Electropolishing—A Practical Method for Accessing Voids in Metal Films for Analyses
Abstract
Featured Application
Abstract
1. Introduction
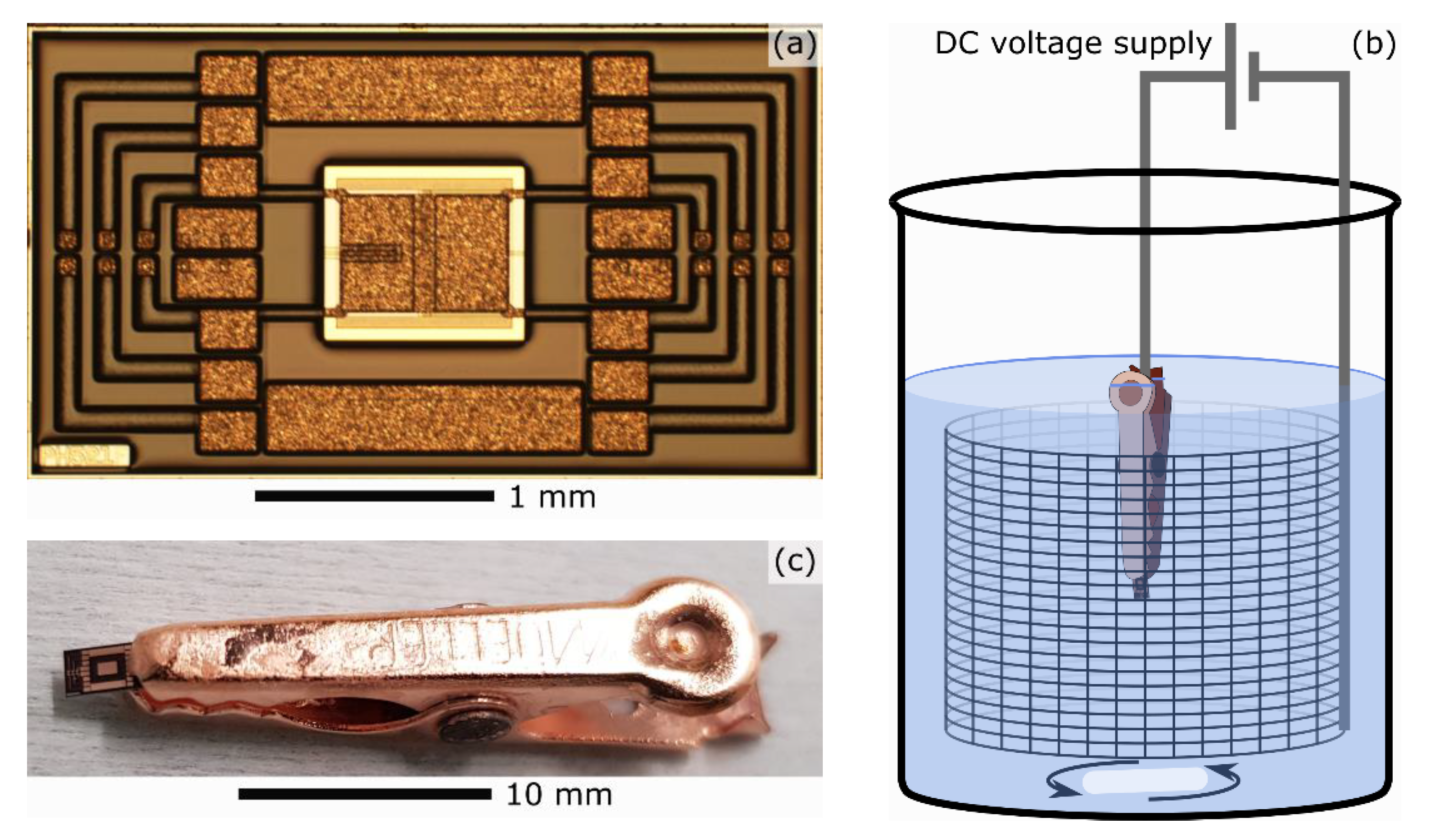
2. Device under Test
3. Electropolishing Setup
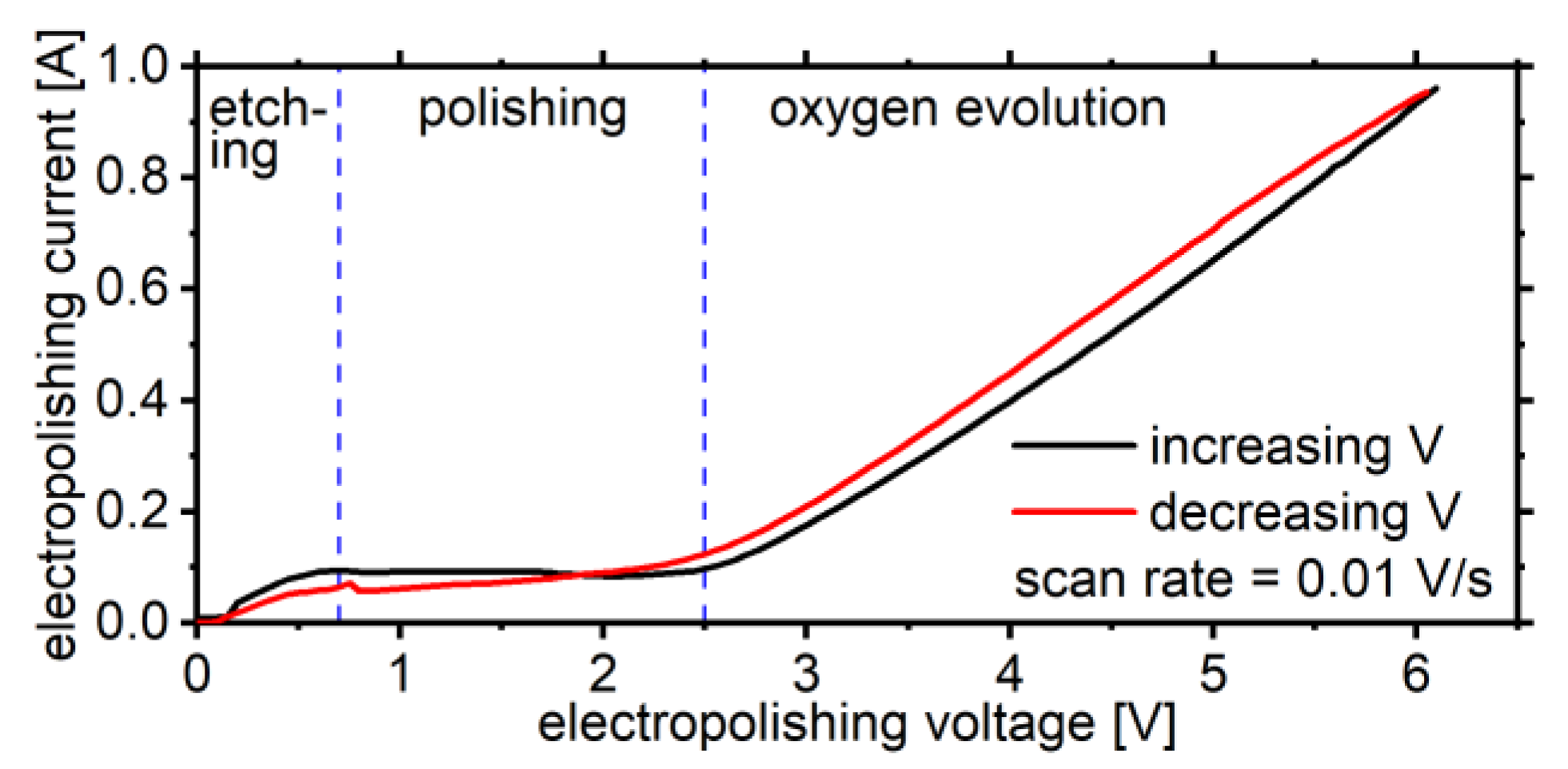
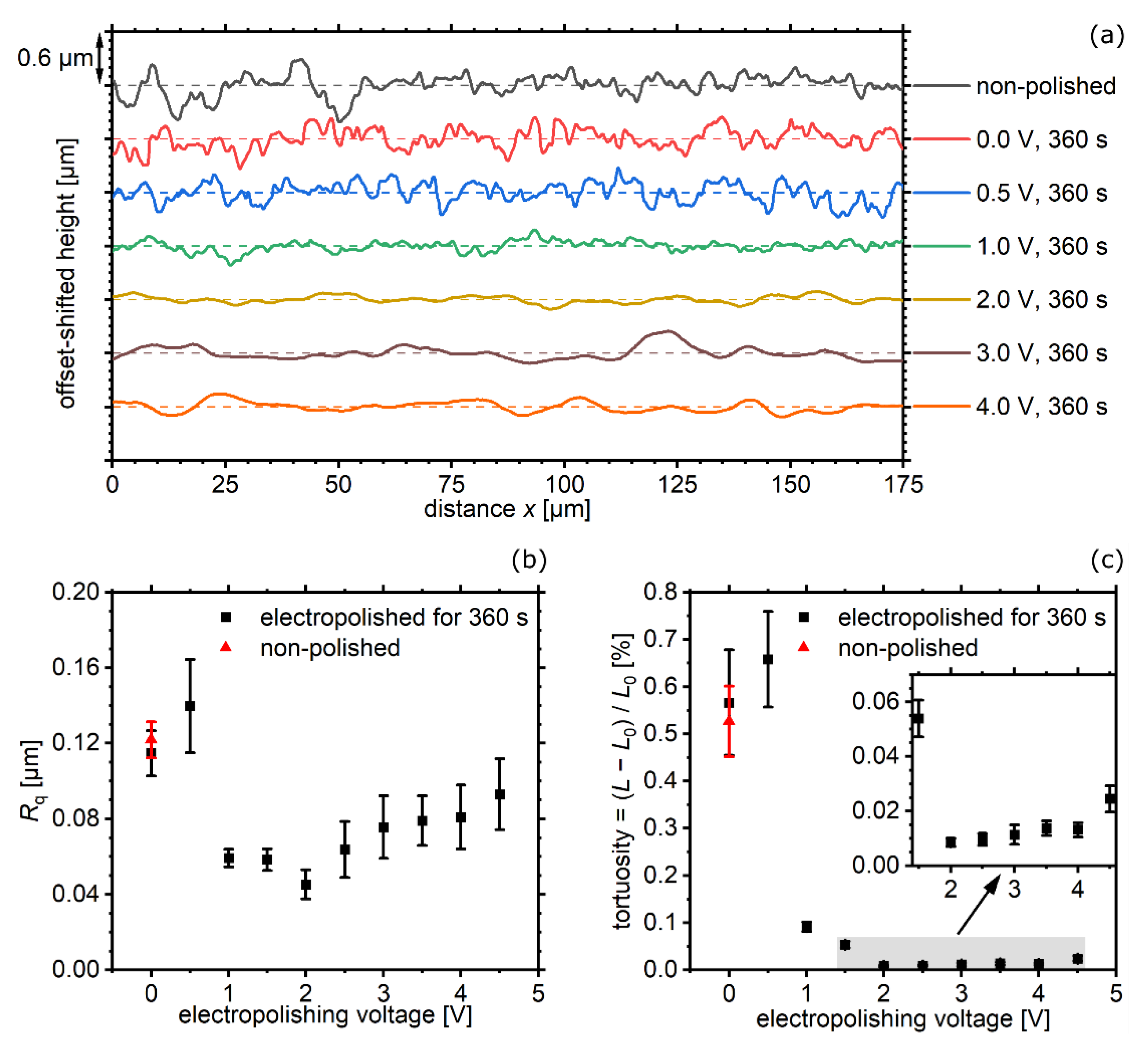
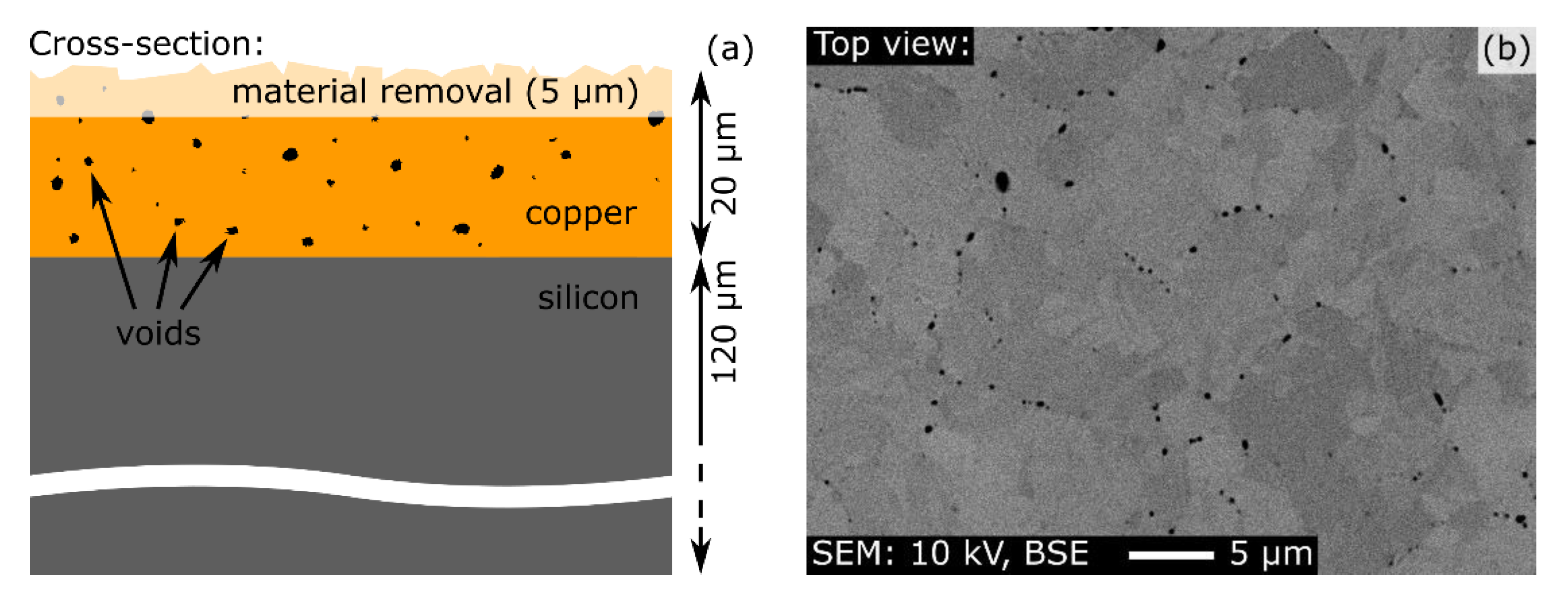
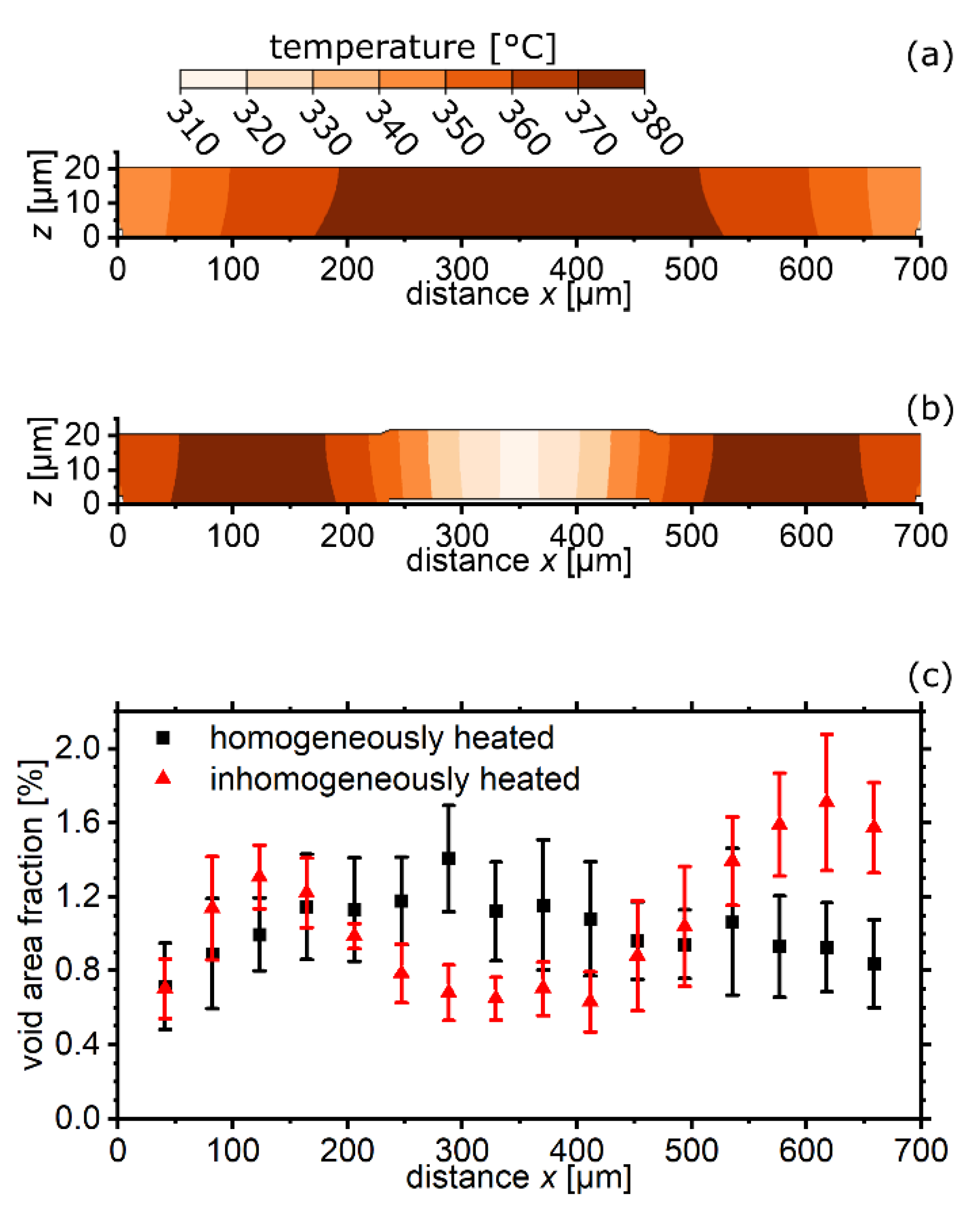
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardoen, T.; Doghri, I.; Delannay, F. Experimental and numerical comparison of void growth models and void coalescence criteria for the prediction of ductile fracture in copper bars. Acta Mater. 1998, 46, 541–552. [Google Scholar] [CrossRef]
- Dzieciol, K.; Borbély, A.; Sket, F.; Isaac, A.; Di Michiel, M.; Cloetens, P.; Buslaps, T.; Pyzalla, A.R. Void growth in copper during high-temperature power-law creep. Acta Mater. 2011, 59, 671–677. [Google Scholar] [CrossRef]
- Mayes, J.L.; Hatfield, S.L.; Gillis, P.P.; House, J.W. Void formation in OFE copper. Int. J. Impact Eng. 1993, 14, 503–508. [Google Scholar] [CrossRef]
- Kraft, O.; Wellner, P.; Hommel, M.; Schwaiger, R.; Arzt, E. Fatigue behavior of polycrystalline thin copper films. Z. Met. Kd. 2002, 93, 392–400. [Google Scholar] [CrossRef]
- Zhang, G.P.; Volkert, C.A.; Schwaiger, R.; Wellner, P.; Arzt, E.; Kraft, O. Length-scale-controlled fatigue mechanisms in thin copper films. Acta Mater. 2006, 54, 3127–3139. [Google Scholar] [CrossRef]
- Liniger, E.; Gignac, L.; Hu, C.-K.; Kaldor, S. In situ study of void growth kinetics in electroplated Cu lines. J. Appl. Phys. 2002, 92, 1803–1810. [Google Scholar] [CrossRef]
