Changes in Elderberry (Sambucus nigra L.) Juice Concentrate Polyphenols during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipments
2.3. Elderberry Juice Concentrate Samples
2.4. Microbiological Analysis
2.5. Measurement of Total Soluble Solids
2.6. Total Phenolic Content
2.7. Total Monomeric Anthocyanins Content
2.8. Color Density, Polymeric Color, and Percent Polymeric Color
2.9. Quantification of Flavan-3-ols
2.10. Quantification of Flavonols
2.11. Determination of Antioxidant Activity
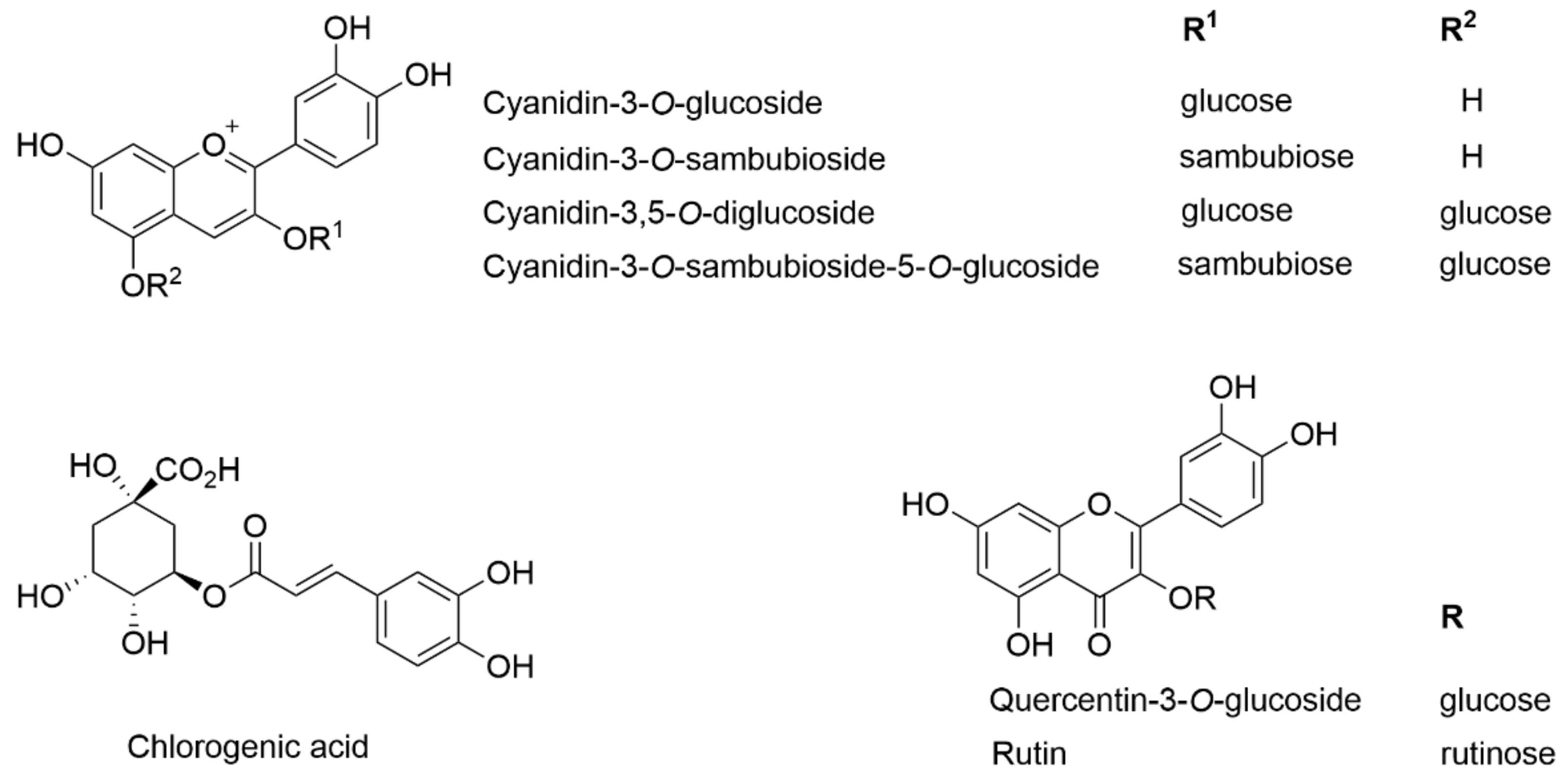
2.12. HPLC-DAD Analysis and Quantification of Polyphenols
3. Results and Discussion
3.1. Characterization of the Initial Eldeberry Concentrates
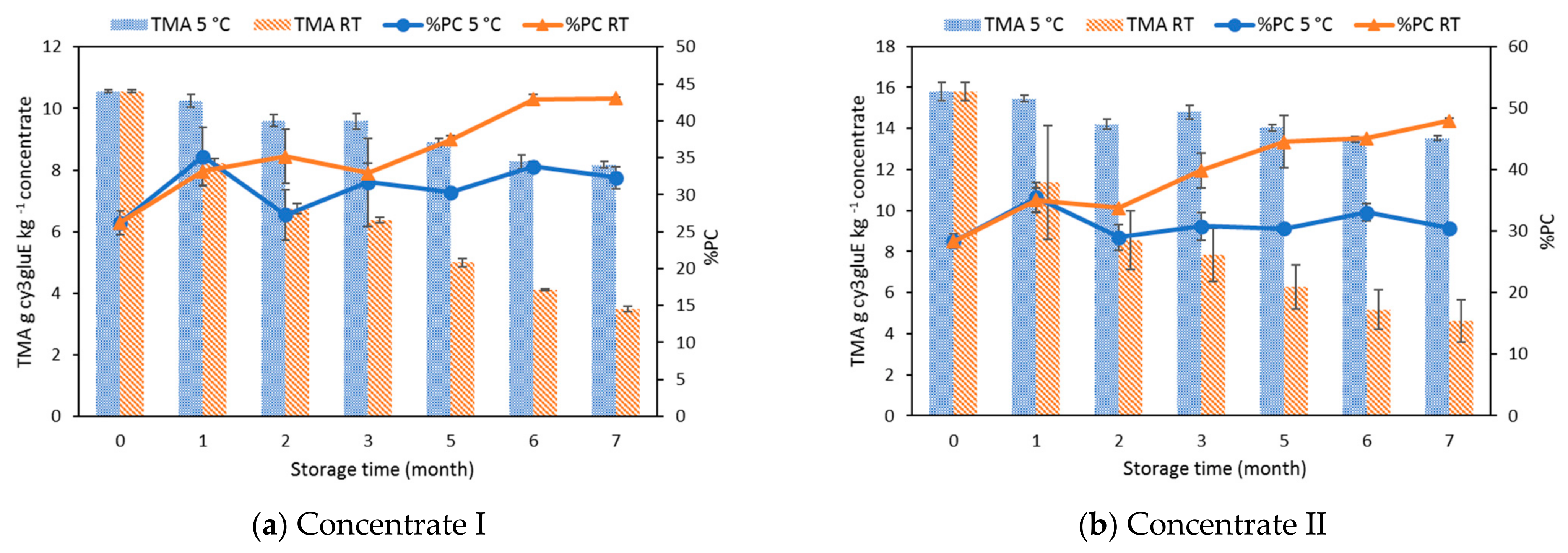
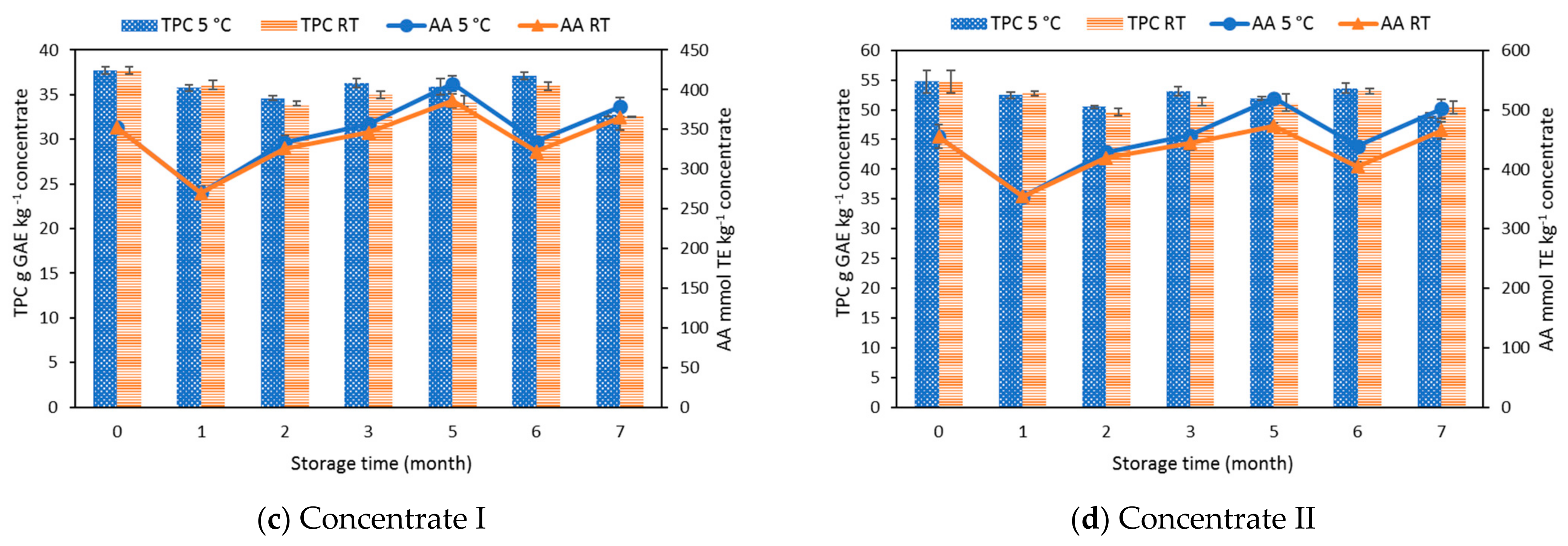
3.2. Effect of Storage on Total Phenolic Content, Total Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Activity
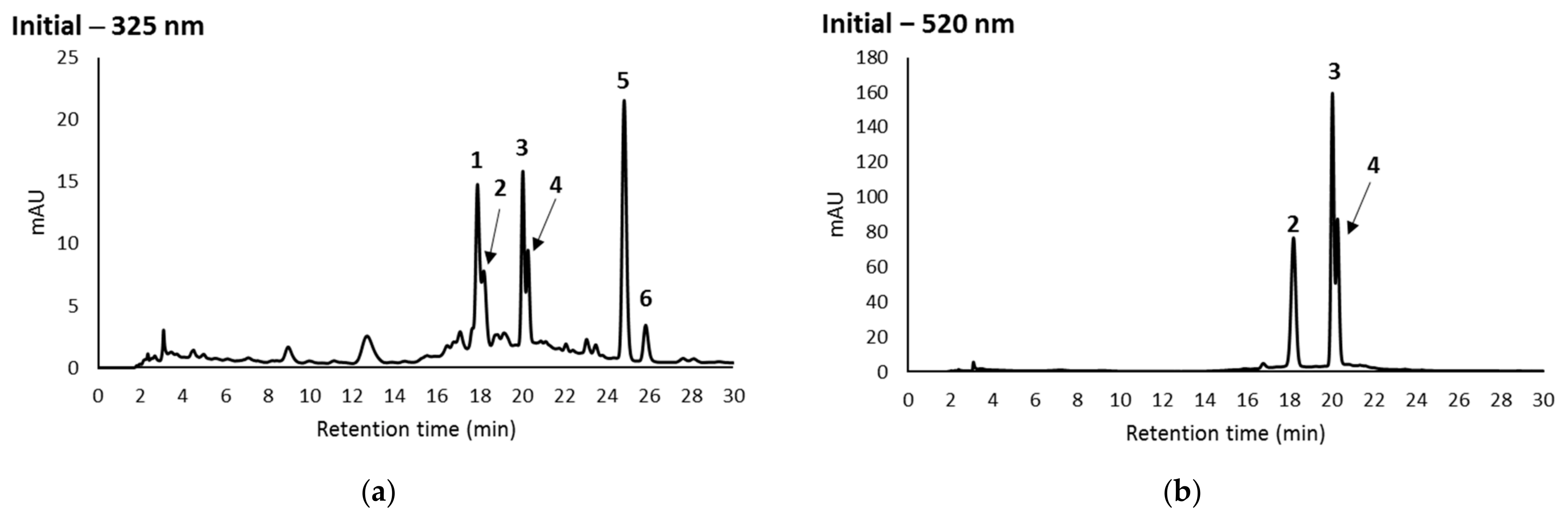
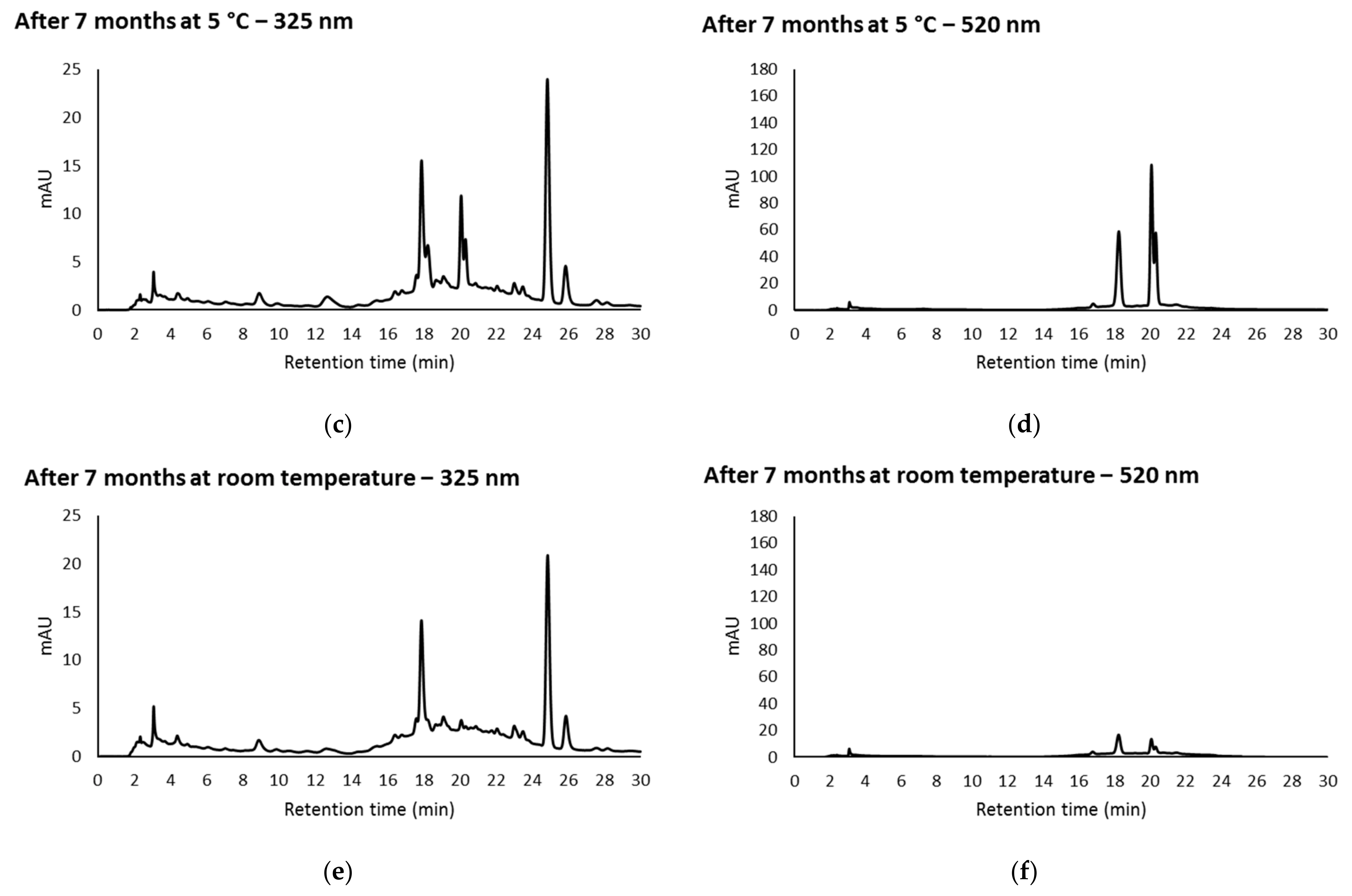
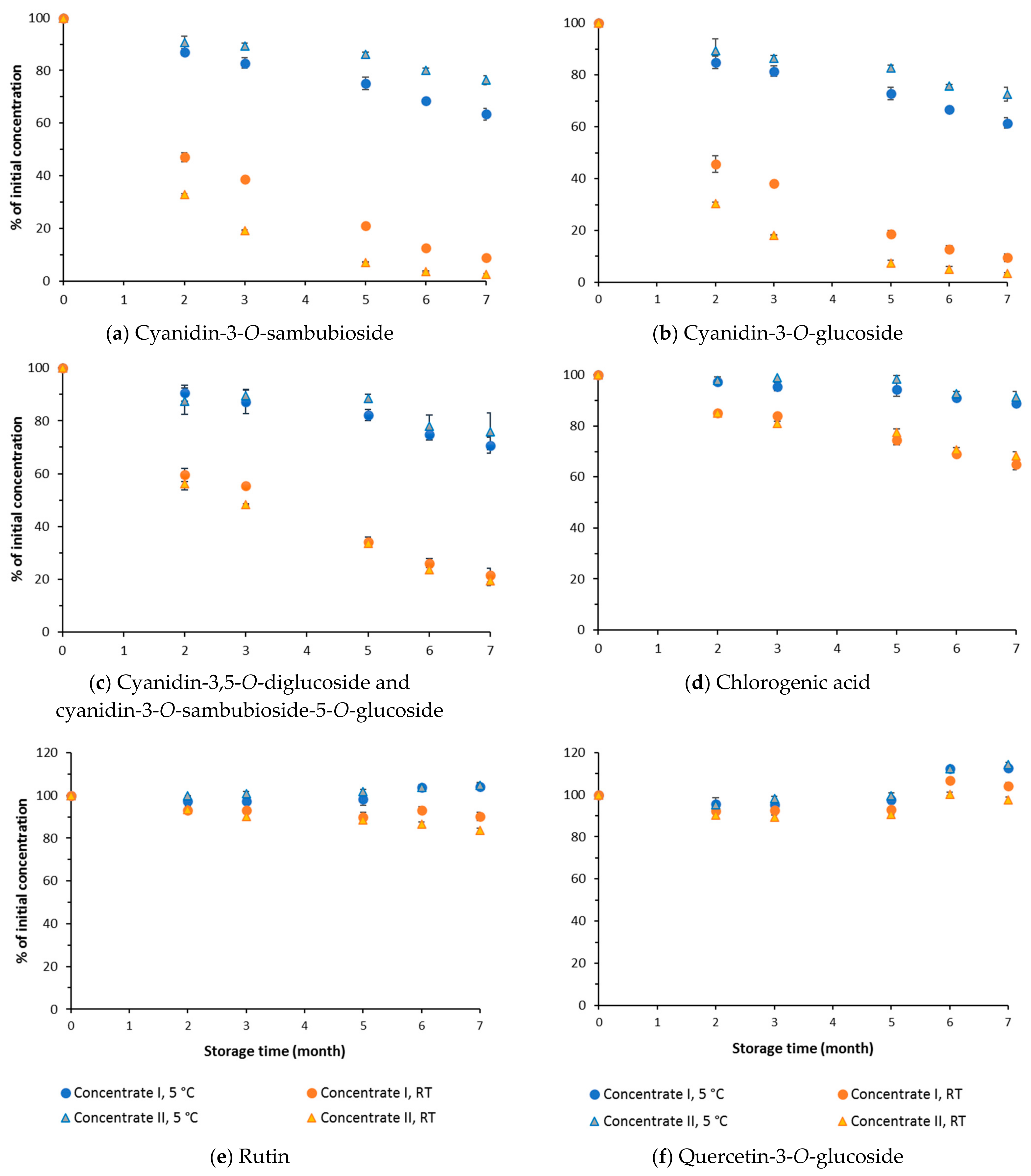
3.3. Effect of Storage on Individual Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schmitzer, V.; Veberic, R.; Stampar, F. European elderberry (Sambucus nigra L.) and American Elderberry (Sambucus canadensis L.): Botanical, chemical and health properties of flowers, berries and their products. In Berries: Properties, Consumption and Nutrition; Tuberoso, C., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2012; ISBN 9781614702573. [Google Scholar]
