Protective Treatments against Endothelial Glycocalyx Degradation in Surgery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
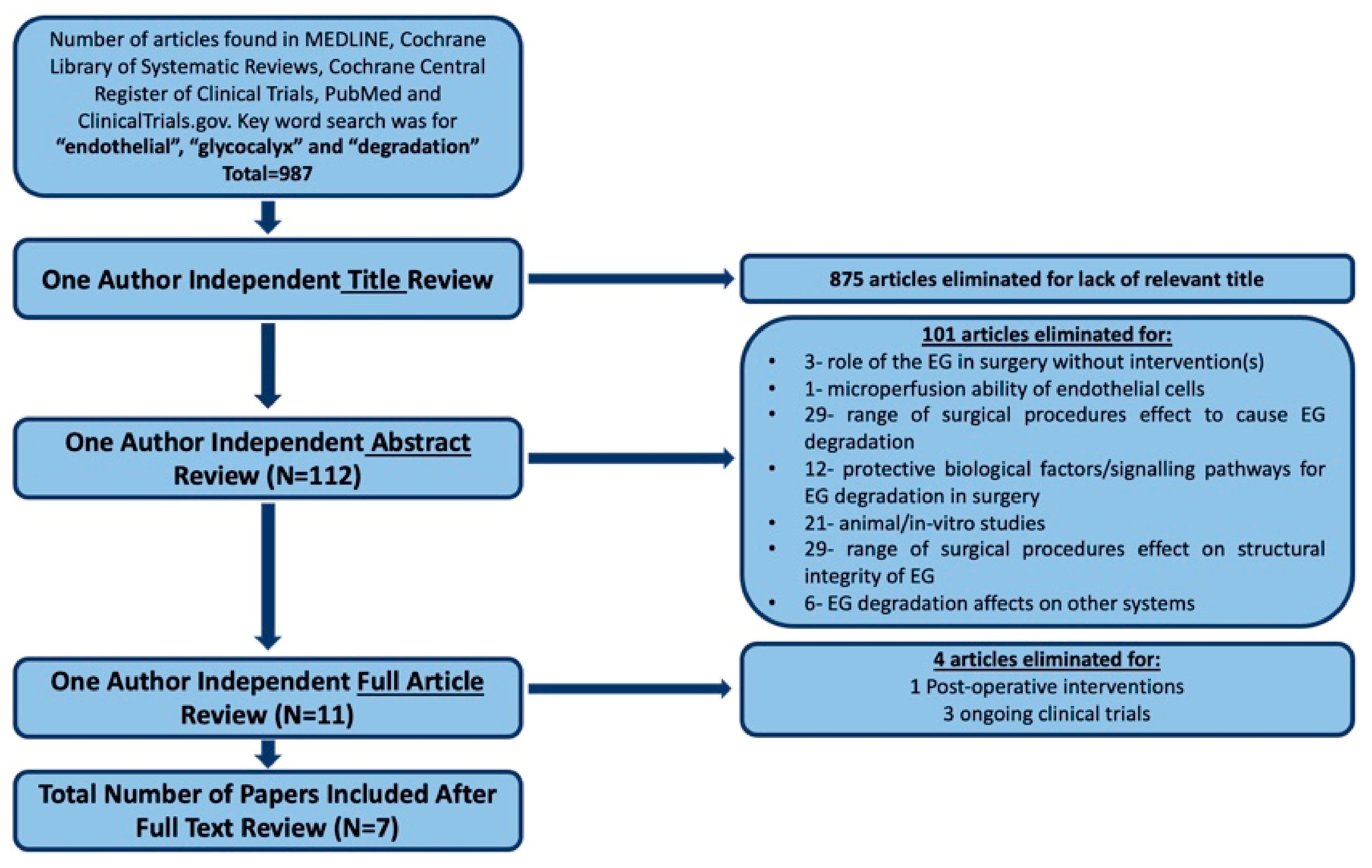
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Statistical Analyses
3. Results
3.1. Description of Included Studies
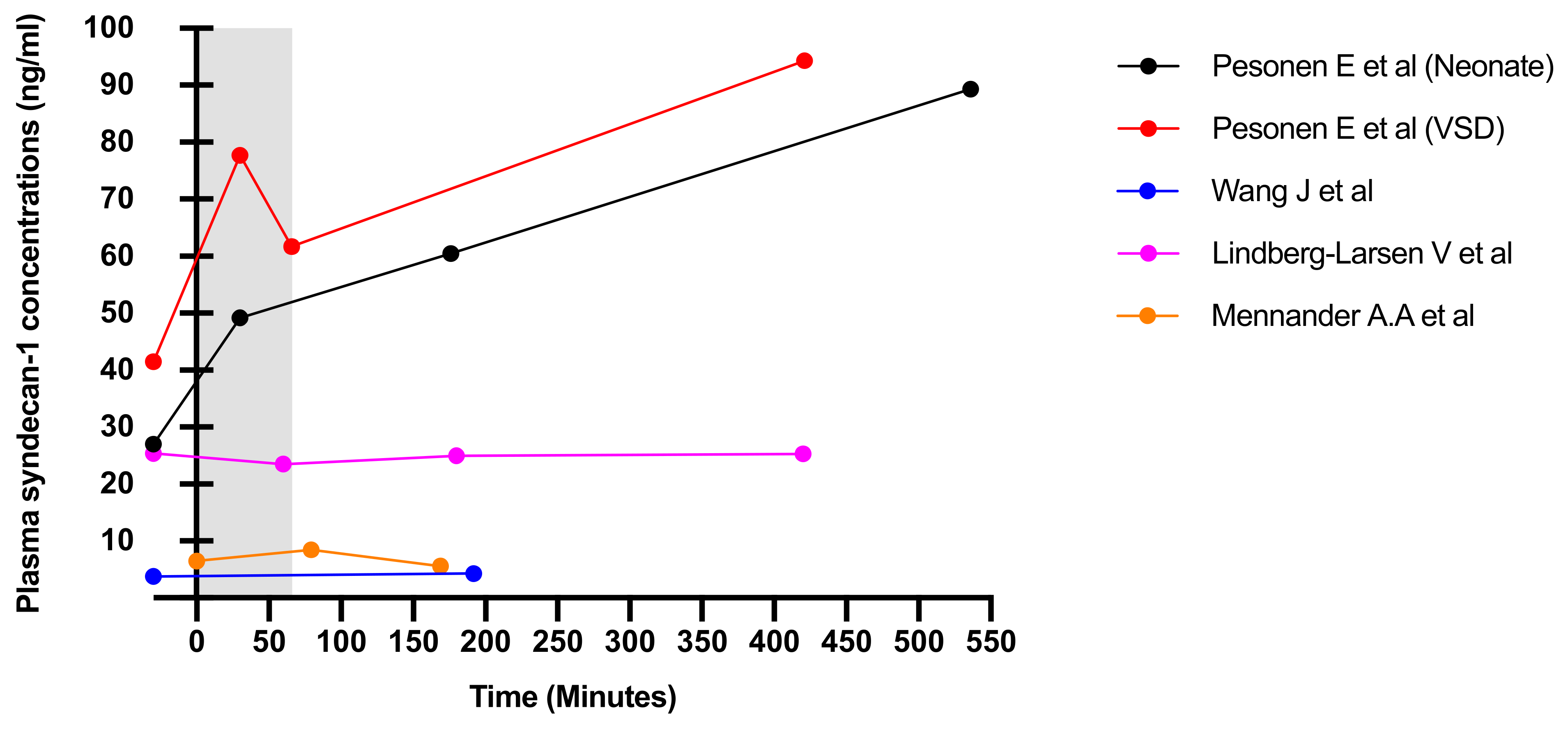
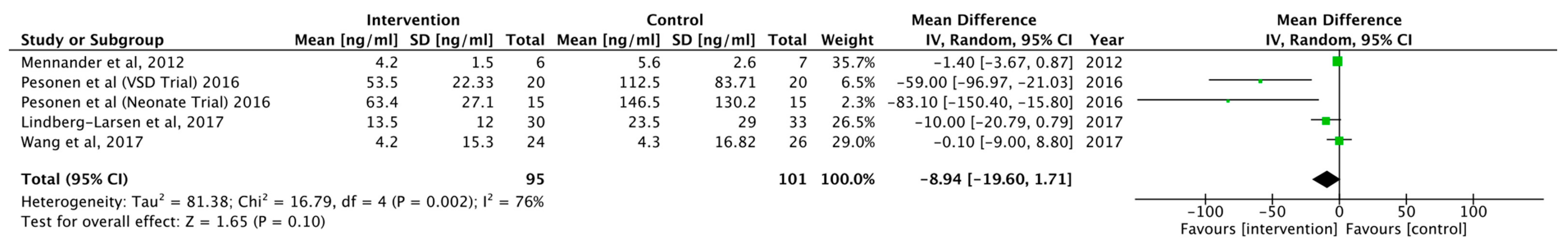
3.2. Outcomes and Results
4. Discussion
- Study design: trials should be a placebo-controlled, double-blind, and a parallel design, with treatment administered pre-operatively or following the induction of anaesthesia but before the start of the surgical procedure. Time-points should be assessed at all stages of the surgical operation (pre-operative, intra-operative and post-operative) to assess trends in which the treatments affect the production of glycocalyx products.
