Abstract
This study aimed to explore the role of preoperative and postoperative C-reactive protein (CRP) levels in mediating the association between the preoperative depression symptoms and postoperative length of stay in patients undergoing coronary artery bypass grafting (CABG). Preoperative depression symptoms of 212 elective CABG patients were measured using the Beck Depression Inventory (BDI-II). The patient’s demographic and clinical parameters were collected from medical records prior to surgery. Patients were followed up during their in-hospital stay to measure early (1–3 days post-surgery) and persistent (4–6 days post-surgery) CRP response to CABG surgery. The higher persistent CRP response was significantly (p < 0.001) associated with a longer postoperative hospital stay. The binary logistic regression analysis confirmed the association of persistent CRP change with prolonged hospital stay (OR = 1.017, 95% CI = 1.005–1.029, p = 0.009). However, when the gender subgroups were analyzed separately, that remained significant (OR = 1.016, 95% CI = 1.004–1.028, p = 0.005) only for the male subgroup. There was no significant association between elevated BDI-II depression scores and longer postoperative hospital stay. Additionally, no significant influence of BDI-II scores on preoperative or postoperative CRP levels, or vice versa, was detected. Further work is needed to explore the extent and pathways through which depression might influence the postoperative recovery of CABG patients.
1. Introduction
Coronary artery disease (CAD) is a major form of heart disease and one of the leading causes of morbidity and mortality worldwide [1]. A commonly used treatment approach is on-pump or off-pump coronary artery bypass grafting (CABG), which bypasses atherosclerotic occlusions and improves blood supply to the heart muscle, thus restoring its function and viability [2]. The traditional risk factors associated with initiation and progression of atherosclerotic lesions in the coronary artery walls include increasing age, gender, unhealthy lifestyle behaviors (physical inactivity, smoking, and unbalanced diet), obesity, high blood cholesterol levels, diabetes, hypertension, family history, and race [3,4]. In addition, the patient’s psychological state in terms of depression, anxiety, anger, and stress has also been implicated as potential risk factor in the development of an increased incidence of morbidity and mortality of CAD patients [5,6,7,8].
Depression is highly prevalent (17% to 27%) in CAD patients, including those with stable CAD, unstable angina, or myocardial infarction [9]. The prevalence of significant depressive symptoms is diagnosed in more than 20% of CAD patients, while many have subsyndromal depressive symptoms below the diagnostic threshold [7,9,10]. Therefore, depressive symptoms are frequently underdiagnosed and consequently left untreated in CAD patients, although they may exist for a long time before the onset of disease [10,11]. The bidirectional mechanisms responsible for the comorbidity of depression and CAD are complex and multifactorial [12,13,14,15,16]. Various pathophysiological triggers, including neuroendocrine dysregulation, activation of the inflammatory response, increased platelet activation and aggregation, oxidative stress, and endothelial dysfunction, may all be involved in the relationship between depressive symptoms and CAD [5,12,13,14,15,16,17,18]. Individuals with depressive disorders tend to have elevated inflammatory markers and pro-inflammatory cytokines that undermine their response to conventional antidepressant therapy [14,19,20,21,22]. Several studies indicate a bidirectional relationship between depression and inflammatory response [23,24]. However, although the depression and elevated inflammatory markers frequently covary, it is still unclear how they relate to each other, and to the onset of CAD [25]. Some reports indicate that depressive symptoms may precede and augment inflammatory processes in clinical entities such as coronary artery disease while others suggest that elevation of inflammatory biomarkers precedes depression [25,26,27,28,29]. Another possibility is that both depression and inflammation may be consequences of another underlying pathologic process [25].
Individuals with depressive disorders tend to have elevated levels of inflammatory markers and pro-inflammatory cytokines that also undermine their response to conventional antidepressant therapy [24]. Among them the C-reactive protein (CRP), a marker of acute phase response, has been used most extensively in clinical practice as a biomarker of systemic inflammation and is routinely measured across medical centers and research laboratories [30,31]. Levels of CRP are used to detect and follow disease in many fields of medicine and its kinetics after major surgery procedure is usually related to inflammatory response to infection [32].