- Lee, J.; Finn, C.E. Anthocyanins and Other Polyphenolics in American Elderberry (Sambucus Canadensis) and European Elderberry (S. nigra) Cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of Harvesting Year and Elderberry Cultivar on the Chemical Composition and Potential Bioactivity: A Three-Year Study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced Research on the Antioxidant and Health Benefit of Elderberry (Sambucus nigra) in Food—A Review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive Properties of Sambucus nigra L. as a Functional Ingredient for Food and Pharmaceutical Industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Goiffon, J.-P.; Mouly, P.P.; Gaydou, E.M. Anthocyanic Pigment Determination in Red Fruit Juices, Concentrated Juices and Syrups Using Liquid Chromatography. Anal. Chim. Acta 1999, 382, 39–50. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A. Evaluation of Commercial Red Fruit Juice Concentrates as Ingredients for Antioxidant Functional Juices. Eur. Food Res. Technol. 2004, 219, 133–141. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of Anthocyanins in Elderberry and Chokeberry Dietary Supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Kaack, K.; Fretté, X.C.; Christensen, L.P.; Landbo, A.-K.; Meyer, A.S. Selection of Elderberry (Sambucus nigra L.) Genotypes Best Suited for the Preparation of Juice. Eur. Food Res. Technol. 2008, 226, 843–855. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Processed Elderberry (Sambucus nigra L.) Products: A Beneficial or Harmful Food Alternative? LWT Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of Thermal Processing on Anthocyanin Stability in Foods; Mechanisms and Kinetics of Degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Buvé, C.; Kebede, B.T.; de Batselier, C.; Carrillo, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; van Loey, A. Kinetics of Colour Changes in Pasteurised Strawberry Juice during Storage. J. Food Eng. 2018, 216, 42–51. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Duxford, UK, 2018; pp. 295–323. [Google Scholar]
- Jackman, R.L.; Yada, R.Y.; Tung, M.A.; Speers, R.A. Anthocyanins as Food Colorants—A Review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of Anthocyanins and Polymeric Color Formation during Heat Treatment of Purple Sweet Potato Extract at Different PH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef]
- Howard, L.R.; Prior, R.L.; Liyanage, R.; Lay, J.O. Processing and Storage Effect on Berry Polyphenols: Challenges and Implications for Bioactive Properties. J. Agric. Food Chem. 2012, 60, 6678–6693. [Google Scholar] [CrossRef]