- Fisher, S.B.; White, R.J.; Miller, K.M. Quantitative analysis of void swelling in pure copper. Philos. Mag. A 1979, 40, 239–255. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Kulcinski, G.L.; Knoll, R.W. Microstructure of copper following high dose 14-MeV Cu ion irradiation. J. Nucl. Mater. 1986, 138, 46–56. [Google Scholar] [CrossRef]
- Hung, C.-C.; Lee, W.-H.; Chang, S.-C.; Wang, Y.-L.; Hwang, G.-J. Investigation of copper scratches and void defects after chemical mechanical polishing. Jpn. J. Appl. Phys. 2008, 47, 7073. [Google Scholar] [CrossRef]
- Nakahara, S. Microscopic mechanism of the hydrogen effect on the ductility of electroless copper. Acta Metall. 1988, 36, 1669–1681. [Google Scholar] [CrossRef]
- Nakahara, S.; Okinaka, Y. Microstructure and Mechanical Properties of Electroless Copper Deposits. Annu. Rev. Mater. Sci. 1991, 21, 93–129. [Google Scholar] [CrossRef]
- Garrison, W.M., Jr.; Moody, N.R. Ductile fracture. J. Phys. Chem. Solids 1987, 48, 1035–1074. [Google Scholar] [CrossRef]
- Benzerga, A.A.; Leblond, J.-B. Ductile Fracture by Void Growth to Coalescence. Adv. Appl. Mech. 2010, 44, 169–305. [Google Scholar] [CrossRef]
- Rudd, R.E.; Belak, J.F. Void nucleation and associated plasticity in dynamic fracture of polycrystalline copper: An atomistic simulation. Comput. Mater. Sci. 2002, 24, 148–153. [Google Scholar] [CrossRef]
- Barnes, R.S. The generation of vacancies in metals. Philos. Mag. 1960, 5, 635–646. [Google Scholar] [CrossRef]
- Goland, A.N. Radiation-enhanced helium precipitation in copper. Philos. Mag. 1961, 6, 189–193. [Google Scholar] [CrossRef]
- Hills, C.R. A Simple Technique for Preparing Bulk Metallographic Cross Sections. Rev. Sci. Instrum. 1971, 42, 1093. [Google Scholar] [CrossRef]
- Guo, Y.B.; Warren, A.W. Microscale mechanical behavior of the subsurface by finishing processes. J. Manuf. Sci. Eng. 2005, 127, 333–338. [Google Scholar] [CrossRef]