The increased concentrations of CRP have been found in a subset of patients with depression compared with the non-depressed control group, often preceding the onset of illness [27,33,34,35,36,37]. CRP is also one of the elevated inflammatory markers in depression that shows reduced variability thus supporting greater homogeneity in terms of corresponding inflammatory phenotype [38]. Several studies have also shown the correlation between peripheral CRP levels and the increased risk of CAD [37,39,40,41,42,43,44]. The relationship between preoperative depression and increased hospital length of stay following CABG procedures was also reported [45,46,47,48,49]. However, more than half of the CABG patients are not routinely assessed and treated for depressive syndromes [45,46]. In addition, only a few studies have assessed the relationship between depression and CRP proteins in CABG patients [29,48,50]. In addition, little is known about the causal relationship between these two covariates in predicting CABG recovery. Furthermore, only one study to date showed that the effect of preoperative depression symptoms on postoperative hospital stay of CABG patients may be mediated by the post-surgery inflammation and elevated CRP levels [48]. Specifically, this association was partially mediated by a greater change in CRP from baseline preoperative values to 4–8 days post-CABG surgery.
Based on these reports, the present study aimed to assess the relationship between preoperative depressive symptoms with postoperative hospital length of stay (LOS) of patients undergoing elective CABG surgery and to examine the extent to which this association could be influenced by preoperative and postoperative CRP values. Specifically, we hypothesized that the presence of preoperative depression symptoms measured using the Beck Depression Inventory (BDI-II) would be associated with the length of postoperative hospital stay and that the effect of preoperative depression score on the length of stay would be mediated by the higher values of preoperative and postoperative CRP levels.
2. Patients, Materials, and Methods
The present study was designed as a prospective observational (non-interventional) study. All participants signed the informed consent. The Ethics Committee of the University Hospital Centre (UHC) Zagreb, Croatia, approved the study protocol (Approval number of the Ethics Committee UHC Zagreb, Class: 8.1-11/84-3).
2.1. Patients
The eligible patients undergoing elective CABG surgery (n = 257) were consecutively recruited at the Department of Cardiac Surgery, UHC Zagreb, Croatia, between December 2014 and March 2016. A flow chart displaying the recruitment and retention of participants through the present study is shown in Figure 1. Eligibility criteria included patients undergoing elective CABG surgery who were 18 years of age or older and could complete questionnaires in the Croatian language. CABG procedures encompass both on-pump and off-pump surgical procedures. Patients who were unwilling to participate (n = 22) were excluded from the study. Some patients (n = 4) died within three days of surgery and were excluded from the study due to a lack of postoperative data. Patients missing some laboratory data were also noted (n = 19) and excluded from the study.
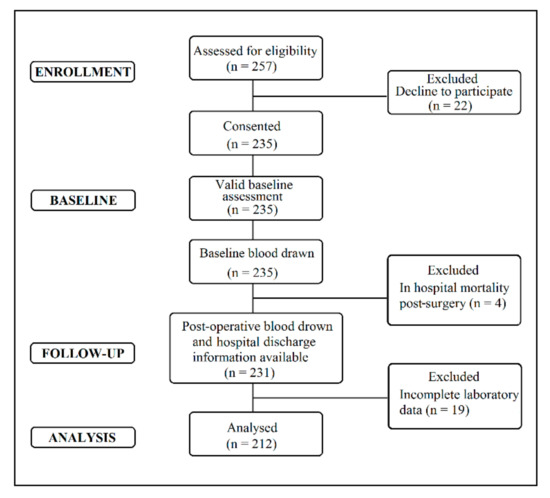
Figure 1.
Flow diagram of coronary artery bypass grafting (CABG) patient’s recruitment and retention.
The lack of laboratory findings in the later patient subgroup can be explained because some of the data are not extracted routinely, especially not every day. Therefore, sometimes without rigorous checking whether some of the findings were extracted, an unwanted omission can occur. Consequently, 212 CABG patients with complete data for all requested variables at baseline and follow-up were included in the present study (Figure 1).
