Genomic Overlap between Platelet Parameters Variability and Age at Onset of Parkinson Disease
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Linkage Disequilibrium Score Regression
2.2. Multitrait Genetic Association Analysis
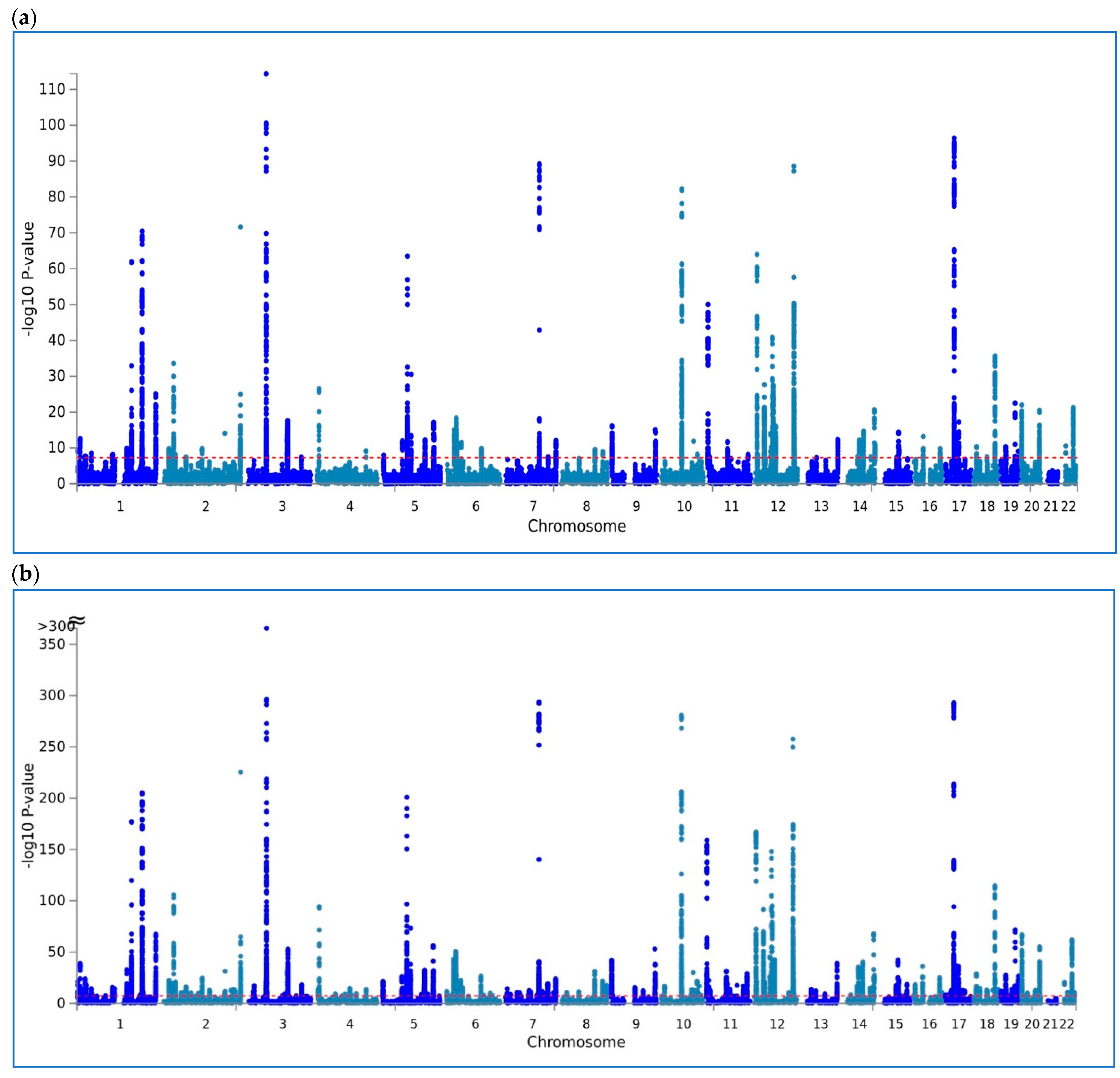
3. Results
4. Discussion
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tirozzi, A.; Izzi, B.; Noro, F.; Marotta, A.; Gianfagna, F.; Hoylaerts, M.F.; Cerletti, C.; Donati, M.B.; De Gaetano, G.; Iacoviello, L.; et al. Assessing genetic overlap between platelet parameters and neurodegenerative disorders. Front. Immunol. 2020, 11, 02127. [Google Scholar] [CrossRef] [PubMed]
- Canobbio, I. Blood platelets: Circulating mirrors of neurons? Res. Pract. Thromb. Haemost. 2019, 3, 564–565. [Google Scholar] [CrossRef] [Green Version]
- Geyik, S.; Yigiter, R.; Akgul, G.P.; Elci, M.; Firat, Y.E. The Relationship between Parkinson’s Disease and Mean Platelet Volume. Park. Hast. Hareket Bozuklukları Derg. 2016, 19, 31–34. [Google Scholar] [CrossRef]
- Koçer, A.; Yaman, A.; Niftaliyev, E.; Dürüyen, H.; Eryılmaz, M.; Koçer, E. Assessment of platelet indices in patients with neurodegenerative diseases: Mean platelet volume was increased in patients with Parkinson’s disease. Curr. Gerontol. Geriatr. Res. 2013, 2013, 986254. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of ge-nome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.K.; Loh, P.R.; Finucane, H.K.; Ripke, S.; Yang, J.; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Bulik-Sullivan, B.; Finucane, H.K.; Anttila, V.; Gusev, A.; Day, F.R.; Loh, P.-R.; Duncan, L.E.; Perry, J.R.; Patterson, N.; Robinson, E.; et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015, 47, 1236–1241. [Google Scholar] [CrossRef] [Green Version]
