.
The rat heart was surgically exposed with a left thoracotomy. After the pericardium was separated, the left anterior descending coronary artery was ligated with a 5–0 silk suture to induce ischemia in the distal regions of the left ventricular myocardium. The infarct site turned white rapidly, indicating successful ligation. PBS and pre-hydrogels were aspirated with 1 mL microinjector for 60 μL. The experimental materials were injected at 3~4 sites on the border of myocardial infarction area in rats. After confirming that the material was successful injected and no malignant arrhythmia was found in the rats, the thoracic cavity was sutured layer by layer, and the ventilating was maintained continuously. During the whole process, the rats were placed on a heating pad to support the body temperature of the rats and promote the recovery of the rats. After the rats recovered, the intravenous catheter was removed. Finally, the rats were transferred to the animal cages for further breeding.
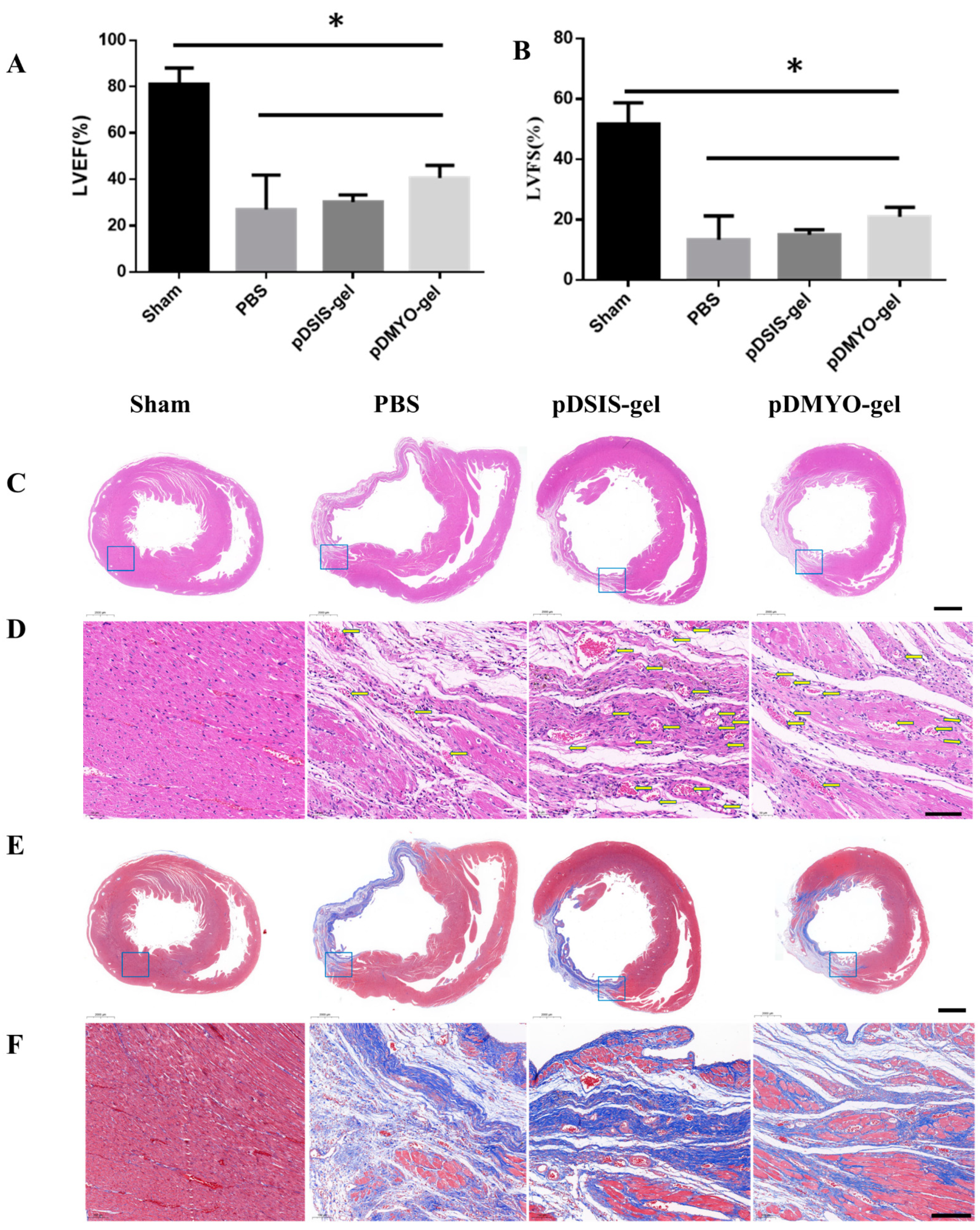
2.12. Echocardiography
Four weeks after surgery, the rats were anesthetized, and then fixed on the rat plate, coated with a small amount of couplant. Echocardiography was performed with small animal ultrasound equipment (Vevo 2100 Imaging System, vevo2100, Canada). Five cardiac cycles were recorded, as well as left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) with which reflecting cardiac function were measured.
2.13. Histology
The rats were sacrificed at four weeks after surgery for histological evaluation, and the whole heart was extracted. The isolated heart was cleaned with PBS and fixed with 4% paraformaldehyde and stored at 4 °C.
The specimens were embedded in paraffin, and were serially sectioned at a thickness of 3 μm. After dewaxing, the slides were stained with H&E and Masson trichrome by Wuhan Servicebio Technology Co., Ltd. (Wuhan, China) and observed and photographed under Nikon eclipse E100. The obtained scanning section images were browsed by caseviewer software and analyzed by ImageJ image analysis software (version No. 1.53c).
2.14. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 6.02 (GraphPad Software Inc., San Diego, CA, USA). Data are shown as mean ± standard deviation. Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The values were considered significantly different at p < 0.05.