2.2. Materials and Methods
2.2.1. Predictors
Assessment of Depressive Symptoms
The severity of the patient’s depressive symptoms at baseline (preoperative assessment) was determined by the Beck Depression Inventory (BDI-II) test [51]. Since all the participants spoke Croatian, validated Croatian versions of BDI-II were used [52]. Both questionnaires were fulfilled by each patient included in the study with the supervision and assistance of the physician when deemed necessary.
The BDI-II depression inventory test represents a 21-question multiple-choice self-report inventory, in which the patients were asked how they felt over the last two weeks [51]. Each answer to the BDI-II test ranges from 1 to 3, and the total ratings were calculated as follows: a score of 0–13 indicates patients with minimal depressive symptoms, 14–19 indicates those with mild depressive symptoms, and a score of 20–28 indicates patients with moderately expressed depressive symptoms, while a 29–63 total rating score designates CABG patients with high depressive symptoms [51]. A binary variable generated according to accepted cut-off values divides patients into a non-depressive symptom group with a BDI-II score ranging from 0 to 13 and a mildly to a severely depressed group with a BDI-II score > 13.
Baseline and Postoperative C-Reactive Protein Levels
The inflammatory activity of CABG patients included in the present study was monitored by measuring CRP values in the patient’s peripheral circulation. Baseline CRP values were determined preoperatively upon admission to the Department of Cardiac Surgery. The CRP values were measured on the first, second, third, fourth, fifth, and sixth postoperative days in the postoperative course. To obtain the samples, 5 mL of peripheral blood from each patient was drawn into plasma separator tubes by vacuum puncture from the forearm at baseline and postoperatively, as designated. Blood was subsequently centrifuged for 10 min at 3700 rpm (revolutions per minute), and the resulting plasma was placed into Eppendorf tubes and frozen at −80 °C until further analysis. CRP measurement was performed using a commercial immunoturbidimetry assay (Tina-quant® C-Reactive Protein Gen.3, Roche Diagnostics, Rotkreuz, Switzerland ) in the Roche Cobas c 501 modules (Roche Diagnostics Rotkreuz, Switzerland,) following the manufacturer’s guidelines. Overall, two summary scores of postoperative CRP values were generated. The first summary score used the mean for the CRP values measured on days 1, 2, and 3 postoperatively, while the second CRP summary score used the mean for the CRP values measured on days 4, 5, and 6 postoperatively. The postoperative CRP responses to CABG surgery were calculated as differential scores by subtracting the baseline CRP value from the mean postoperative CRP scores and termed as “early” and “persistent”, as previously described [48].
2.2.2. Outcome: Length of Stay Measure
As a measure of clinical recovery, the duration of postoperative hospital LOS reported in days was collected from the patient’s clinical records. Similar to some other clinical institutions, the policy of the Department of Cardiac Surgery, University Hospital Centre Zagreb, was to discharge patients with an uncomplicated course within seven days of the CABG procedure [48,53]. There were no changes to this discharge policy during the period of data collection. CABG patients with the poorest recovery and the most significant in-hospital complications are expected to have the longest in-hospital length of stay after a CABG procedure. The patient’s intensive care unit (ICU) length of stay was also recorded.
2.2.3. Covariates: Demographic and Clinical Measures
The patient’s clinical and demographic data were collected during the interview following admission and from the patient’s clinical records. Recorded characteristics included: age, gender, preoperative body mass index (BMI; kg/m2), preoperative hemoglobin levels, smoking status (current smoker/non-smoker), and prescribed medications, including the use of antidepressants (underlying psychiatric conditions), statins, acetylsalicylic acid, angiotensin-converting enzyme (ACE) inhibitors, and beta-blockers. Patient-related or a family history of cardiovascular disease and the existence of preoperative myocardial infarction (MI), diabetes I and II, hyperlipidemia, and hypertension were also recorded to capture the number of comorbidities in participants with coronary artery disease. The assessment of cardiac surgical risk (i.e., mortality after undergoing cardiac surgery procedures) for individual patients was assessed using the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II [54] and the corresponding EuroSCORE II online calculator (http://www.euroscore.org/calc.html, accessed on 25 July 2022). In addition, the risks relative to the operative procedure, including a systolic function of the myocardium, number of grafts received intraoperatively, cardiopulmonary bypass procedure, extubating time, and extracorporeal circulation length, were recorded as well. The (post)operative infections that may cause a persistent elevation in CRP responses were also recorded.