- Janssen, P.; Hoefnagels, J.; Keijser, T.d.; Geers, M. Processing induced size effects in plastic yielding upon miniaturisation. J. Mech. Phys. Solids 2008, 56, 2687–2706. [Google Scholar] [CrossRef]
- Brett, J.; Seigle, L. Shrinkage of voids in copper. Acta Metall. 1963, 11, 467–474. [Google Scholar] [CrossRef]
- Dow, W.-P.; Huang, H.-S. Roles of Chloride Ion in Microvia Filling by Copper Electrodeposition: I. Studies Using SEM and Optical Microscope. J. Electrochem. Soc. 2005, 152, C67. [Google Scholar] [CrossRef]
- Wu, X.; Sha, W. Experimental study of the voids in the electroless copper deposits and the direct measurement of the void fraction based on the scanning electron microscopy images. Appl. Surf. Sci. 2009, 255, 4259–4266. [Google Scholar] [CrossRef]
- Smorodin, T.; Wilde, J.; Alpern, P.; Stecher, M. Investigation and Improvement of Fast Temperature-Cycle Reliability for DMOS-Related Conductor Path Design. In Proceedings of the 2007 IEEE International Reliability Physics Symposium, 45th Annual, Phoenix, AZ, USA, 15–19 April 2007. [Google Scholar] [CrossRef]
- Nelhiebel, M.; Illing, R.; Schreiber, C.; Wöhlert, S.; Lanzerstorfer, S.; Ladurner, M.; Kadow, C.; Decker, S.; Dibra, D.; Unterwalcher, H. A reliable technology concept for active power cycling to extreme temperatures. Microelectron. Reliab. 2011, 51, 1927–1932. [Google Scholar] [CrossRef]
- Zhang, M.; Yoshihisa, Y.; Furuya, K.; Imai, Y.; Hatasako, K.; Ipposhi, T.; Maegawa, S. Reliability study of thermal cycling stress on smart power devices. Jpn. J. Appl. Phys. 2014, 53, 04EP12. [Google Scholar] [CrossRef]
