On the Control Strategy to Improve the Salt Rejection of a Thin-Film Composite Reverse Osmosis Membrane
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
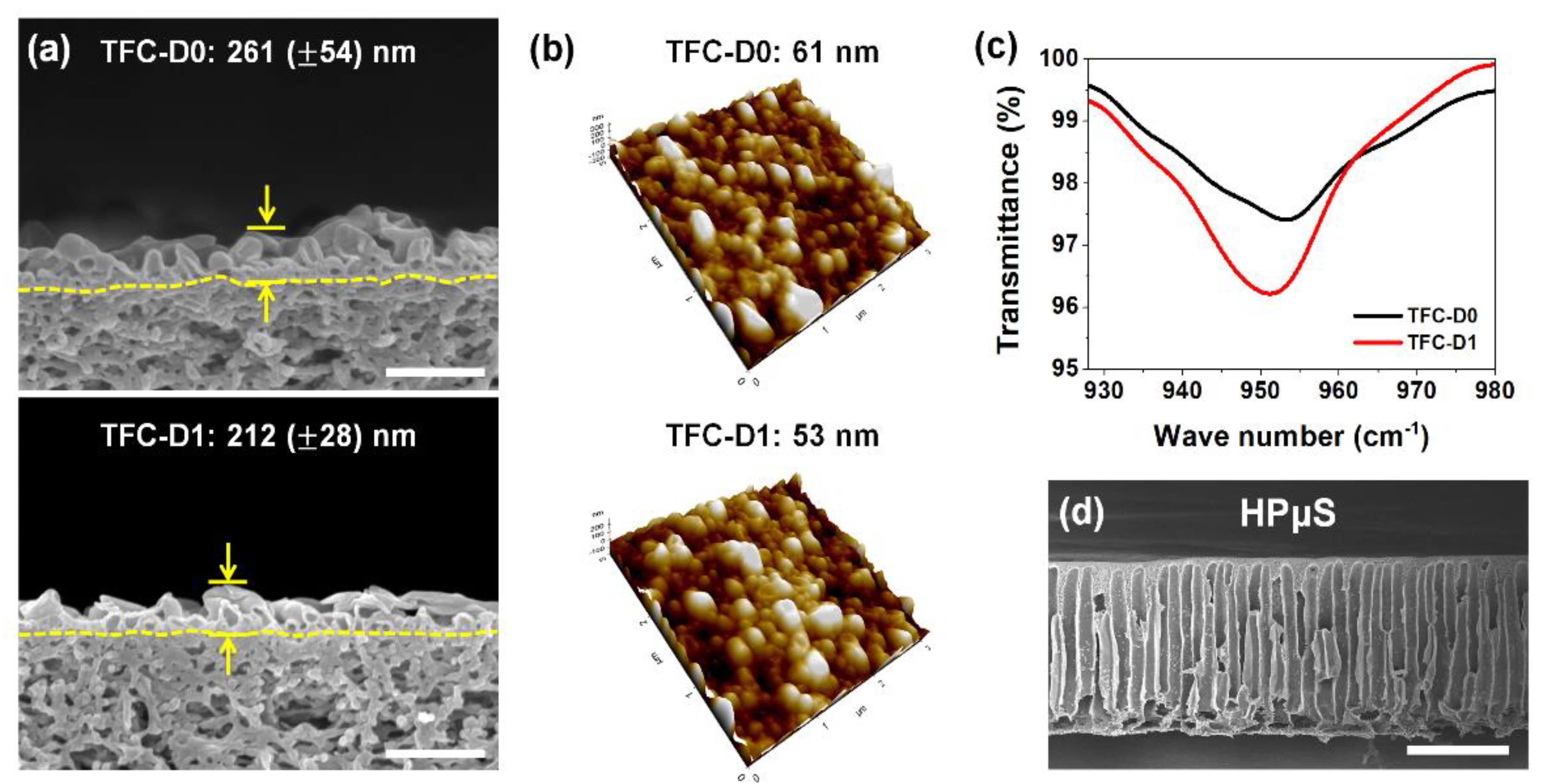
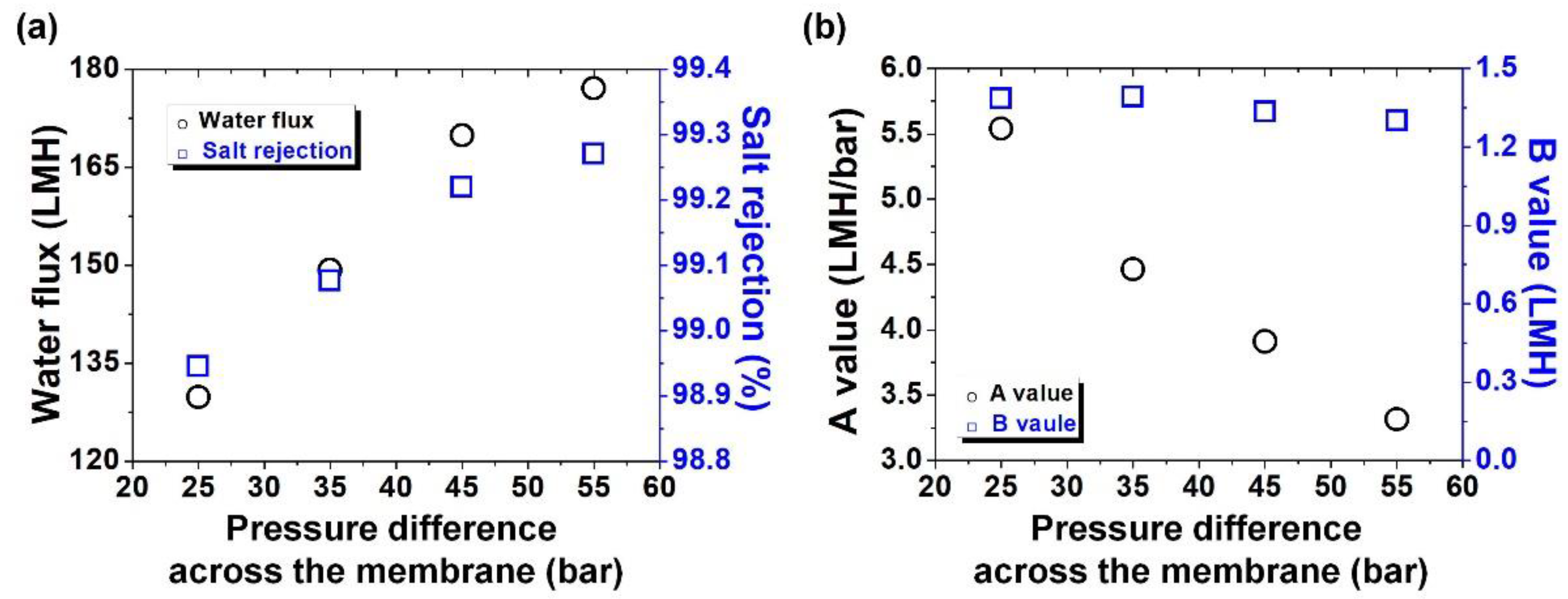
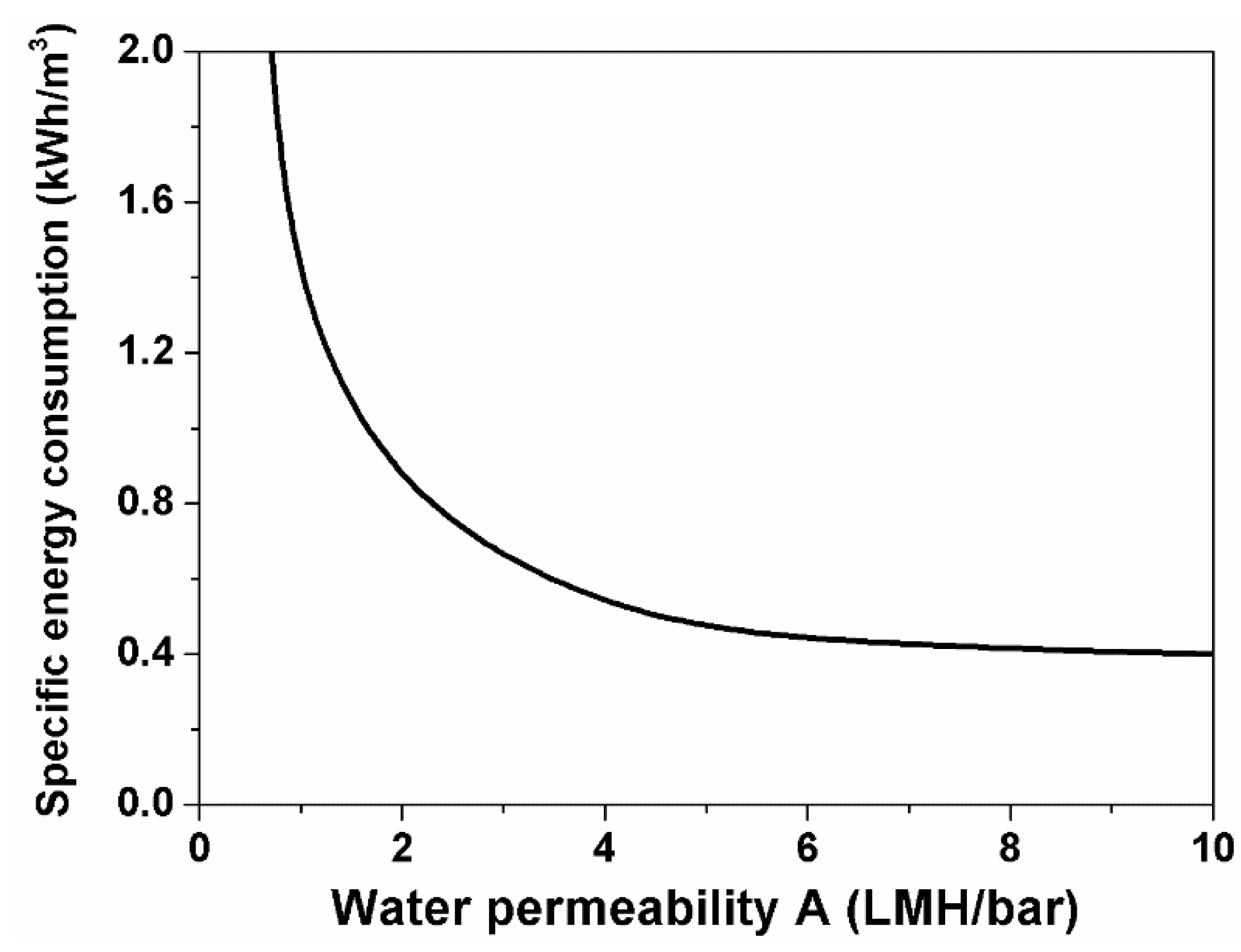
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, J.; Jang, J.H.; Chae, H.-R.; Lee, S.H.; Lee, C.-H.; Park, P.-K.; Won, Y.-J.; Kim, I.-C. A facile route to enhance the water flux of a thin-film composite reverse osmosis membrane: Incorporating thickness-controlled graphene oxide into a highly porous support layer. J. Mater. Chem. A 2015, 3, 22053–22060. [Google Scholar] [CrossRef]
- Lee, J.; Zhou, F.; Baek, K.; Kim, W.; Su, H.; Kim, K.; Wang, R.; Bae, T.-H. Use of rigid cucurbit[6]uril mediating selective water transport as a potential remedy to improve the permselectivity and durability of reverse osmosis membranes. J. Membr. Sci. 2021, 623, 119017. [Google Scholar] [CrossRef]
- Sethunga, G.; Lee, J.; Wang, R.; Bae, T.-H. Influences of operating parameters and membrane characteristics on the net energy production in dense, porous, and composite hollow fiber membrane contactors for dissolved biomethane recovery. J. Membr. Sci. 2020, 610, 118301. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, S.M.; Wang, R.; Lee, J. Emerging Materials to Prepare Mixed Matrix Membranes for Pollutant Removal in Water. Membranes 2021, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A. European Green Deal–legal and financial challenges of the climate change. ERA Forum 2021, 21, 681–697. [Google Scholar] [CrossRef]
- Alam, S.; Kumar, A.; Dawes, L. Roughness optimization of road networks: An option for carbon emission reduction by 2030. J. Transp. Eng. Part. B Pavements 2020, 146, 04020062. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.J.; Lee, K.; Lee, C.-H.; Lee, H.H.; Kim, I.-C.; Lee, J.-M. Graphene Oxide Nanoplatelets Composite Membrane with Hydrophilic and Antifouling Properties for Wastewater Treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Lee, J.; Won, Y.-J.; Choi, D.-C.; Lee, S.; Park, P.-K.; Choo, K.-H.; Oh, H.-S.; Lee, C.-H. Micro-patterned membranes with enzymatic quorum quenching activity to control biofouling in an MBR for wastewater treatment. J. Membr. Sci. 2019, 592, 117365. [Google Scholar] [CrossRef]