2.3. Statistical Analysis
The normality of data distribution was analyzed by the Shapiro–Wilk test. The data for continuous variables are presented as mean ± SD (standard deviation), when normally distributed, or median and interquartile range (IQR) in cases of skewed distribution, and as frequencies (percentages) for categorical variables. Differences in variables between groups were compared using a t-test for normally distributed or a Mann–Whitney U test for continuous variables with skewed distribution. In addition, the Pearson χ2 test was used for group comparisons of categorical variables. The correlations between variables were analyzed by Spearman’s correlation analysis. To test the association between baseline depression (predictor) and postoperative hospital LOS (outcome variable), we analyzed the baseline BDI-II depression scores using the binary cut-off of 13 (≤13 vs. >13) and modeled associations between baseline depression and postoperative hospital LOS using the binary logistic regressions analysis. Furthermore, a binary cut-off of 7 days was implemented for the postoperative hospital LOS, with prolonged hospitalization defined as LOS > 7 days [48,53]. The binary logistic regression analysis results are presented as OR (odds ratios) with 95% CI (confidence interval). The covariates that might relate to the postoperative hospital LOS were included in the models of binary logistic regressions based on the statistical significance (p-value < 0.05) of Spearman’s correlation analysis and Mann–Whitney test for continuous data or the Pearson χ2 test for categorical variables. Model 2 of adjusted binary logistic regression analysis included only age and sex as covariables. Model 3 further included other demographic and clinical variables (current smoking status, EuroSCORE-II, preoperative hemoglobin levels, the use of β-blockers, extubation/ventilation time, and postoperative atrial fibrillation) that could confound or mediate the association between depressive symptoms and postoperative hospital LOS. However, to avoid double adjustment, variables such as age, gender, or insulin-dependent diabetes mellitus that are already included in the EuroSCORE-II calculation were not included in Model 3 of adjusted binary logistic regression. The same approach for non-adjusted and adjusted binary logistic regression was used to analyze the association of preoperative and post-operative CRP values (“early“ and “persistent“ post-operative CRP response) with postoperative hospital LOS (outcome variable). In addition, baseline CRP values, as a separate variable, were also included in Models 2 and 3 of binary logistic regression to assess the relative contribution of both baseline and post-operative CRP values on postoperative hospital LOS as previously described [48]. The association between depression symptoms and preoperative and postoperative CRP plasma levels was also assessed by the linear regression model. Covariates included in the linear regression analysis for changes in preoperative CRP levels and postoperative CRP response were mainly the same as the variables included in Model 3 of binary logistic regression, using the same inclusion criteria as described above. The only difference was the introduction of infection as additional covariates to control for its possible contribution to the postoperative elevation of CRP levels. To examine the hypothesis in which the relationship between the independent (BDI-II depression score) and dependent (postoperative hospital LOS) variables is affected by a third variable (CRP response), that is, to assess the indirect relationship between the predictor and outcome variable, mediation analysis was performed. For that purpose, Hayes SPSS PROCESS macro v.4.1 was used [55,56,57,58]. The 95% percentile bootstrap confidence intervals (CIs) based on 5000 samples were used to test the significance of indirect effects of depression in all analyses. In this analysis, the upper and lower CIs must be entirely above or below zero for the indirect effect to demonstrate statistical significance [59,60]. Covariates included in the mediation analysis were the same as those included in Model 3 of binary logistic regression with the addition of (post)operative infection to adjust for its impact on CRP response. All statistical analysis was performed using Statistical Product and Service Solutions (SPSS) version 29.0 (IBM Corp., Armonk, NY, USA). The two-tailed p < 0.05 was considered significant and corrected according to the Bonferroni correction (the corrected level of significance was: Pc = 0.05/N; N—number of independent tests). All reported p values are uncorrected unless stated otherwise.
