Synchrotron X-ray Irradiation of a Rat’s Head Model: Monte Carlo Study of Chromatic Gel Dosimetry
Abstract
:1. Introduction
2. Materials and Methods
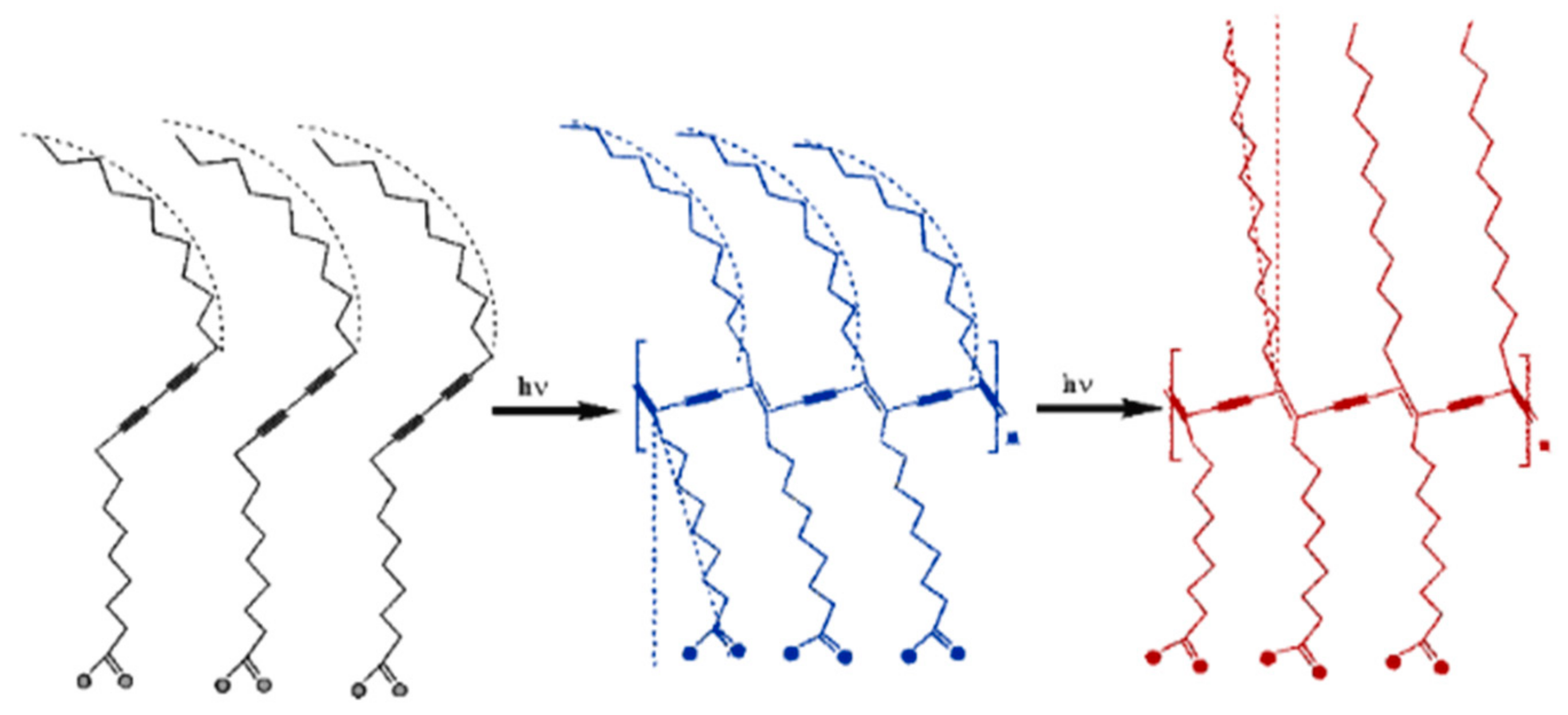
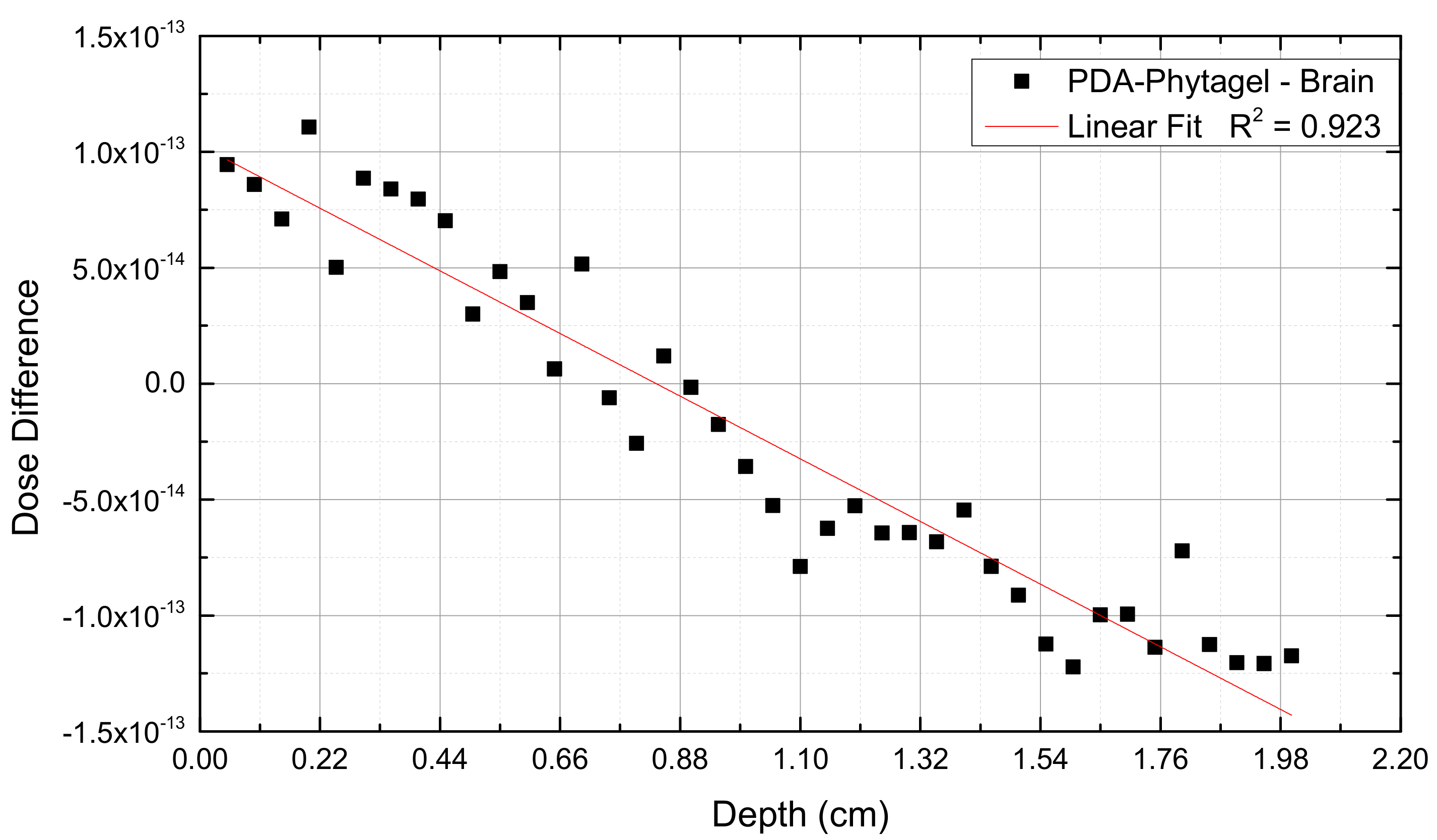
2.1. PDA-Phytagel Dosimeter Properties
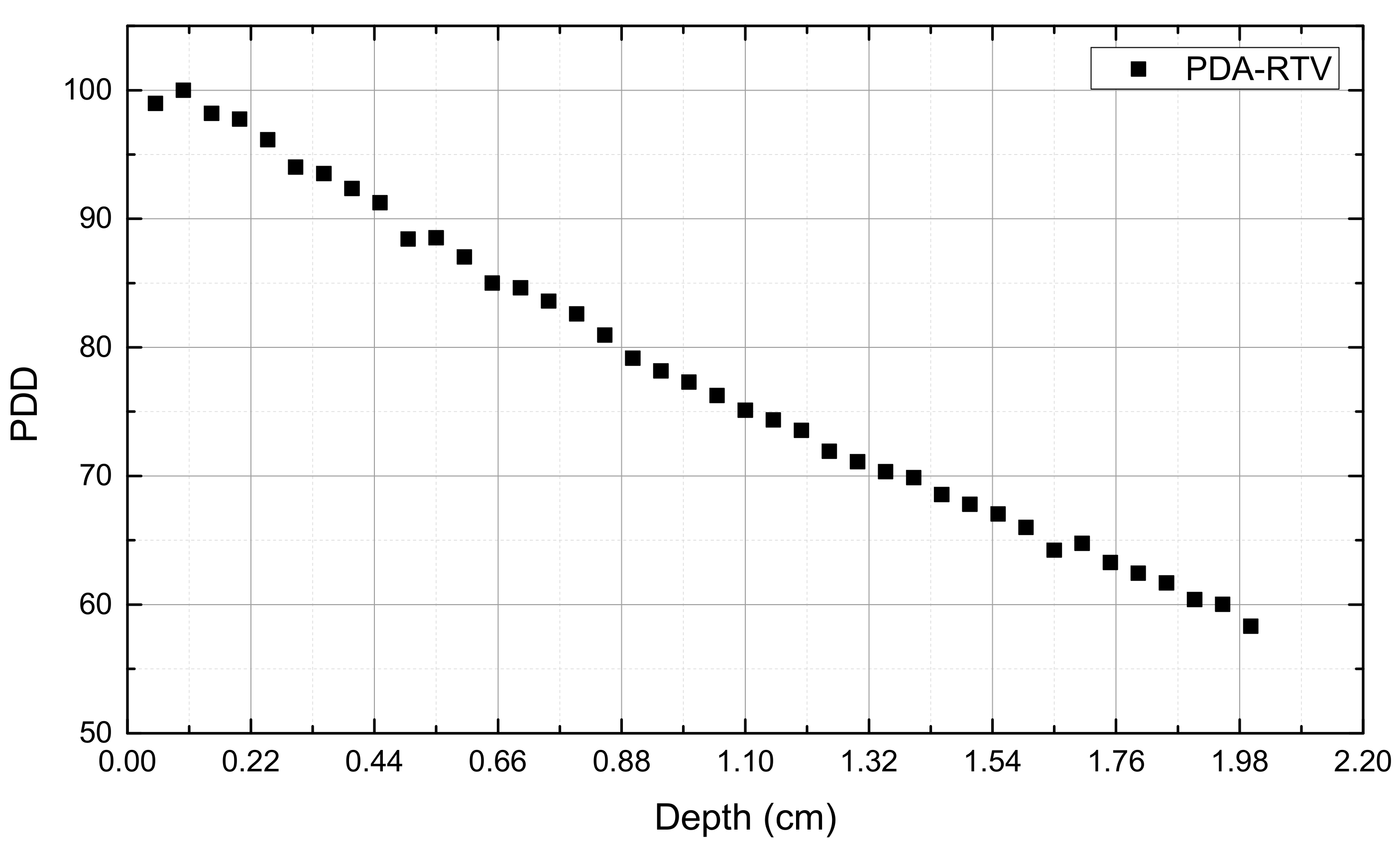
2.2. PDA-RTV Silicone Rubber Dosimeter Properties
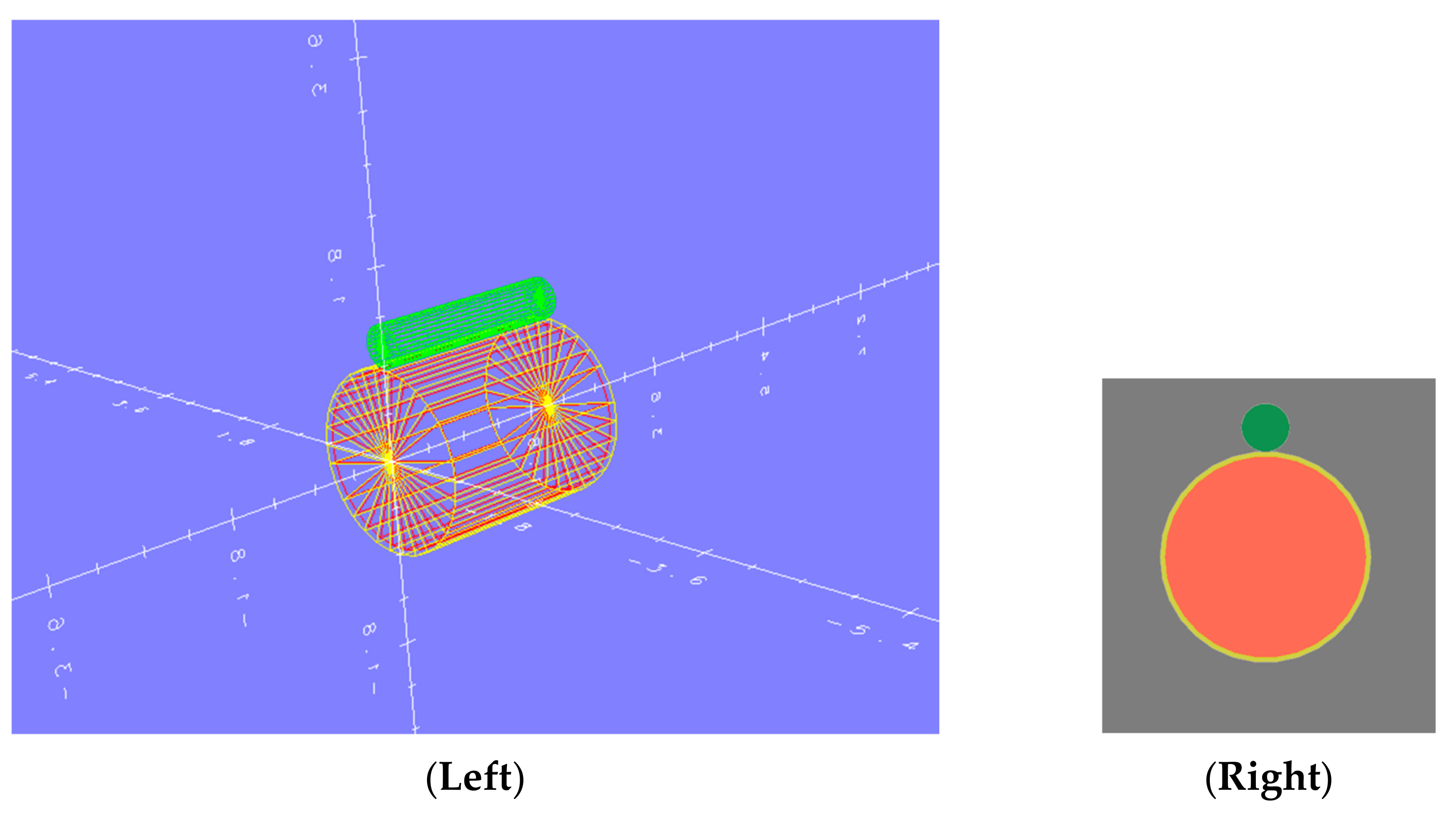
2.3. Rat’s Head Modeling and Geometry
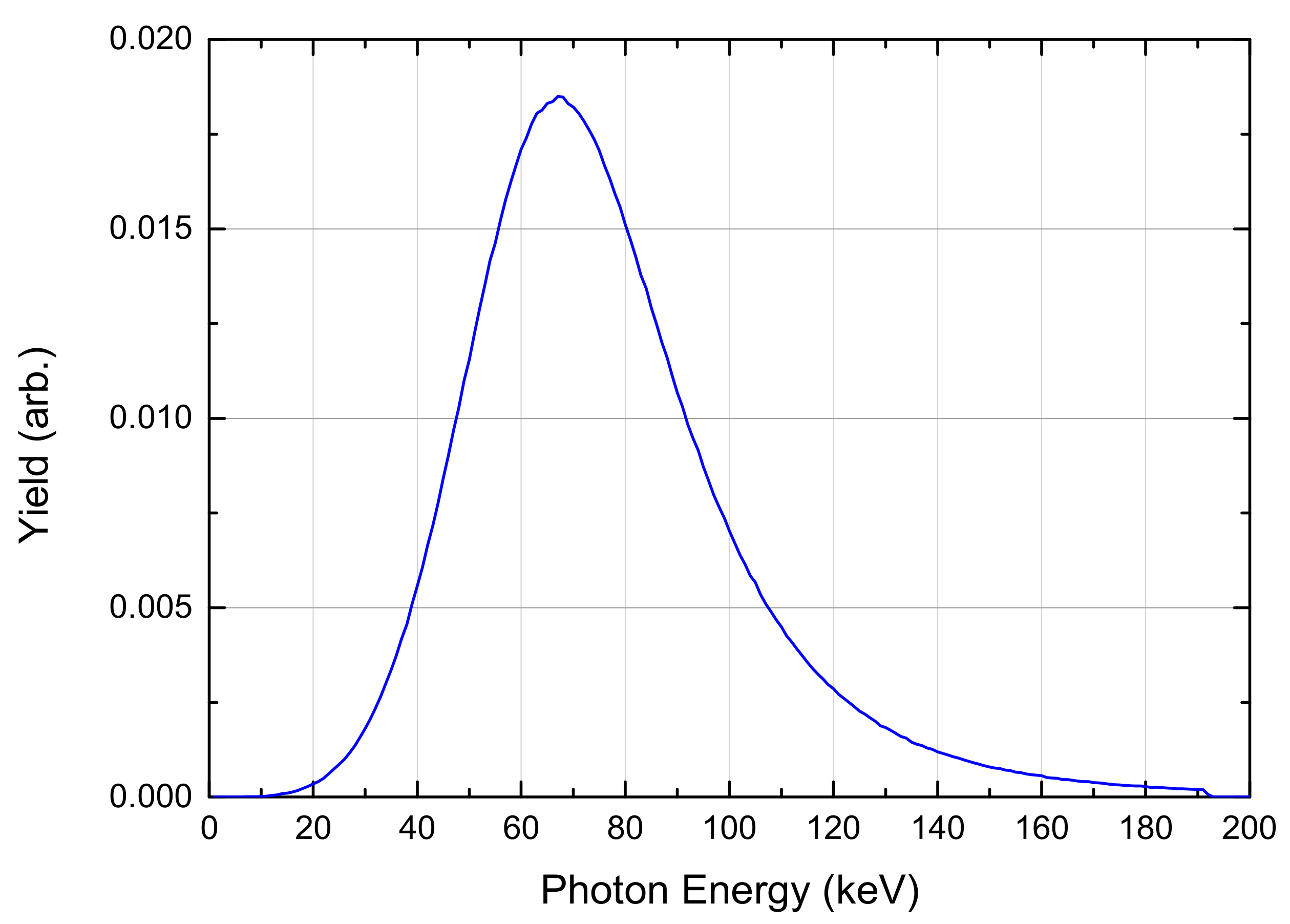
2.4. The Radiation Source
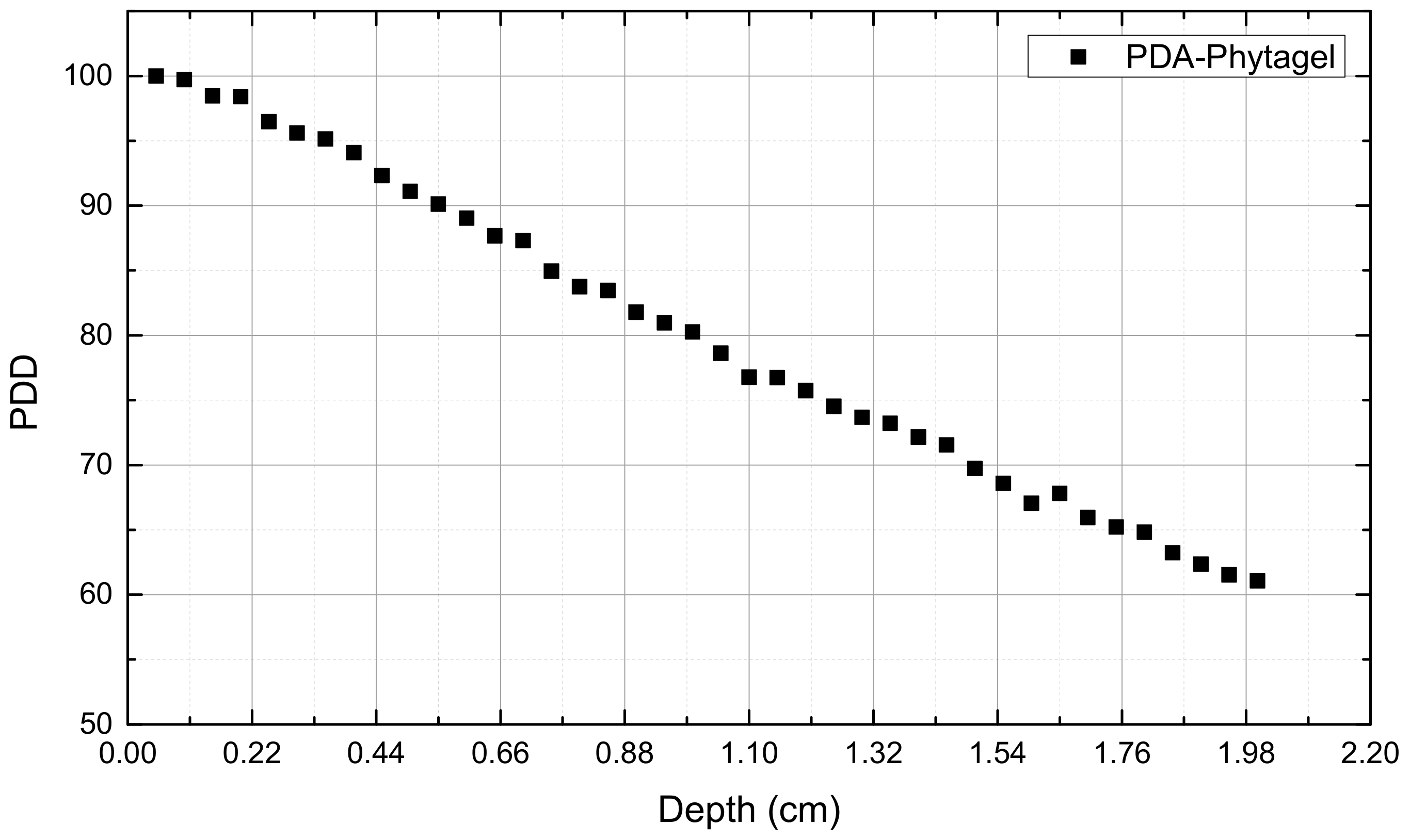
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Maryanski, M.J.; Schulz, R.J.; Ibbott, G.S.; Gatenby, J.C.; Xie, J.; Horton, D.; Gore, J.C. Magnetic resonance imaging of radiation dose distributions using a polymer-gel dosimeter. Phys. Med. Biol. 1994, 39, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C. Review of gel dosimetry: A personal reflection. J. Phys. Conf. Ser. 2017, 777, 012029. [Google Scholar] [CrossRef]
- Venning, A.J.; Hill, B.; Brindha, S.; Healy, B.J.; Baldock, C. Investigation of the PAGAT polymer gel dosimeter using magnetic resonance imaging. Phys. Med. Biol. 2005, 50, 3875–3888. [Google Scholar] [CrossRef] [PubMed]
- Walg, Y.P.; Silveira, M.A.; Eafergan, N.; Krutman, Y.; Baffa, O.; Berman, A.; Orion, I. Characterization of novel polydiacetylene gel dosimeter for radiotherapy. Biomed. Phys. Eng. Express 2020, 6, 055017. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, Y.; Golan, Y.; Konovalov, O.; Berman, A. Structural transitions in polydiacetylene langmuir films. Langmuir 2009, 25, 4469–4477. [Google Scholar] [CrossRef] [PubMed]
- Upcher, A.; Lifshitz, Y.; Zeiri, L.; Golan, Y.; Berman, A. Effect of metal cations on polydiacetylene langmuir films. Langmuir 2012, 28, 4248–4258. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.M.; Gibbons, J.P.; Sperduto, P.W. Khan’s Treatment Planning in Radiation Oncology, 4th ed.; Wolters Kluwer: Alfen am Rhein, The Netherlands, 2016. [Google Scholar]
- Chetty, I.J.; Curran, B.; Cygler, J.E.; Demarco, J.J.; Ezzell, G.; Faddegon, B.A.; Kawrakow, I.; Keall, P.J.; Liu, H.; Ma, C.-M.C.; et al. Report of the AAPM task group No. 105: Issues associated with clinical implementation of monte carlo-based photon and electron external beam treatment planning. Med. Phys. 2007, 34, 4818–4853. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.W.O.; Bielajew, A.F.; Kase, K.R. Monte Carlo Techniques of Electron and Photon Transport for Radiation Dosimetry; Bjarngard, B.E., Attix, F.H., Eds.; National Research Council of Canada: Ottawa, ON, Canada, 1990. [Google Scholar]