- Astle, W.; Elding, H.; Jiang, T.; Allen, D.; Ruklisa, D.; Mann, A.; Mead, D.; Bouman, H.; Riveros-Mckay, F.; Kostadima, M.A.; et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 2016, 167, 1415–1429.e19. [Google Scholar] [CrossRef] [Green Version]
- Gialluisi, A.; Reccia, M.G.; Tirozzi, A.; Nutile, T.; Lombardi, A.; De Sanctis, C.; Varanese, S.; Pietracupa, S.; Modugno, N.; Simeone, A.; et al. Whole exome sequencing study of parkinson disease and related endophenotypes in the Italian population. Front. Neurol. 2020, 10, 1362. [Google Scholar] [CrossRef] [Green Version]
- Hapmap3. Available online: https://www.sanger.ac.uk/resources/downloads/human/hapmap3.html (accessed on 23 July 2021).
- LDSCORE. Available online: https://data.broadinstitute.org/alkesgroup/LDSCORE/w_hm3.snplist (accessed on 23 July 2021).
- MTAG. Available online: https://github.com/omeed-maghzian/mtag (accessed on 23 July 2021).
- Turley, P.; Walters, R.K.; Maghzian, O.; Okbay, A.; Lee, J.J.; Fontana, M.A.; Nguyen-Viet, T.A.; Wedow, R.; Zacher, M.; Furlotte, N.A.; et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat. Genet. 2018, 50, 229–237. [Google Scholar] [CrossRef]
- De Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; Van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef] [Green Version]
- Blauwendraat, C.; Heilbron, K.; Vallerga, C.L.; Bandres-Ciga, S.; Von Coelln, R.; Pihlstrøm, L.; Simón-Sánchez, J.; Schulte, C.; Sharma, M.; Krohn, L.; et al. Parkinson’s disease age at onset genome-wide association study: Defining heritability, genetic loci, and α-synuclein mechanisms. Mov. Disord. 2019, 34, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensembl. Available online: https://grch37.ensembl.org/info/docs/tools/vep/index.html (accessed on 23 July 2021).