3. Results
3.1. Patient Demographic and Clinical Data
The patient demographic, preoperative, and postoperative clinical characteristics are listed in Table 1. The mean age of the patient cohort at the time of surgery was 61.61 ± 7.94 years, with an age range of 39–80 years. The majority of patients were male (80.7%), overweight (BMI > 25 = 80.7%), and hypertensive (87.3%), with diabetes mellitus present in more than a third (36.3%) of participants. Additionally, most of the patients were on prescribed beta-blockers, acetylsalicylic acid, ACE inhibitors, and statin medications, while only two participants (0.9%) were on antidepressant therapy (Table 1).

Table 1.
Demographic, clinical, and depression characteristics of CABG patients (N = 212).
More than half of the patients (52.4%) included in the present study received three coronary artery bypass grafts. The mean postoperative length of stay was 9.3 ± 0.3 days, with a range of 6–36 days, while the mean ICU LOS was 2.6 ± 0.1 days with a range of 1–10 days. The majority of patients were within a normal score range for baseline depressive symptoms (BDI-II ≤ 13; 87.7%). However, for 26 (12.3%) patients, the BDI-II baseline score was > 13. The median baseline plasma CRP level was 2.3 (1.0–4.7) mg/dL (Table 1).
Depressive symptoms measured by the Beck Depression Inventory were significantly associated only with the higher EuroSCORE-II values (Mann–Whitney U test, p < 0.001) (Table 2). Furthermore, 13 (6.1%) patients in our CABG cohort had a postoperative infection, with only 1 of them belonging to the group with a higher BDI-II (>13) score. Additionally, the majority of patients with elevated BDI-II (>13) scores (73.1%) had prolonged postoperative hospital LOS (>7 days), compared with 56.5% of participants within the non-depressive symptom group.

Table 2.
Association between BDI-II depression symptom scores and patients’ demographic and clinical data.
The prolonged postoperative hospital LOS was significantly associated only with older age at admission (t = 4.301, p < 0.001), lower preoperative hemoglobin levels (Mann–Whitney U test, p < 0.001), higher EuroSCORE II (Mann–Whitney U test, p < 0.001), persistent CRP change (Mann–Whitney U test, p < 0.001), and higher frequency of postoperative atrial fibrillation (χ2 = 12.45, p < 0.001) (Table 3).

Table 3.
Association between a postoperative hospital stay and patients’ demographic and clinical data.
Regarding the association of the patient’s depressive symptoms with the postoperative length of stay, no significant association between postoperative length of stay and binary BDI-II depression scores was detected (Table 3). Only the continuous BDI-II scores at baseline were associated (p = 0.014) with a prolonged hospital LOS. However, that association did not remain significant after the Bonferroni correction was applied. Interestingly, although only two participants in our patient cohort were under antidepressant therapy, both of them were in the non-depressive symptom group (BDI-II ≤ 13) (Table 2). On the contrary, they were equally distributed in subgroups stratified according to the extent of postoperative hospital LOS (Table 3).
3.2. The Association between Depression Symptoms, and CRP with the Length of Postoperative Hospital Stay
Table 4 shows the results of the binary logistic regression analysis used to predict the impact of baseline depression on post-operative hospital LOS.

Table 4.
Predictive role of depression scores for the postoperative in-hospital length of stay.
Similar to the non-adjusted model of binary logistic regression (Model 1), the age and gender-adjusted model (Model 2) showed that patients in the mild to severe depression group (BDI-II > 13) had more than two times greater odds of a prolonged postoperative hospital LOS (>7 days) when compared to non-depressive symptom group.
However, the only variable significantly predicting the prolonged hospital LOS in Model 2 was the patient’s age (OR = 1.08, 95% CI = 1.04–1.13, p < 0.001). Model 3 of binary logistic regression included the following covariables significantly correlated (Spearman’s correlation analysis) with postoperative hospital LOS: current smoking (r = −0.213, p = 0.002), preoperative hemoglobin levels (r = −0.230, p = 0.001), the use of β-blockers (r = −0.163, p = 0.018), EuroSCORE-II (r = 0.318, p < 0.001), ventilation time (r = 0.165, p = 0.016) and postoperative atrial fibrillation (r = 0.242, p < 0.001).
Although the CABG patients in the mild to severe depression group showed a 1.21 times higher odds of prolonged hospital LOS when compared with the non-depressive symptoms group, that was not statistically significant (Table 4).