- Gonçalves, F.J.; Rocha, S.M.; Coimbra, M.A. Study of the Retention Capacity of Anthocyanins by Wine Polymeric Material. Food Chem. 2012, 134, 957–963. [Google Scholar] [CrossRef]
- Mónica Giusti, M.; Wrolstad, R.E. Anthocyanins. In Handbook of Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 2, pp. 5–69. ISBN 9780471709084. [Google Scholar]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2‘-Diphenyl-1-Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Kader, F.; Rovel, B.; Girardin, M.; Metche, M. Mechanism of Browning in Fresh Highbush Blueberry Fruit (Vaccinium corymbosum L.) Role of Blueberry Polyphenol Oxidase, Chlorogenic Acid and Anthocyanins. J. Sci. Food Agric. 1997, 74, 31–34. [Google Scholar] [CrossRef]
- Kader, F.; Irmouli, M.; Nicolas, J.P.; Metche, M. Involvement of Blueberry Peroxidase in the Mechanisms of Anthocyanin Degradation in Blueberry Juice. J. Food Sci. 2002, 67, 910–915. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamamoto, A.; Nagai, S.; Terabe, S. Analysis of Elderberry Pigments in Commercial Food Samples by Micellar Electrokinetic Chromatography. Anal. Sci. 1998, 14, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Buckow, R.; Kastell, A.; Terefe, N.S.; Versteeg, C. Pressure and Temperature Effects on Degradation Kinetics and Storage Stability of Total Anthocyanins in Blueberry Juice. J. Agric. Food Chem. 2010, 58, 10076–10084. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blueberry Products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef]