- Patient population: RCTs should evaluate the work of Johansson study [12] to assess EG degradation in patients with greater operative stress. Mean age, concomitant medication, incidence and common co-morbidities should be collected and reported for each treatment group.
- Clinical outcomes: syndecan-1 and heparan sulphate should be used as primary markers to measure the degree of EG degradation. In addition, inflammatory markers (CRP, white blood cells) as well as capillary leakage of albumin (HGB and albumin) should be measured and correlated to identify relationships with glycocalyx shedding. Future work should use either Hedin and Hahn [42] or Hasselgren [43] technique to measure capillary leakage.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beyer, A.M.; Gutterman, D.D. Regulation of the human coronary microcirculation. J. Mol. Cell. Cardiol. 2012, 52, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Yau, J.W.; Teoh, H.T.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovas. Disord. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The endothelial glycocalyx: Composition, functions, and visualization. Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Gilepsie, G.P.; Muller, U. Mechanotransduction by hair Cells: Models, molecules, and mechanisms. Cell 2009, 139, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Squire, J.M.; Chew, M.; Nneji, G.; Barry, J.; Michel, C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: A possible explanation for molecular filtering. J. Struct. Biol. 2001, 136, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Tarbell, J.M. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Robich, M.; Ryzhov, S.; Kacer, D.; Palmeri, M.; Peterson, S.M.; Quinn, R.D.; Carter, D.; Shephard, F.; Hayes, T.; Sawyer, D.B.; et al. Prolonged cardiopulmonary bypass is associated with endothelial glycocalyx degradation. J. Surg. Res. 2020, 251, 287–295. [Google Scholar] [CrossRef]
- Chappell, D.; Hofmann-Kiefer, K.; Jacob, M.; Rehm, M.; Briegel, J.; Welsch, U.; Conzen, P.; Becker, B.F. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res. Cardiol. 2009, 104, 78–89. [Google Scholar] [CrossRef]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantafyllou, C.; Nikolao, M.; Ikonomidis, I.; Bamias, G.; Kouretas, D.; Andreadou, I.; Tsoumani, M.; Thymis, J.; Papaconstantinou, I. Effects of anti-inflammatory treatment and surgical intervention on endothelial glycocalyx, peripheral and coronary microcirculatory function and myocardial deformation in inflammatory bowel disease patients: A two-arms two-stage clinical trial. Diagnostics 2021, 11, 993. [Google Scholar] [CrossRef]
- Johansson, P.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef]