- Wurster, S.; Bigl, S.; Cordill, M.J.; Kiener, D. Accelerated thermo-mechanical fatigue of copper metallizations studied by pulsed laser heating. Microelectron. Eng. 2017, 167, 110–118. [Google Scholar] [CrossRef]
- Wijaya, A.; Eichinger, B.; Rose, J.; Sartory, B.; Mischitz, M.; Brunner, R. 3D Microanalysis of Porous Copper Using FIB-Tomography in Combination with X-ray Computed Tomography. Microsc. Microanal. 2017, 23, 254–255. [Google Scholar] [CrossRef][Green Version]
- Jacquet, P.A. Electrolytic Method for obtaining Bright Copper Surfaces. Nature 1935, 135, 1076. [Google Scholar] [CrossRef]
- Jacquet, P.A. On the Anodic Behavior of Copper in Aqueous Solutions of Orthophosphoric Acid. Trans. Electrochem. Soc. 1936, 69, 629. [Google Scholar] [CrossRef]
- Kauczor, E. Elektrolytisches Polieren und Ätzen. In Metallographische Arbeitsverfahren; Kauczor, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1957; pp. 39–54. ISBN 978-3-540-02231-2. [Google Scholar]
- Buhlert, M. Elektropolieren: Elektrolytisches Glänzen, Glätten und Entgraten von Edelstahl, Stahl, Messing, Kupfer, Aluminium, Magnesium und Titan, 2. Auflage; Leuze: Bad Saulgau, Germany, 2017; ISBN 9783874802987. [Google Scholar]
- Elmore, W.C. Electrolytic Polishing. J. Appl. Phys. 1939, 10, 724. [Google Scholar] [CrossRef]
- Walton, H.F. The anode layer in the electrolytic polishing of copper. J. Electrochem. Soc. 1950, 97, 219. [Google Scholar] [CrossRef]
- Edwards, J. The Mechanism of Electropolishing of Copper in Phosphoric Acid Solutions: I. Processes Preceding the Establisment of Polishing Conditions. J. Electrochem. Soc. 1953, 100, 189C. [Google Scholar] [CrossRef]
- Edwards, J. The mechanism of electropolishing of copper in phosphoric acid solutions: II. The mechanism of smoothing. J. Electrochem. Soc. 1953, 100, 223C. [Google Scholar] [CrossRef]