- Briesmeister, J.f. MCNP-A General Monte Carlo Code for Neutron and Photon Transport; Los Alamos National Laboratory: Los Alamos, NM, USA, 1993. [Google Scholar]
- Seltzer, S.M. Electron-photon monte carlo calculations: The ETRAN Code. Appl. Radiat. Isot. Int. J. Radiat. Appl. Instrum. Part A 1991, 42, 917–941. [Google Scholar] [CrossRef]
- Hirayama, H.; Namito, Y.; Nelson, W.R.; Bielajew, A.F.; Wilderman, S.J.; Michigan, U. The EGS5 Code System, in KEK Reports; United States Department of Energy: Washington, DC, USA, 2005. [Google Scholar]
- Chandrasekaran, S.; Shanmugasundaram, S. Optimization of variance reduction techniques used in EGSnrc monte carlo codes. J. Med. Phys. 2018, 43, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, K. Review of fast Monte Carlo codes for dose calculation in radiation therapy treatment planning. J. Med. Signals Sens. 2011, 1, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Orion, I.; Rosenfeld, A.; Dilmanian, F.A.; Telang, F.; Ren, B.; Namito, Y. Monte Carlo simulation of dose distributions from a synchrotron-produced microplanar beam array using the EGS4 code system4. Phys. Med. Biol. 2000, 45, 2497–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gefen, A.; Gefen, N.; Zhu, Q.; Raghupathi, R.; Margulies, S.S. Age-dependent changes in material properties of the brain and braincase of the rat. J. Neurotrauma 2003, 20, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Photon Sciences Division. High Energy Engineering X-ray Scattering (HEX) Facility. In Preliminary Design Report for the NSLS-II