- Hager, A.; Howard, L.R.; Prior, R.L.; Brownmiller, C. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Black Raspberry Products. J. Food Sci. 2008, 73, H134–H140. [Google Scholar] [CrossRef]
- Casati, C.B.; Baeza, R.; Sanchez, V.; Catalano, A.; López, P.; Zamora, M.C. Thermal Degradation Kinetics of Monomeric Anthocyanins, Colour Changes and Storage Effect in Elderberry Juices. J. Berry Res. 2015, 5, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.; Guyot, S.; Marnet, N.; Delgadillo, I.; Renard, C.M.G.C.; Coimbra, M.A. Composition of Phenolic Compounds in a Portuguese Pear (Pyrus communis L. Var. S. bartolomeu) and Changes after Sun-Drying. J. Agric. Food Chem. 2002, 50, 4537–4544. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Huang, H.-P.; Huang, T.-C. Relationship between Anthocyanin Patterns and Antioxidant Capacity in Mulberry Wine during Storage. J. Food Qual. 2004, 27, 497–505. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–Activity Relationships of Anthocyanidin Glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Martí, N.; Pérez-Vicente, A.; García-Viguera, C. Influence of Storage Temperature and Ascorbic Acid Addition on Pomegranate Juice. J. Sci. Food Agric. 2002, 82, 217–221. [Google Scholar] [CrossRef]