In addition, current smoking (OR = 0.21, 95% CI = 0.08–0.55, p = 0.002) and use of β-blockers (OR = 0.22, 95% CI = 0.08–0.64, p = 0.006) were the only predictors of prolonged postoperative hospital LOS in Model 3 of binary logistic regression that remained significant after Bonferroni correction was applied.
Interestingly, when the gender groups of our cohort were analyzed separately (data not shown) the mild to severe depression group of female patients showed a 4.04 times higher odds of prolonged hospital LOS compared with the non-depressive symptom group, while in the male patient group the odds of mild to severe depression group for prolonged hospital LOS was 0.793. Nevertheless, in both cases the observed values were nonsignificant.
Table 5 presents the binary logistic regression results used to predict the impact of baseline CRP levels and changes in early and persistent CRP response on postoperative hospital LOS.

Table 5.
Predictive role of early and persistent C-reactive protein levels on the postoperative in-hospital length of stay.
Model 3 of binary logistic regression for the predictive role of persistent CRP change (4–6 days post-operatively) showed that for every unit increase in CRP levels, there was a significant 1.7% increase in the odds of prolonged hospital LOS (p = 0.005). There were no other significant predictors in this model. The baseline CRP levels, and early CRP response were non-significant predictors of postoperative hospital LOS in the fully adjusted model (Model 3) of binary logistic regression. In addition, current smoking (OR = 0.21, 95% CI = 0.08–0.55, p = 0.002) and the use of β-blockers (OR = 0.22, 95% CI = 0.08–0.65, p = 0.006) were proven as the only significant predictor of postoperative hospital LOS in Model 3 of binary logistic regression analyzing the impact of baseline CRP levels. In addition, current smoking (OR = 0.22, 95% CI = 0.09–0.60, p = 0.003) was also a significant predictor of postoperative hospital LOS in Model 3 of binary logistic regression related to early CRP changes. Interestingly, in the age (OR = 1.08, CI = 1.04–1.13, p < 0.001) and gender adjusted model for the predictive role of persistent CRP change on the length of stay, female patients had more than three times (OR = 3.20; CI = 1.38–7.39, p = 0.007) greater odds for prolonged length of stay. Age was also the only predictive factor in the age and gender adjusted model for predictive role of baseline (OR = 1.08; CI = 1.04–1.13, p < 0.001) and early CRP change (OR = 1.09; CI = 1.04–1.13, p < 0.001) on the postoperative length of stay.
Interestingly, when the gender groups of our patient cohort were analyzed separately (data not shown), the baseline CRP levels and early CRP response were also non-significant predictors of postoperative hospital LOS in the fully adjusted model (Model 3) of binary logistic regression, while for every unit increase in CRP levels, there was a significant 1.6% increase in the odds of prolonged hospital LOS (p = 0.009). On the contrary, in the female subgroup of our cohort, all three measured CRP values (baseline, early and persistent) were non-significant predictors of postoperative LOS in the fully adjusted Model 3 of binary logistic regression analysis.
3.3. The Impact of Depression on the Baseline and Postoperative CRP Levels
Since Spearman’s correlation analysis revealed a significant association of infection with CRP levels measured on days 4 (r = 0.141, p = 0.043) 5 (r = 0.194, p = 0.005) and 6 (r = 0.190, p = 0.006) this variable, together with baseline CRP values, was added as an additional covariate in models examining the impact of depression scores on early and persistent postoperative CRP change. Linear regression analysis performed to examine the cross-sectional relationship of baseline BDI-II depression score with baseline CRP levels (t = 0.35, p = 0.729) and postoperative “early”: (t = 1.0, p = 0.318) and “persistent” (t = 0.13, p = 0.899) CRP response resulted in no significant association. There were no significant predictors in the linear regression model of cross-sectional relationship between the BDI-II depression score and basal CRP levels. Preoperative hemoglobin levels (t = 2.85, p = 0.005) were the only significant predictors of early CRP change (Table 6), while current smoking (t = −3.09, p = 0.002) and (post)operative infection (t = 3.31, p = 0.001) were the only predictors of persistent CRP change after Bonferroni correction was applied (Table 7).