- Wagner, C. Contribution to the theory of electropolishing. J. Electrochem. Soc. 1954, 101, 225. [Google Scholar] [CrossRef]
- Landolt, D. Fundamental aspects of electropolishing. Electrochim. Acta 1987, 32, 1–11. [Google Scholar] [CrossRef]
- Vidal, R.; West, A.C. Copper Electropolishing in Concentrated Phosphoric Acid: I. Experimental Findings. J. Electrochem. Soc. 1995, 142, 2682. [Google Scholar] [CrossRef]
- Vidal, R.; West, A.C. Copper Electropolishing in Concentrated Phosphoric Acid: II. Theoretical Interpretation. J. Electrochem. Soc. 1995, 142, 2689. [Google Scholar] [CrossRef]
- Yang, G.; Wang, B.; Tawfiq, K.; Wei, H.; Zhou, S.; Chen, G. Electropolishing of surfaces: Theory and applications. Surf. Eng. 2017, 33, 149–166. [Google Scholar] [CrossRef]
- Han, W.; Fang, F. Fundamental aspects and recent developments in electropolishing. Int. J. Mach. Tools Manuf. 2019, 139, 1–23. [Google Scholar] [CrossRef]
- Ramasawmy, H.; Blunt, L. 3D surface topography assessment of the effect of different electrolytes during electrochemical polishing of EDM surfaces. Int. J. Mach. Tools Manuf. 2002, 42, 567–574. [Google Scholar] [CrossRef]
- Awad, A.M.; Ghany, N.A.; Dahy, T.M. Removal of tarnishing and roughness of copper surface by electropolishing treatment. Appl. Surf. Sci. 2010, 256, 4370–4375. [Google Scholar] [CrossRef]
- Pircher, E.; Martinez, M.R.; Hansal, S.; Hansal, W. Electropolishing of copper alloys in phosphoric acid solutions with alcohols. Plat. Surf. Finish. 2003, 90, 74–79. [Google Scholar]
- Datta, M.; Landolt, D. Fundamental aspects and applications of electrochemical microfabrication. Electrochim. Acta 2000, 45, 2535–2558. [Google Scholar] [CrossRef]