- Yilmaz, O.; Afsar, B.; Ortiz, A.; Kanbay, M. The role of endothelial glycocalyx in health and disease. Clin. Kidney J. 2019, 12, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, E.; Cardenas, J.C.; Baimukanova, G.; Usadi, B.; Bruhn, R.; Pati, S.; Ostrowski, S.R.; Johansson, P.I.; Holcomb, J.B.; Wade, C.E. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J. Transl. Med. 2015, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, S.; Yun, J.; Adamson, G.; Galvez, J.; Jialal, I. C-reactive protein impairs the endothelial glycocalyx resulting in endothelial dysfunction. Cardiovas. Res. 2009, 84, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappell, D.; Bruegger, D.; Potzel, J.; Jacob, M.; Brettner, F.; Vogeser, M.; Conzen, P.; Becker, B.F.; Rehm, M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit. Care 2014, 18, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, M.; Bruegger, D.; Christ, F.; Conzen, P.; Thiel, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Reichart, B.; et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007, 116, 1896–1906. [Google Scholar] [CrossRef] [Green Version]
- Gybel-Brask, M.; Rasmussen, R.; Stensballe, J.; Johansson, P.A.; Ostrowski, S.R. Effect of delayed onset prostacyclin on markers of endothelial function and damage after subarachnoid haemorrhage. Acta. Neurochir. 2017, 159, 1073–1078. [Google Scholar] [CrossRef]
- Halenarova, K. Effects of corticosteroids on endothelial dysfunction in cardiac surgery patients. Crit. Care Med. 2019, 47, 94. [Google Scholar] [CrossRef]
- Tosif, S. Preoperative microvascular protection in patients undergoing major abdominal surgery. Unpublished work. 2018. [Google Scholar]
- Yonsei University. The effect of balanced crystalloid versus 5% albumin on endothelial glycocalyx degradation in patients undergoing off-pump coronary artery bypass surgery. Unpublished work. 2019. [Google Scholar]
- Brettner, F.; Chappell, D.; Nebelsiek, T.; Hauera, D.; Schellinga, G.; Becker, B.F.; Rehm, M.; Weis, F. Preinterventional hydrocortisone sustains the endothelial glycocalyx in cardiac surgery. Clin. Hemorheol. Microcirc. 2019, 71, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Nam, K.; Cho, Y.J.; Min, J.J.; Hong, Y.J.; Park, K.U.; Hong, D.M.; Jeon, Y. Microvascular reactivity and endothelial glycocalyx degradation when administering hydroxyethyl starch or crystalloid during off-pump coronary artery bypass graft surgery: A randomised trial. Anaesthesia 2017, 72, 204–213. [Google Scholar] [CrossRef]
- Lindberg-Larsen, V.; Ostrowski, S.R.; Lindberg-Larsen, M.; Rovsing, M.L.; Johansson, P.I.; Kehlet, H. The effect of pre-operative methylprednisolone on early endothelial damage after total knee arthroplasty: A randomised, double-blind, placebo-controlled trial. Anaesthesia 2017, 72, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
- Mennander, A.A.; Shalaby, A.; Oksala, N.; Leppanen, T.; Hamalainen, M.; Huovinen, S.; Zhao, F.; Moilanen, E.; Tarkka, M. Diazoxide may protect endothelial glycocalyx integrity during coronary artery bypass grafting. Scand. Cardiovasc. J. 2012, 46, 339–344. [Google Scholar] [CrossRef]
- Nemme, J.; Krizhanovskii, C.; Ntika, S.; Sabelnikovs, O.; Vanags, I.; Hahn, R.G. Hypervolemia does not cause degradation of the endothelial glycocalyx layer during open hysterectomy performed under sevoflurane or propofol anesthesia. Acta. Anaesthesiol. Scand. 2019, 64, 538–545. [Google Scholar] [CrossRef]