- Adams, B.; Nunes, J.M.; Page, M.; Roberts, T.; Carr, J.; Nell, T.; Kell, D.; Pretorius, E. Parkinson’s Disease: A systemic inflammatory disease accompanied by bacterial inflammagens. Front. Aging Neurosci. 2019, 11, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korniluk, A.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Kemona, H.; Dymicka-Piekarska, V. Mean Platelet Volume (MPV): New perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediat. Inflamm. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Tarelli, R. Parkinson’s disease and systemic inflammation. Park. Dis. 2011, 2011, 436813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csencsits-Smith, K.; Suescun, J.; Li, K.; Luo, S.; Bick, D.L.; Schiess, M. Serum lymphocyte-associated cytokine concentrations change more rapidly over time in multiple system atrophy compared to Parkinson Disease. Neuroimmunomodulation 2016, 23, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Jovanova, O.S.; Nedeljkovic, I.; Spieler, D.; Walker, R.M.; Liu, C.; Luciano, M.; Bressler, J.; Brody, J.; Drake, A.J.; Evans, K.L.; et al. DNA Methylation signatures of depressive symptoms in middle-aged and elderly persons: Meta-analysis of multiethnic epigenome-wide studies. JAMA Psychiatry 2018, 75, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Parnell, E.; Shapiro, L.P.; Voorn, R.A.; Forrest, M.P.; Jalloul, H.A.; Loizzo, D.D.; Penzes, P. KALRN: A central regulator of synaptic function and synaptopathies. Gene 2021, 768, 145306. [Google Scholar] [CrossRef]
- Gialluisi, A.; Izzi, B.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Revisiting the link between platelets and depression through genetic epidemiology: New insights from platelet distribution width. Haematologica 2020, 105, e246–e248. [Google Scholar] [CrossRef] [Green Version]
- Penzes, P.; Remmers, C. Kalirin signaling: Implications for synaptic pathology. Mol. Neurobiol. 2011, 45, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.-C.; Riess, O.; Soehn, A.S.; Nguyen, H.P. The Guanine Nucleotide exchange factor Kalirin-7 is a novel Synphilin-1 interacting protein and modifies Synphilin-1 aggregate transport and formation. PLoS ONE 2012, 7, e51999. [Google Scholar] [CrossRef]
- Izzi, B.; Tirozzi, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Hoylaerts, M.F.; Iacoviello, L.; Gialluisi, A. Beyond Haemostasis and Thrombosis: Platelets in Depression and Its Co-Morbidities. Int. J. Mol. Sci. 2020, 21, 8817. [Google Scholar] [CrossRef] [PubMed]
- Gialluisi, A.; Izzi, B.; Di Castelnuovo, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PLoS ONE 2018, 13, e0192121. [Google Scholar]
- Merlini, M.; Rafalski, V.A.; Coronado, P.E.R.; Gill, T.M.; Ellisman, M.; Muthukumar, G.; Subramanian, K.S.; Ryu, J.K.; Syme, C.A.; Davalos, D.; et al. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer’s disease model. Neuron 2019, 101, 1099–1108.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Platelet Parameter | Variants before/after QC | rg | se | z | p |