- Yao, W.; Wang, Z.; Wang, X. Detachment mechanism and energy consumption model for the ex-situ rinsing process in membrane bioreactors. J. Membr. Sci. 2020, 615, 118462. [Google Scholar] [CrossRef]
- Rongwong, W.; Lee, J.; Goh, K.; Karahan, H.E.; Bae, T.-H. Membrane-based technologies for post-treatment of anaerobic effluents. NPJ Clean Water 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Sethunga, G.; Lee, J.; Wang, R.; Bae, T.-H. Influence of membrane characteristics and operating parameters on transport properties of dissolved methane in a hollow fiber membrane contactor for biogas recovery from anaerobic effluents. J. Membr. Sci. 2019, 589, 117263. [Google Scholar] [CrossRef]
- Sethunga, G.; Karahan, H.E.; Wang, R.; Bae, T.-H. Wetting-and fouling-resistant hollow fiber membranes for dissolved methane recovery from anaerobic wastewater treatment effluents. J. Membr. Sci. 2021, 617, 118621. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication–A review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Chong, T.H.; Loo, S.-L.; Krantz, W.B. Energy-efficient reverse osmosis desalination process. J. Membr. Sci. 2015, 473, 177–188. [Google Scholar] [CrossRef]
- Chong, T.H.; Loo, S.-L.; Fane, A.G.; Krantz, W.B. Energy-efficient reverse osmosis desalination: Effect of retentate recycle and pump and energy recovery device efficiencies. Desalination 2015, 366, 15–31. [Google Scholar] [CrossRef]
- Chong, T.H.; Krantz, W.B. Process economics and operating strategy for the energy-efficient reverse osmosis (EERO) process. Desalination 2018, 443, 70–84. [Google Scholar] [CrossRef]
- Zhu, A.; Christofides, P.D.; Cohen, Y. On RO membrane and energy costs and associated incentives for future enhancements of membrane permeability. J. Membr. Sci. 2009, 344, 1–5. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Nuez, I. Performance evaluation and boron rejection in a SWRO system under variable operating conditions. Comput. Chem. Eng. 2021, 153, 107441. [Google Scholar] [CrossRef]