- Huo, J.; Solanki, R.; McAndrew, J. Electrochemical Polishing of Copper for Microelectronic Applications. Surf. Eng. 2003, 19, 11–16. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shieh, J.-M.; Chen, C.; Dai, B.-T.; Hensen, K.; Cheng, S.-S. Two-Additive Electrolytes for Superplanarizing Damascene Cu Metals. Electrochem. Solid-State Lett. 2005, 8, C47. [Google Scholar] [CrossRef]
- Liu, S.-H.; Shieh, J.-M.; Chen, C.; Hensen, K.; Cheng, S.-S. Roles of additives in damascene copper electropolishing. J. Electrochem. Soc. 2006, 153, C428. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.; Yoo, B. Electrochemical Polishing of Cu Redistribution Layers for Fan-Out Wafer Level Packaging. J. Nanosci. Nanotechnol. 2019, 19, 6512–6515. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.-M.; Chang, S.-C.; Wang, Y.-L.; Dai, B.-T.; Cheng, S.-S.; Ting, J. Reduction of etch pits of electropolished Cu by additives. J. Electrochem. Soc. 2004, 151, C459. [Google Scholar] [CrossRef]
- Moser, S.; Zernatto, G.; Kleinbichler, M.; Nelhiebel, M.; Zechner, J.; Cordill, M.J.; Pippan, R. A Novel Setup for In Situ Monitoring of Thermomechanically Cycled Thin Film Metallizations. JOM 2019, 71, 3399–3406. [Google Scholar] [CrossRef]
- Kondo, S.; Sakuma, N.; Homma, Y.; Ohashi, N. Slurry Chemical Corrosion and Galvanic Corrosion during Copper Chemical Mechanical Polishing. Jpn. J. Appl. Phys. 2000, 39, 6216. [Google Scholar] [CrossRef]
- Wang, T.C.; Wang, Y.L.; Hsieh, T.E.; Chang, S.C.; Cheng, Y.L. Copper voids improvement for the copper dual damascene interconnection process. J. Phys. Chem. Solids 2008, 69, 566–571. [Google Scholar] [CrossRef]