Table 6.
The predictive value of the BDI-II depression score on early CRP response.

Table 7.
The predictive value of the BDI-II scores on persistent CRP response.
3.4. Mediation Analysis
The mediation models were performed to illustrate to the extent to which patients’ postoperative hospital LOS (dependent outcome variable) related to their BDI-II depressive symptom scores (independent, predictor variable) and whether the relationship was mediated by early and persistent postoperative CRP changes (mediator variables) or if patients’ postoperative early (1–3 days postoperatively) and persistent (4–6 days post-operatively) CRP changes affected the relationship between their BDI-II depression symptom score and the postoperative hospital LOS. In Figure 2 and Figure 3, pathway c’ represents the direct effect of the BDI-II score on postoperative hospital LOS after controlling for mediators, while pathway a1b1 represents the total indirect pathway of the mediators. Both the direct and indirect pathways in the presented models were non-significant. This indicates the lack of association of the BDI-II depression score with the length of postoperative hospital LOS and the non-existence of the mediation role of postoperative CRP levels. Nevertheless, a higher persistent CRP change (Figure 3) was proven as a significant predictor of postoperative hospital LOS in both mediation models as presented. As shown in Figure 2 and Figure 3, significant contributors to early and persistent CRP response were smoking status and postoperative atrial fibrillation and, in the case of persistent CRP change, concomitant (post)operative infection.
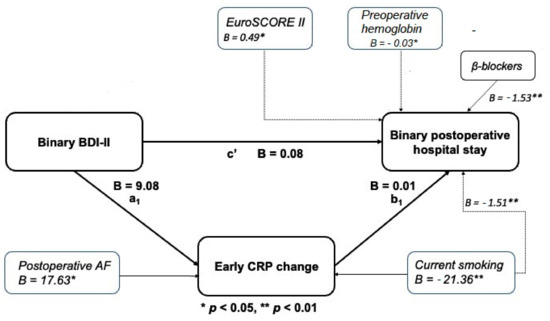
Figure 2.
Mediation model of baseline BDI-II score and length of stay through change in early CRP.
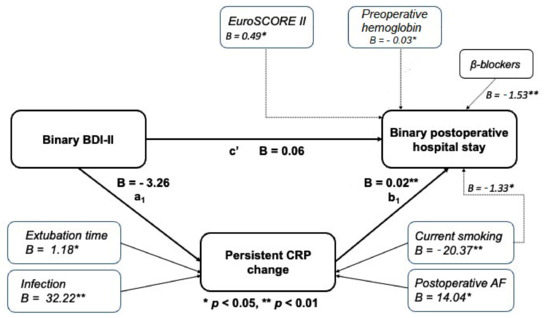
Figure 3.
Mediation model of baseline BDI-II score and length of stay through change in persistent CRP.
4. Discussion
The present study aimed to examine the role of preoperative and postoperative C-reactive protein (CRP) levels in mediating the association between preoperative depression symptoms and postoperative length of stay in patients undergoing coronary artery bypass grafting (CABG). No significant association between elevated BDI-II (>13) depression symptom scores and more extended (>7 days) postoperative hospital LOS was detected. Although the higher persistent CRP response was significantly associated with a longer postoperative hospital stay, no significant effect of higher BDI-II scores on early (1–3 days post-surgery) or persistent (4–6 days post-surgery) CRP response, or vice versa, was found. In our cohort, 12.3% of patients exhibited mild-to-severe depressive symptoms measured preoperatively by the BDI-II depression inventory. The obtained frequencies are within the range of 0.6% to 60% of previously published data on the prevalence of depression symptoms before CABG surgery [10,45,46,47,48,50]. This variability in reported estimates throughout the corresponding literature may be attributed to one or more of the following causes: clinical and demographic differences between patient cohorts, diverse inclusion and exclusion criteria, differences in questionnaires or clinical interviews used for measurement, different timing of measurement, and different cut-off values used for determining high or low levels of depression.