- Pesonen, E.; Keski-Nisula, J.; Andersson, S.; Palo, R.; Salminen, J.; Suominen, P.K. High-dose methylprednisolone and endothelial glycocalyx in paediatric heart surgery. Acta. Anaesthesiol. Scand. 2016, 60, 1386–1394. [Google Scholar] [CrossRef]
- Wang, J.; Wu, A.; Wu, Y. Endothelial Glycocalyx Layer: A Possible Therapeutic Target for Acute Lung Injury during Lung Resection. BioMed Res. Int. 2017, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bruegger, D.; Rehm, M.; Abicht, J.; Paul, J.O.; Stoeckelhuber, M.; Pfirrmann, M.; Reichart, B.; Becker, B.F.; Christ, F. Shedding of the endothelial glycocalyx during cardiac surgery: On-pump versus off-pump coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2009, 138, 1445–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, B.A.; Oragui, E.E.; Dung, N.M.; Loan, H.T.; Chau, N.V.; Farrar, J.J.; Levin, M. Size and charge characteristics of the protein leak in dengue shock syndrome. J. Infect. Dis. 2004, 190, 810–818. [Google Scholar] [CrossRef]
- Mulivor, A.W.; Lipowsky, H.H. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, 1282–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keski-Nisula, J.; Pesonen, E.; Olkkola, K.T.; Peltola, K.; Neuvonen, P.J.; Tuominen, N.; Sairanen, H.; Andersson, S.; Suominen, P.K. Methylprednisolone in neonatal cardiac surgery: Reduced inflammation without improved clinical outcome. Ann. Thorac. Surg. 2013, 95, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Bruegger, D.; Rehm, M.; Conzen, P.; Welsch, U.; Becker, B.F. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 2007, 107, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Chappell, D.; Dorfler, N.; Jacob, M.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010, 34, 133–139. [Google Scholar] [CrossRef]
- Kilger, E.; Weis, F.; Briegel, J.; Frey, L.; Goetz, A.E.; Reuter, D.; Nagy, A.; Schuetz, A.; Lamm, P.; Knoll, A.; et al. Stress doses of hydrocortisone reduce severe systemic inflammatory response syndrome and improve early outcome in a risk group of patients after cardiac surgery. Crit. Care Med. 2003, 31, 1068–1074. [Google Scholar] [CrossRef]
- Checchia, P.A.; Bronicki, R.A.; Costello, J.M.; Costello, J.M.; Nelson, D.P. Steroid use before pediatric cardiac operations using cardiopulmonary bypass: An international survey of 36 centers. Pediatr. Crit. Care Med. 2005, 6, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Norberg, A.; Hahn, R.G.; Li, H.; Olsson, J.; Prough, D.S.; Børsheim, E.; Wolf, S.; Minton, R.K.; Svensén, C.H. Population volume kinetics predicts retention of 0.9% saline infused in awake and isoflurane-anesthetized volunteers. Anesthesiology 2007, 107, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Bruegger, D.; Rehm, M.; Welsch, U.; Conzen, P. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology 2006, 104, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2013, 111, 1805–1812. [Google Scholar] [CrossRef]