- Mendez, J.; Akolkar, R.; Andryushchenko, T.; Landau, U. A Mechanistic Model for Copper Electropolishing in Phosphoric Acid. J. Electrochem. Soc. 2007, 155, D27. [Google Scholar] [CrossRef]
- Kwon, G.D.; Kim, Y.W.; Moyen, E.; Keum, D.H.; Lee, Y.H.; Baik, S.; Pribat, D. Controlled electropolishing of copper foils at elevated temperature. Appl. Surf. Sci. 2014, 307, 731–735. [Google Scholar] [CrossRef]
- Hoar, T.P.; Rothwell, G.P. The influence of solution flow on anodic polishing. Copper in aqueous o-phosphoric acid. Electrochim. Acta 1964, 9, 135–150. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Loftis, J.D. Comparison of the Electrochemical Polishing of Copper and Aluminum in Acid and Acid-free Media. ECS Trans. 2009, 25, 327. [Google Scholar] [CrossRef]
- Petzow, G. Metallographic Etching, Techniques for Metallography, Ceramography, Plastography, 2nd ed.; ASM International: Materials Park, OH, USA, 1999; ISBN 9781615032204. [Google Scholar]
- Chang, S.-C.; Shieh, J.-M.; Dai, B.-T.; Feng, M.-S.; Li, Y.-H.; Shih, C.H.; Tsai, M.H.; Shue, S.L.; Liang, R.S.; Wang, Y.-L. Superpolishing for Planarizing Copper Damascene Interconnects. Electrochem. Solid-State Lett. 2003, 6, G72. [Google Scholar] [CrossRef]
- Wimmer, A.; Heinz, W.; Leitner, A.; Detzel, T.; Robl, W.; Kirchlechner, C.; Dehm, G. Micro-tension study of miniaturized Cu lines at variable temperatures. Acta Mater. 2015, 92, 243–254. [Google Scholar] [CrossRef]
- Wimmer, A.; Heinz, W.; Detzel, T.; Robl, W.; Nellessen, M.; Kirchlechner, C.; Dehm, G. Cyclic bending experiments on free-standing Cu micron lines observed by electron backscatter diffraction. Acta Mater. 2015, 83, 460–469. [Google Scholar] [CrossRef]