Project; National Synchrotron Light Source II (NSLS-II), Brookhaven National Laboratory: New York, NY, USA, 2018. [Google Scholar]
- Einfeld, D.; Stuck, D. Synchrotron radiation as an absolute standard source. Nucl. Instrum. Methods 1980, 172, 101–106. [Google Scholar] [CrossRef]





| Dosimeter | Composition (%wt) | Density (gr/cm3) | |
|---|---|---|---|
| PDA-Phytagel | H | 66.66 | 1.02 |
| O | 33.27 | ||
| C | 0.07 | ||
| PDA-RTV | H | 62.16 | 1.03 |
| O | 5.41 | ||
| C | 21.62 | ||
| Si | 10.81 | ||
| Body Zone | Radius (cm) | Length (cm) | Thickness (cm) |
|---|---|---|---|
| Brain | 0.84 | 2.00 | 0.84 |
| Skull | 0.88 | 2.08 | 0.04 |
| Tumor/dosimeter | 0.20 | 2.00 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peleg Walg, Y.; Krutman, Y.; Berman, A.; Orion, I. Synchrotron X-ray Irradiation of a Rat’s Head Model: Monte Carlo Study of Chromatic Gel Dosimetry. Appl. Sci. 2021, 11, 7389. https://doi.org/10.3390/app11167389
Peleg Walg Y, Krutman Y, Berman A, Orion I. Synchrotron X-ray Irradiation of a Rat’s Head Model: Monte Carlo Study of Chromatic Gel Dosimetry. Applied Sciences. 2021; 11(16):7389. https://doi.org/10.3390/app11167389
Chicago/Turabian StylePeleg Walg, Yarden, Yanai Krutman, Amir Berman, and Itzhak Orion. 2021. "Synchrotron X-ray Irradiation of a Rat’s Head Model: Monte Carlo Study of Chromatic Gel Dosimetry" Applied Sciences 11, no. 16: 7389. https://doi.org/10.3390/app11167389
APA StylePeleg Walg, Y., Krutman, Y., Berman, A., & Orion, I. (2021). Synchrotron X-ray Irradiation of a Rat’s Head Model: Monte Carlo Study of Chromatic Gel Dosimetry. Applied Sciences, 11(16), 7389. https://doi.org/10.3390/app11167389