- Rehm, M.; Haller, M.; Orth, V.; Kreimeier, U.; Jacob, M.; Dressel, H.; Mayer, S.; Brechtelsbauer, H.; Finsterer, U. Changes in blood volume and hematocrit during acute perioperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology 2001, 95, 849–856. [Google Scholar] [CrossRef]
- Hahn, R.G.; Hasselgren, E.; Håkan, B.; Markus, Z.; Zdolsek, J. Biomarkers of endothelial injury in plasma are dependent on kidney function. Clin. Hemorheol. Microcirc. 2019, 72, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Hedin, A.; Hahn, R.G. Volume expansion and plasma protein clearance during intravenous infusion of 5% albumin and autologous plasma. Clin. Sci. 2005, 106, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, E.; Zdolsek, M.; Zdolsek, J.; Björne, H.; Krizhanovskii, C.; Stelia, N.; Hahn, R.G. Long intravascular persistence of albumin 20% in postoperative patients. Anesth. Analg. 2019, 129, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
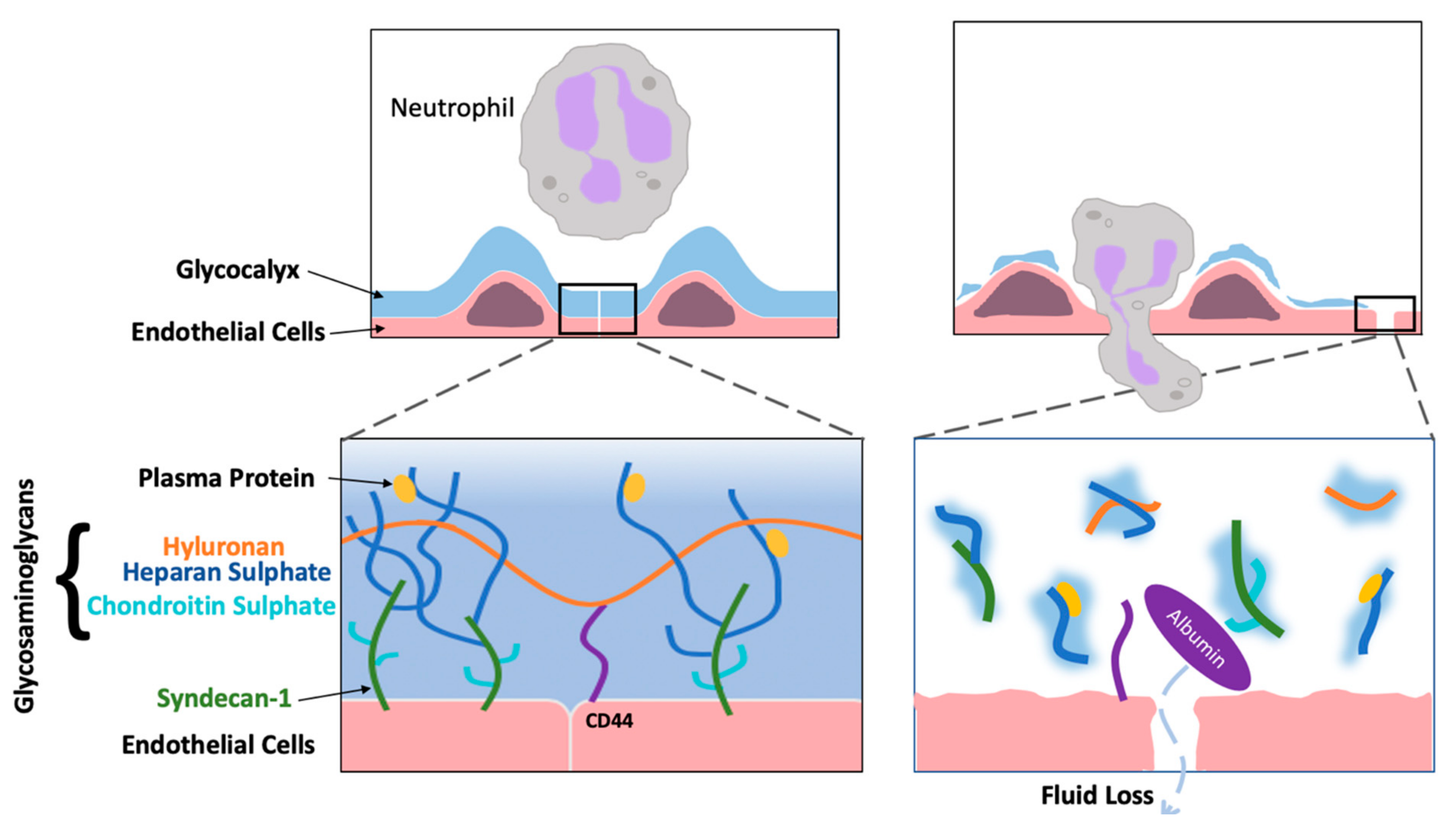
| Reference | Sample Size | Study | Controlled | Comparative | Patients Age | Syndecan-1 Marker | Surgery | Anaesthesia | Treatment (Drug Class) |
|---|---|---|---|---|---|---|---|---|---|
| Pesonen et al. (2016) [Neonates] Pesonen et al. (2016) [VSD] | 40 45 | Prospective | No | Yes | 7 (1–27) 0.37 (0.15–1.36) | Yes Yes | CPB | Sufentanil, Pancuronium, S-ketamine Maintained-> Sevoflurane | Methylprednisolone (Corticosteroid) |
| Brettner et al. (2019) | 30 | Prospective | No | No | 65 (57.3–74) | Yes | CPB | Midazolam, Sufentanil, Pancuronium Maintained-> Sufentanil | Hydrocortisone (Corticosteroid) |
| Wang et al. (2017) | 50 | Prospective | No | Yes | 58.56 | Yes | VATS Lobectomy | Midazolam, Propofol, Sufentanil-> Propofol, Remifentanil, Rocuronium | Ulinastatin (UTI, anti-inflammatory agent) |
| Lindberg-Larsen et al. (2017) | 63 | Prospective | No | Yes | 63.27 | Yes | Unilateral Total Knee Arthroplasty | Standard procedure | Methylprednisolone (Corticosteroid) |
| Nemme et al. (2019) | 24 | Prospective | No | No | 47(5) 46(4) | Yes | Hysterectomy | Midazolam, Fentanyl, Propofol | Ringers Lactate (Fluid Therapy) |
| Mennander et al. (2012) | 13 | Prospective | No | Yes | Not stated | Yes | CPB | Propofol, Sufentanil, cis-atracurium Maintained-> Sevoflurane | Diazoxide (Thiazide) |
| Kim et al. (2017) | 120 | Prospective | No | No | 67.8 (9.9) 65.3 (10.5) | Yes | CPB | Midazolam, Sufentanil, Vecuronium Miantained0> Remifentanil, Propofol | Hydroxyl Starch (Crystalloid Starch Fluid Therapy) |
| Reference | Timepoints | Syndecan-1 Levels | Timepoints of Statistical Significance | Heparan Sulphate | Inflammatory Markers | HGB and Albumin | Hyluronan |
|---|---|---|---|---|---|---|---|
| Pesonen et al. (2016) [Neonates] Pesonen et al. (2016) [VSD] | T1: induction of anaesthesia T2: 30-min on CPB T3: weaning of CPB T4: 6-h post-operative | Significant lowering in intervention group to none in control group, when comparing to baseline values None | T1->T2 N/A | N/A N/A | N/A N/A | N/A N/A | N/A N/A |