- Clennell, M.B. Tortuosity: A guide through the maze. Geol. Soc. Spec. Publ. 1997, 122, 299–344. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Karpinski, T.; Lukianowicz, C. The evaluation of electrolytically polished surfaces. Wear 1977, 45, 335–343. [Google Scholar] [CrossRef]
- Hoffmann, P.; Moser, S.; Kofler, C.; Nelhiebel, M.; Tscharnuter, D.; Karunamurthy, B.; Pettermann, H.E.; Todt, M. Thermomechanical Fatigue Damage Modeling and Material Parameter Calibration for Thin Film Metallizations. Int. J. Fatigue. under review.
- Kleinbichler, M.; Kofler, C.; Stabentheiner, M.; Reisinger, M.; Moser, S.; Zechner, J.; Nelhiebel, M.; Kozeschnik, E. Void Initiation in Thermo-Mechanical Fatigue of Polycrystalline Copper Films and its Relation to Grain Structure. Microelectron. Reliab. under review.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moser, S.; Kleinbichler, M.; Kubicek, S.; Zechner, J.; Cordill, M.J. Electropolishing—A Practical Method for Accessing Voids in Metal Films for Analyses. Appl. Sci. 2021, 11, 7009. https://doi.org/10.3390/app11157009
Moser S, Kleinbichler M, Kubicek S, Zechner J, Cordill MJ. Electropolishing—A Practical Method for Accessing Voids in Metal Films for Analyses. Applied Sciences. 2021; 11(15):7009. https://doi.org/10.3390/app11157009
Chicago/Turabian StyleMoser, Sebastian, Manuel Kleinbichler, Sabine Kubicek, Johannes Zechner, and Megan J. Cordill. 2021. "Electropolishing—A Practical Method for Accessing Voids in Metal Films for Analyses" Applied Sciences 11, no. 15: 7009. https://doi.org/10.3390/app11157009
APA StyleMoser, S., Kleinbichler, M., Kubicek, S., Zechner, J., & Cordill, M. J. (2021). Electropolishing—A Practical Method for Accessing Voids in Metal Films for Analyses. Applied Sciences, 11(15), 7009. https://doi.org/10.3390/app11157009