- Brauch, J.E.; Kroner, M.; Schweiggert, R.M.; Carle, R. Studies into the Stability of 3-O-Glycosylated and 3,5-O-Diglycosylated Anthocyanins in Differently Purified Liquid and Dried Maqui (Aristotelia Chilensis (Mol.) Stuntz) Preparations during Storage and Thermal Treatment. J. Agric. Food Chem. 2015, 63, 8705–8714. [Google Scholar] [CrossRef] [PubMed]
- Timberlake, C.F.; Bridle, P. Anthocyanins: Colour Augmentation with Catechin and Acetaldehyde. J. Sci. Food Agric. 1977, 28, 539–544. [Google Scholar] [CrossRef]
- Kırca, A.; Özkan, M.; Cemeroğlu, B. Effects of Temperature, Solid Content and PH on the Stability of Black Carrot Anthocyanins. Food Chem. 2007, 101, 212–218. [Google Scholar] [CrossRef]
| Concentrate I | Concentrate II | |
|---|---|---|
| Thermal processing step | Heating of mash at 80 °C | No |
| Total soluble solids (°Brix) | 55.8 ± 0.8 | 80.6 ± 1.0 |
| Total phenolic content (g GAE kg−1) | 37.7 ± 0.4 | 54.7 ± 1.9 |
| Total monomeric anthocyanins (g Cy3gluE kg−1) | 10.6 ± 0.1 | 15.8 ± 0.4 |
| Flavonols (g QE kg−1) | 3.1 ± 0.04 | 4.8 ± 0.2 |
| Flavan-3-ols (g EpiE kg−1) | 1.0 ± 0.6 | 1.5 ± 0.3 |
| Polymeric color (%) | 26.2 ± 1.6 | 28.3 ± 1.2 |
| Antioxidant activity (mmol TE kg−1) | 352.3 ± 2.6 | 455.0 ± 19.4 |
| Compounds | Concentrate I | Concentrate II |
|---|---|---|
| Cyanidin-3-O-sambubioside | 3.30 ± 0.06 | 4.69 ± 0.04 |
| Cyanidin-3-O-glucoside | 1.99 ± 0.07 | 2.86 ± 0.02 |