- Ruiz-García, A.; León, F.; Ramos-Martín, A. Different boron rejection behavior in two RO membranes installed in the same full-scale SWRO desalination plant. Desalination 2019, 449, 131–138. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Shin, M.G.; Seo, J.Y.; Park, H.; Park, Y.-I.; Lee, J.-H. Overcoming the permeability-selectivity trade-off of desalination membranes via controlled solvent activation. J. Membr. Sci. 2021, 620, 118870. [Google Scholar] [CrossRef]
- Gai, W.; Zhang, Y.; Zhao, Q.; Chung, T.-S. Highly permeable thin film composite hollow fiber membranes for brackish water desalination by incorporating amino functionalized carbon quantum dots and hypochlorite treatment. J. Membr. Sci. 2021, 620, 118952. [Google Scholar] [CrossRef]
- Li, W.-X.; Yang, Z.; Liu, W.-L.; Huang, Z.-H.; Zhang, H.; Li, M.-P.; Ma, X.-H.; Tang, C.Y.; Xu, Z.-L. Polyamide reverse osmosis membranes containing 1D nanochannels for enhanced water purification. J. Membr. Sci. 2021, 618, 118681. [Google Scholar] [CrossRef]
- Lee, J.; Wang, R.; Bae, T.-H. High-performance reverse osmosis membranes fabricated on highly porous microstructured supports. Desalination 2018, 436, 48–55. [Google Scholar] [CrossRef]
- Lee, J.; Wang, R.; Bae, T.-H. A comprehensive understanding of co-solvent effects on interfacial polymerization: Interaction with trimesoyl chloride. J. Membr. Sci. 2019, 583, 70–80. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, H.; Yoo, J.H.; Choi, D.-C.; Nahm, C.H.; Lee, S.H.; Chae, H.-R.; Kim, Y.H.; Lee, C.-H.; Park, P.-K. Influence of the sublayer structure of thin-film composite reverse osmosis membranes on the overall water flux. Environ. Sci. Water Res. Technol. 2018, 4, 1912–1922. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, J.; Bae, T.-H.; Torres, J.; Wang, R. Feasibility and performance of a thin-film composite seawater reverse osmosis membrane fabricated on a highly porous microstructured support. J. Membr. Sci. 2020, 611, 118407. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, L.; Zhu, X.; Xu, D.; Li, G.; Liang, H.; Wiesner, M.R. The role of carboxylated cellulose nanocrystals placement in the performance of thin-film composite (TFC) membrane. J. Membr. Sci. 2021, 617, 118581. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Wang, Y.-C.; Wang, W.-J.; Tao, S.-N.; Chen, Y.-F.; Tang, M.; Shao, D.-D.; Xing, W.; Sun, S.-P. Designing scalable dual-layer composite hollow fiber nanofiltration membranes with fully cross-linked ultrathin functional layer. J. Membr. Sci. 2021, 628, 119243. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, X.; Wang, G.-R.; Lu, T.-D.; Shi, Q.; Sun, S.-P. Inner-selective coordination nanofiltration hollow fiber membranes from assist-pressure modified substrate. J. Membr. Sci. 2021, 626, 119186. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Ge, Y.; Yu, S.; Liu, M.; Gao, C. A combined interfacial polymerization and in-situ sol-gel strategy to construct composite nanofiltration membrane with improved pore size distribution and anti-protein-fouling property. J. Membr. Sci. 2021, 623, 119097. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Lai, G.S.; Ng, C.Y.; Torres, J.; Wang, R. Fast water transport through biomimetic reverse osmosis membranes embedded with peptide-attached (pR)-pillar [5] arenes water channels. J. Membr. Sci. 2021, 628, 119276. [Google Scholar] [CrossRef]
- Ruiz-García, A.; de la Nuez Pestana, I. Feed spacer geometries and permeability coefficients. Effect on the performance in BWRO spriral-wound membrane modules. Water 2019, 11, 152. [Google Scholar] [CrossRef] [Green Version]
- Koutsou, C.; Kritikos, E.; Karabelas, A.; Kostoglou, M. Analysis of temperature effects on the specific energy consumption in reverse osmosis desalination processes. Desalination 2020, 476, 114213. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 6343. [Google Scholar] [CrossRef] [Green Version]
- Karabelas, A.; Koutsou, C.; Kostoglou, M.; Sioutopoulos, D. Analysis of specific energy consumption in reverse osmosis desalination processes. Desalination 2018, 431, 15–21. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; McGovern, R.K.; Dave, S.H.; Lienhard, J.H.; Grossman, J.C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. 