| Brettner et al. (2019) | T0: preoperative T1: induction of anaesthesia T2: 30 min after onset of CPB T3: weaning of CPB T4: 1-h post-operative T5: 4-h post-operative | None | N/A | T2–3 | Higher CRP post-operatively on Days 1,2,3 in control to intervention group, when comparing to baseline values Higher IL-6 on Day 2 post-op in control to intervention group, when comparing to baseline values | N/A | N/A |
| Wang et al. (2017) | T0: preoperative T1: end of surgery | Control group showed a significant increase with none in intervention group, when comparing to baseline values | T0->T1 | None | N/A | Significant lowering of albumin in control to intervention group, when comparing to baseline values | N/A |
| Lindberg-Larsen et al. (2017) | T0: pre-operative T1: 2-h post-operative T2: 6-h post-operative T3: 24-h post-operative | Control group showed a significant increase with none in intervention group | T0->T3 | N/A | No effect on sE, prevent significant drop in thrombomodulin, Reduced increase in VEGF in intervention to control, CRP increased less so in intervention group, when comparing to baseline values | N/A | N/A |
| Nemme et al. (2019) | T0: pre-operative T1: 30-min intra-operative T2: 60-min intra-operative T3: 90-min intra-operative T4: 2-h post-operative | Significant increase from baseline values in both groups | T3->T4 | T3->T4 | Some patients showed raised CRP post-operatively However, only results for some were significant | Albumin showed similar trends to HB but lower No significant differences at time points The greatest difference of values was at 20 min but lowered significantly at 90 min | N/A |
| Mennander et al. (2012) | T1: induction of anaesthesia T2: after aortic clamp removal T3: 60-min intra-operative T4: closure of skin wound | Significant drop found in intervention group of which control group did not show, when comparing to baseline values | T2->T3 T3->T4 | N/A | N/A | N/A | Similar changes to syndecan-1 levels |
| Kim et al. (2017) | T1: induction of anaesthesia T2: 60-min after coronary artery anastomosis T3: upon infusion of HES/crystalloid T4: skin closure T5: 12-h after ICU admission | None | N/A | N/A | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.Q.R.B.; Reilly, G.C. Protective Treatments against Endothelial Glycocalyx Degradation in Surgery: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 6994. https://doi.org/10.3390/app11156994
Khan HQRB, Reilly GC. Protective Treatments against Endothelial Glycocalyx Degradation in Surgery: A Systematic Review and Meta-Analysis. Applied Sciences. 2021; 11(15):6994. https://doi.org/10.3390/app11156994
Chicago/Turabian StyleKhan, Hasnain Q. R. B., and Gwendolen C. Reilly. 2021. "Protective Treatments against Endothelial Glycocalyx Degradation in Surgery: A Systematic Review and Meta-Analysis" Applied Sciences 11, no. 15: 6994. https://doi.org/10.3390/app11156994
APA StyleKhan, H. Q. R. B., & Reilly, G. C. (2021). Protective Treatments against Endothelial Glycocalyx Degradation in Surgery: A Systematic Review and Meta-Analysis. Applied Sciences, 11(15), 6994. https://doi.org/10.3390/app11156994