|---|---|---|---|---|---|
| Plt | 1,180,459/904,009 | 0.149 | 0.075 | 1.99 | 0.047 |
| MPV | 1,180,437/903,998 | −0.215 | 0.082 | −2.61 | 0.009 |
| PDW | 1,180,156/903,772 | −0.126 | 0.087 | −1.45 | 0.146 |
| (a) | |||||||||||||
| SNP | CHR:BP | A1 | A2 | Consequence | IMPACT | Gene | A1freq | Beta (SE)-MTAG | p-MTAG | Beta (SE)-PD_AAO | P-PD_AAO | Beta(SE)-MPV | P-MPV |
| rs73186248 | 7:106423109 | A | G | upstream_gene_variant | M | RP5−884M6.1 | 0.09 | −0.93 (0.16) | 5.57 × 10−9 | −1084 (0.258) | 0.00001781 | −0.037 (0.007) | 7.36 × 10−8 |
| rs73186270 | 7:106444515 | A | G | intron_variant,non_coding_transcript_variant | M | RP5−884M6.1 | 0.1 | −0.885 (0.152) | 5.74 × 10−9 | −1045 (0.242) | 0.0000212 | −0.033 (0.006) | 5.83 × 10−8 |
| rs73186233 | 7:106413337 | A | G | downstream_gene_variant | M | RP5−884M6.1 | 0.09 | −0.933 (0.161) | 5.98 × 10−9 | −1091 (0.26) | 0.00001618 | −0.037 (0.007) | 1.21 × 10−7 |
| rs17477708 | 7:106433932 | A | G | downstream_gene_variant | M | RP5−884M6.1 | 0.09 | −0.929 (0.16) | 6.31 × 10−9 | −1106 (0.258) | 0.00002551 | −0.036 (0.007) | 5.57 × 10−8 |
| rs73186245 | 7:106422325 | T | C | intron_variant,non_coding_transcript_variant | M | RP5−884M6.1 | 0.09 | −0.933 (0.16) | 6.37 × 10−9 | −1105 (0.258) | 0.00001776 | −0.037 (0.007) | 8.85 × 10−8 |
| rs73186239 | 7:106417063 | A | G | intron_variant,non_coding_transcript_variant | M | RP5−884M6.1 | 0.09 | −0.914 (0.158) | 6.96 × 10−9 | −1069 (0.253) | 0.00002631 | −0.036 (0.007) | 7.21 × 10−8 |
| rs73186269 | 7:106441672 | T | C | intergenic_variant | M | - | 0.09 | −0.919 (0.158) | 6.99 × 10−9 | −1081 (0.254) | 0.00002455 | −0.036 (0.007) | 6.81 × 10−8 |
| rs75209503 | 7:106435698 | T | C | intergenic_variant | M | - | 0.09 | −0.909 (0.158) | 7.97 × 10−9 | −1067 (0.253) | 0.00002527 | −0.036 (0.007) | 8.06 × 10−8 |
| (b) | |||||||||||||
| SNP | CHR:BP | A1 | A2 | Consequence | IMPACT | Gene | A1freq | Beta (SE)-MTAG | P-MTAG | Beta (SE)-MPV | P-MPV | Beta(SE)-PD_AAO | P-PD_AAO |
| rs17477708 | 7:106433932 | A | G | intron_variant,non_coding_transcript_variant | M | LINC02577 | 0.27 | −0.022 (0.004) | 3.26 × 10−8 | −0.215 (0.134) | 2.55 × 10−5 | −0.022 (0.004) | 5.57 × 10−8 |
| rs73186270 | 7:106444515 | A | G | intron_variant,non_coding_transcript_variant | M | LINC02577 | 0.4 | −0.02 (0.004) | 3.39 × 10−8 | −0.33 (0.133) | 2.12 × 10−5 | −0.02 (0.004) | 5.83 × 10−8 |
| rs7310366 | 12:123069722 | T | C | intron_variant | M | KNTC1 | 0.4 | −0.02 (0.004) | 3.81 × 10−8 | −0.321 (0.133) | 0.008285 | −0.02 (0.004) | 5.09 × 10−8 |
| rs73186269 | 7:106441672 | T | C | intron_variant,non_coding_transcript_variant | M | LINC02577 | 0.97 | 0.057 (0.01) | 3.98 × 10−8 | 0.773 (0.398) | 2.46 × 10−5 | 0.056 (0.01) | 6.81 × 10−8 |
| rs13333879 | 16:526435 | T | C | intron_variant | M | RAB11FIP3 | 0.89 | −0.032 (0.006) | 4.04 × 10−8 | −0.412 (0.209) | 0.005233 | −0.032 (0.006) | 5.26 × 10−8 |