2014, 7, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Q.; Kang, D.-Y.; Hu, S.; Lin, L.-C. Exploiting interior surface functionalization in reverse osmosis desalination membranes to mitigate permeability–selectivity trade-off: Molecular simulations of nanotube-based membranes. Desalination 2020, 491, 114537. [Google Scholar] [CrossRef]
- Savage, D.F.; Egea, P.F.; Robles-Colmenares, Y.; III, J.D.O.C.; Stroud, R.M. Architecture and selectivity in aquaporins: 2.5 Å X-ray structure of aquaporin Z. PLoS Biol. 2003, 1, e72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, C.; Li, X.; Vararattanavech, A.; Shen, W.; Torres, J.; Helix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization. J. Membr. Sci. 2012, 423, 422–428. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Hoek, E.M.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
| Material | Pore Size | Other Features | Incorporation Type | Control Membrane’s Salt Rejection | Effect of Porous Nanomaterials | Ref. |
|---|---|---|---|---|---|---|
| CB[6] | 3.9 Å |
|
| 97.8% | Improved selectivity | [2] |
| Peptide-appended pillar[5]arene | ~5 Å |
|
| 98.2% | Improved permeability | [34] |
| Aquaporin Z | 2.8 Å [41] |
|
| 96% | Improved permeability | [42] |
| Zeolite | 4 Å |
|
| 93.4% | Improved permeability | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lim, Y.J. On the Control Strategy to Improve the Salt Rejection of a Thin-Film Composite Reverse Osmosis Membrane. Appl. Sci. 2021, 11, 7619. https://doi.org/10.3390/app11167619
Lee J, Lim YJ. On the Control Strategy to Improve the Salt Rejection of a Thin-Film Composite Reverse Osmosis Membrane. Applied Sciences. 2021; 11(16):7619. https://doi.org/10.3390/app11167619
Chicago/Turabian StyleLee, Jaewoo, and Yu Jie Lim. 2021. "On the Control Strategy to Improve the Salt Rejection of a Thin-Film Composite Reverse Osmosis Membrane" Applied Sciences 11, no. 16: 7619. https://doi.org/10.3390/app11167619
APA StyleLee, J., & Lim, Y. J. (2021). On the Control Strategy to Improve the Salt Rejection of a Thin-Film Composite Reverse Osmosis Membrane. Applied Sciences, 11(16), 7619. https://doi.org/10.3390/app11167619