| Cyanidin-3,5-diglucoside + cyanidin-3-O-sambubioside-5-O-glucoside | 4.39 ± 0.15 | 6.49 ± 0.08 |
| Rutin | 2.51 ± 0.04 | 3.70 ± 0.04 |
| Quercentin-3-O-glucoside | 0.79 ± 0.01 | 0.96 ± 0.01 |
| Chlorogenic acid | 0.78 ± 0.02 | 1.11 ± 0.01 |
| Sample | Mesophiles at 30 °C (CFU mL−1) | Fungi (CFU mL−1) |
|---|---|---|
| Concentrate I before storage | ˂1 | ˂1 |
| Concentrate I stored at 5 °C | ˂1 | ˂1 |
| Concentrate I stored at room temperature | 2.1 × 102 | ˂1 |
| Concentrate II before storage | ˂1 | ˂1 |
| Concentrate II stored at 5 °C | 2.2 × 10 | 1.0 × 10 |
| Concentrate II stored at room temperature | 1.1 × 103 | 1.1 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, C.M.B.; Pinto, A.; Gonçalves, F.; Wessel, D.F. Changes in Elderberry (Sambucus nigra L.) Juice Concentrate Polyphenols during Storage. Appl. Sci. 2021, 11, 6941. https://doi.org/10.3390/app11156941
Neves CMB, Pinto A, Gonçalves F, Wessel DF. Changes in Elderberry (Sambucus nigra L.) Juice Concentrate Polyphenols during Storage. Applied Sciences. 2021; 11(15):6941. https://doi.org/10.3390/app11156941
Chicago/Turabian StyleNeves, Cláudia M. B., António Pinto, Fernando Gonçalves, and Dulcineia F. Wessel. 2021. "Changes in Elderberry (Sambucus nigra L.) Juice Concentrate Polyphenols during Storage" Applied Sciences 11, no. 15: 6941. https://doi.org/10.3390/app11156941