| rs16835687 | 3:124327247 | A | G | intron_variant | M | KALRN | 0.17 | 0.027 (0.005) | 4.08 × 10−8 | 0.483 (0.189) | 0.01256 | 0.027 (0.005) | 5.20 × 10−8 |
| rs11818135 | 10:126323224 | A | C | intron_variant | M | FAM53B | 0.27 | −0.022 (0.004) | 4.17 × 10−8 | −0.288 (0.162) | 0.01054 | −0.022 (0.004) | 5.24 × 10−8 |
| rs11065565 | 12:121853164 | T | C | intron_variant | M | RNF34 | 0.92 | −0.037 (0.007) | 4.22 × 10−8 | −0.71 (0.254) | 0.01601 | −0.037 (0.007) | 5.24 × 10−8 |
| rs73186239 | 7:106417063 | A | G | intron_variant,non_coding_transcript_variant | M | LINC02577 | 0.1 | 0.033 (0.006) | 4.23 × 10−8 | 0.519 (0.208) | 2.63 × 10−5 | 0.032 (0.006) | 7.21 × 10−8 |
| rs3817544 | 12:121882819 | T | C | intron_variant | M | KDM2B | 0.28 | −0.022 (0.004) | 4.25 × 10−8 | −0.215 (0.131) | 0.01663 | −0.022 (0.004) | 5.26 × 10−8 |
| rs73186248 | 7:106423109 | A | G | downstream_gene_variant | M | LINC02577 | 0.92 | −0.038 (0.007) | 4.25 × 10−8 | −1.084 (0.258) | 1.78 × 10−5 | −0.037 (0.007) | 7.36 × 10−8 |
| rs6761213 | 2:223870120 | A | G | intergenic_variant | M | - | 0.74 | −0.023 (0.004) | 4.29 × 10−8 | −0.26 (0.146) | 0.03182 | −0.023 (0.004) | 5.09 × 10−8 |
| rs76123955 | 10:64855929 | A | G | intergenic_variant | M | - | 0.92 | −0.037 (0.007) | 4.42 × 10−8 | −0.711 (0.255) | 0.05053 | −0.036 (0.007) | 5.03 × −8 |
| rs113899647 | 10:64850074 | T | C | intergenic_variant | M | - | 0.12 | −0.031 (0.006) | 4.49 × 10−8 | −0.441 (0.22) | 0.05195 | −0.031 (0.006) | 5.09 × 10−8 |
| rs74760108 | 6:25473784 | A | G | intron_variant | M | CARMIL1 | 0.42 | −0.02 (0.004) | 4.49 × 10−8 | −0.231 (0.124) | 0.03488 | −0.02 (0.004) | 5.35 × 10−8 |
| rs35041742 | 17:20036253 | T | C | intron_variant | M | SPECC1 | 0.42 | −0.02 (0.004) | 4.60 × 10−8 | −0.231 (0.124) | 0.06307 | −0.02 (0.004) | 5.17 × 10−8 |
| rs62261974 | 3:107296969 | A | G | intron_variant | M | BBX | 0.4 | −0.02 (0.004) | 4.60 × 10−8 | −0.32 (0.133) | 0.0677 | −0.02 (0.004) | 5.03 × 10−8 |
| rs4081775 | 12:121847579 | A | G | intron_variant | M | RNF34 | 0.58 | 0.02 (0.004) | 4.64 × 10−8 | 0.227 (0.124) | 0.01254 | 0.02 (0.004) | 5.86 × 10−8 |
| rs1765710 | 1:45936700 | T | C | intron_variant | M | TESK2 | 0.7 | 0.022 (0.004) | 4.66 × 10−8 | 0.278 (0.143) | 0.07405 | 0.022 (0.004) | 5.14 × 10−8 |
| rs11900857 | 2:191659011 | T | C | intron_variant,non_coding_transcript_variant | M | - | 0.4 | −0.02 (0.004) | 4.66 × 10−8 | −0.333 (0.133) | 0.07622 | −0.02 (0.004) | 5.04 × 10−8 |
| rs75209503 | 7:106435698 | T | C | intron_variant,non_coding_transcript_variant | M | LINC02577 | 0.19 | 0.026 (0.005) | 4.72 × 10−8 | 0.334 (0.183) | 2.53 × 10−5 | 0.026 (0.005) | 8.06 × 10−8 |
| rs4003797 | 17:20288900 | T | C | intron_variant,non_coding_transcript_variant | M | CCDC144CP | 0.56 | −0.02 (0.004) | 4.75 × 10−8 | −0.312 (0.141) | 0.0674 | −0.02 (0.004) | 5.31 × 10−8 |
| rs958753 | 2:223995281 | A | C | intron_variant,non_coding_transcript_variant | M | KCNE4 | 0.19 | −0.025 (0.005) | 4.78 × 10−8 | −0.337 (0.157) | 0.1045 | −0.025 (0.005) | 5.09 × 10−8 |
| rs34210276 | 5:77060737 | T | C | intron_variant | M | TBCA | 0.35 | 0.021 (0.004) | 4.80 × 10−8 | 0.329 (0.124) | 0.04518 | 0.021 (0.004) | 5.42 × 10−8 |
| rs6446546 | 4:6875714 | T | C | intron_variant | M | KIAA0232 | 0.93 | −0.038 (0.007) | 4.83 × 10−8 | −1.091 (0.26) | 0.02727 | −0.037 (0.007) | 5.69 × 10−8 |
| rs4028 | 3:124261869 | A | G | intron_variant | M | KALRN | 0.92 | −0.038 (0.007) | 4.85 × 10−8 | −1.105 (0.258) | 0.05091 | −0.037 (0.007) | 5.50 × 10−8 |
| rs11585488 | 1:45617312 | A | G | intron_variant | M | ZSWIM5 | 0.92 | −0.037 (0.007) | 4.86 × 10−8 | −1.069 (0.253) | 0.04904 | −0.036 (0.007) | 5.56 × 10−8 |
| rs28678897 | 16:527485 | A | G | intron_variant | M | RAB11FIP3 | 0.92 | −0.037 (0.007) | 4.86 × 10−8 | −1.081 (0.254) | 0.005302 | −0.036 (0.007) | 6.31 × 10−8 |
| rs10072700 | 5:131816903 | A | C | downstream_gene_variant | M | IRF1 | 0.16 | −0.027 (0.005) | 4.86 × 10−8 | −0.344 (0.163) | 0.1091 | −0.027 (0.005) | 5.22 × 10−8 |
| rs36051592 | 17:20035020 | T | C | intron_variant | M | SPECC1 | 0.92 | −0.037 (0.007) | 4.87 × 10−8 | −1.067 (0.253) | 0.0624 | −0.036 (0.007) | 5.49 × 10−8 |
| rs169632 | 12:121588545 | T | C | intron_variant,NMD_transcript_variant | M | P2RX7 | 0.97 | 0.057 (0.01) | 4.93 × 10−8 | 0.775 (0.396) | 0.1002 | 0.056 (0.01) | 5.34 × 10−8 |
| rs10849880 | 12:121859844 | A | C | intron_variant | M | RNF34 | 0.23 | −0.024 (0.004) | 4.99 × 10−8 | −0.179 (0.137) | 0.01321 | −0.024 (0.004) | 6.28 × 10−8 |
| rs79502317 | 11:61736136 | T | G | upstream_gene_variant | M | FTH1 | 0.87 | 0.03 (0.005) | 5.00 × 10−8 | 0.302 (0.186) | 0.191 | 0.029 (0.005) | 5.06 × 10−8 |
| (a) | ||||||
| NGENES | BETA | BETA STD | SE | p | Pbonf | FULL NAME |
| 12 | 2.04 | 0.05 | 0.30 | 5.42 × 10−12 | 8.39 × 10−8 | GO: thrombin-activated receptor signalling pathway |
| 323 | 0.35 | 0.05 | 0.05 | 1.68 × 10−11 | 2.60 × 10−7 | GO: coagulation |
| 591 | 0.23 | 0.04 | 0.04 | 7.28 × 10−10 | 1.13 × 10−5 | Curated gene sets: reactome hemostasis |
| 161 | 0.43 | 0.04 | 0.08 | 4.87 × 10−9 | 7.55 × 10−5 | Curated gene sets: reactome factors involved in megakaryocyte development and platelet production |
| 475 | 0.23 | 0.04 | 0.04 | 5.03 × 10−8 | 7.78 × 10−4 | GO: regulation of body fluid levels |
| 518 | 0.21 | 0.03 | 0.04 | 5.68 × 10−8 | 8.79 × 10−4 | GO: wound healing |
| 5 | 2.32 | 0.04 | 0.44 | 7.37 × 10−8 | 1.14 × 10−3 | GO: thrombin-activated receptor activity |
| 178 | 0.34 | 0.03 | 0.07 | 1.08 × 10−7 | 1.67 × 10−3 | Curated gene sets: wierenga stat5a targets dn |
| 5 | 2.22 | 0.04 | 0.43 | 1.43 × 10−7 | 2.21 × 10−3 | GO: protein localization to juxtaparanode region of axon |
| 7 | 2.06 | 0.04 | 0.40 | 1.46 × 10−7 | 2.26 × 10−3 | Curated gene sets: reactome erythropoietin activates phospholipase c gamma plcg |
| 627 | 0.19 | 0.03 | 0.04 | 2.14 × 10−7 | 3.31 × 10−3 | GO: response to wounding |
| 7 | 2.05 | 0.04 | 0.41 | 3.81 × 10−7 | 5.89 × 10−3 | GO: positive regulation of fc receptor-mediated stimulatory signalling pathway |
| 5 | 2.13 | 0.03 | 0.45 | 1.05 × 10−6 | 1.63 × 10−2 | GO: type 5 metabotropic glutamate receptor binding |
| 9 | 1.70 | 0.04 | 0.37 | 2.73 × 10−6 | 4.22 × 10−2 | GO: exocyst localization |
| 25 | 0.87 | 0.03 | 0.19 | 2.86 × 10−6 | 4.43 × 10−2 | GO: cellular protein-containing complex × localization |
| 18 | 0.99 | 0.03 | 0.22 | 2.91 × 10−6 | 4.50 × 10−2 | GO: platelet morphogenesis |
| (b) | ||||||
| NGENES | BETA | BETA STD | SE | p | Pbonf | FULL NAME |
| 323 | 0.42 | 0.05 | 0.07 | 1.86 × 10−9 | 2.87 × 10−5 | GO: coagulation |
| 161 | 0.60 | 0.06 | 0.10 | 2.82 × 10−9 | 4.37 × 10−5 | Curated gene sets: reactome factors involved in megakaryocyte development and platelet production |
| 158 | 0.57 | 0.05 | 0.10 | 4.08 × 10−9 | 6.32 × 10−5 | GO: rho gtpase binding |
| 591 | 0.29 | 0.05 | 0.05 | 5.47 × 10−9 | 8.46 × 10−5 | Curated gene sets: reactome hemostasis |
| 496 | 0.31 | 0.05 | 0.06 | 1.45 × 10−8 | 2.25 × 10−4 | GO: gtpase binding |
| 438 | 0.34 | 0.05 | 0.06 | 1.72 × 10−8 | 2.66 × 10−4 | GO: regulation of gtpase activity |
| 403 | 0.33 | 0.05 | 0.06 | 3.92 × 10−8 | 6.07 × 10−4 | GO: small gtpase binding |
| 371 | 0.34 | 0.05 | 0.07 | 1.04 × 10−7 | 1.61 × 10−3 | GO: positive regulation of gtpase activity |
| 7 | 2.77 | 0.05 | 0.55 | 2.13 × 10−7 | 3.30 × 10−3 | Curated gene sets: reactome erythropoietin activates phospholipase c gamma plcg |
| 178 | 0.45 | 0.04 | 0.09 | 3.15 × 10−7 | 4.88 × 10−3 | Curated gene sets: wierenga stat5a targets dn |
| 59 | 0.83 | 0.05 | 0.17 | 3.70 × 10−7 | 5.72 × 10−3 | GO: rac gtpase binding |
| 275 | 0.36 | 0.04 | 0.08 | 9.67 × 10−7 | 1.50 × 10−2 | GO: gtpase regulator activity |
| 475 | 0.28 | 0.04 | 0.06 | 1.03 × 10−6 | 1.60 × 10−2 | GO: regulation of body fluid levels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirozzi, A.; Parisi, R.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Gialluisi, A. Genomic Overlap between Platelet Parameters Variability and Age at Onset of Parkinson Disease. Appl. Sci. 2021, 11, 6927. https://doi.org/10.3390/app11156927
Tirozzi A, Parisi R, Cerletti C, Donati MB, de Gaetano G, Iacoviello L, Gialluisi A. Genomic Overlap between Platelet Parameters Variability and Age at Onset of Parkinson Disease. Applied Sciences. 2021; 11(15):6927. https://doi.org/10.3390/app11156927
Chicago/Turabian StyleTirozzi, Alfonsina, Roberta Parisi, Chiara Cerletti, Maria Benedetta Donati, Giovanni de Gaetano, Licia Iacoviello, and Alessandro Gialluisi. 2021. "Genomic Overlap between Platelet Parameters Variability and Age at Onset of Parkinson Disease" Applied Sciences 11, no. 15: 6927. https://doi.org/10.3390/app11156927
APA StyleTirozzi, A., Parisi, R., Cerletti, C., Donati, M. B., de Gaetano, G., Iacoviello, L., & Gialluisi, A. (2021). Genomic Overlap between Platelet Parameters Variability and Age at Onset of Parkinson Disease. Applied Sciences, 11(15), 6927. https://doi.org/10.3390/app11156927