Poole et al. reported that CABG patients with elevated preoperative Beck Depression Inventory (BDI) scores had significantly higher odds of a prolonged hospital stay of greater than one week when compared with the non-depressive symptom group [48]. However, no Bonferroni or any other correction for multiple comparisons was applied in their study. The cut-off value for the BDI-II depression score was set at ≤10 for the non-depressive and >10 for the depressive CABG patient group. Notably, no statistically significant association between binary BDI-II values and hospital LOS was detected when the same cut-off values were applied to our cohort of CABG patients. The negative influence of higher depressive symptom scores on CABG patient outcomes and longer hospital stay was also reported in several other studies [45,46,47]. Thus, AbuRuz et al. (2021.) reported that every one-unit increase in preoperative depressive symptoms in their patients’ cohort resulted in an increased length of hospital stay by 0.37 days [45]. Furthermore, in their cohort, the female patients had higher levels of depressive symptoms compared to male patients, while being female increased the hospital LOS by 0.18 days [45,46]. The higher rate of preoperative depression among female CABG patients was also reported by Yang et al. (2012.) and Poole et al. (2014.) [48,50].
In our patient cohort, female CABG patients were also more prone to preoperative depression and extended hospital stay than male patients. However, that gender discrepancy was not statistically significant after applying the Bonferroni correction for multiple comparisons. Although non-significant, gender related association of prolonged hospital stay with higher depression score was also underlined by separate analysis of male and female patient subgroups within our cohort of CABG patients with females having more than a four times higher odds ratio for prolonged hospital stay compared with the male subgroup that exhibited a negative association. This coincides with the differences in the prevalence of depression between men and women and confirms the theory of the gender gap in depression etiology [10,48,50].
According to the available literature, the presence of preoperative depression increased the odds of other adverse outcomes for CABG patients, including a higher incidence of postoperative inflammation, decreased wound healing, and depressed patient resistance, all of which have a negative impact on their postoperative hospital LOS [11,49,61]. These adverse outcomes have a strong correlation with the level of CRP and other inflammatory markers [48,62]. As already stated, inflammation, the key regulator of CRP synthesis, has a prominent role in the etiology of atherosclerotic cardiovascular diseases, including CAD [12,13].
In the study by Poole et al., (2014) the association of a higher BDI score with increased odds of prolonged hospital stay was partially mediated by elevated early (baseline to 1–3 days post-operatively) and persistent (baseline to 4–8 days post-operatively) CRP response [48]. One increase in early CRP response in their patient cohort resulted in a 1% increase in the greater odds of a more extended hospital stay. In contrast, the same increase in persistent CRP response resulted in 1.3% higher odds. On the contrary, in our CABG cohort, only the persistent CRP levels were associated with a prolonged hospital LOS, with an increase of one unit in persistent CRP resulting in a 1.7% increase in the higher odds for an in-hospital stay greater than one week. Interestingly, separate analysis of gender subgroups within our cohort of CABG patients revealed significant association of persistent CRP change only among the male subgroup while none of CRP measures was associated with prolonged hospital stay in female patient subgroup.
Elevated baseline CRP has also been associated with extended postoperative hospital LOS of CABG patients [63]. In the study reported by Yang et al. (2012), elevated baseline CRP levels were identified as an independent predictor for depression (assessed with the PHQ-9 test score) present preoperatively and up to six months after the CABG procedure [50]. However, no such association was detected in our cohort of CABG patients. Poole et al. also reported the lack of association between BDI score and preoperative CRP levels [49].
In our cohort of CABG patients, smoking status was one of the major intensity indicators in early and persistent CRP response. At the same time, the presence of (post)operative infection was statistically associated only with the persistent CRP response.
In the present study, smoking status and EuroSCORE-II were associated with a postoperative hospital stay longer than one week. The association between depression and smoking in CABG patients has been reported in several studies [10,64,65]. In addition, Abbasi et al. reported that cigarette smoking was significantly more frequent in depressed young male patients with documented CAD [65].
Most of the variables included in the EuroSCORE-II have been related to anxiety and depression. Thus, EuroSCORE II operational risk values might also be accountable for risks originating from preoperative anxiety and depressive symptoms [66]. This link between baseline depression and patients’ EuroSCORE risk stratification should be further explored. Furthermore, Cromhout et al. (2019) also believe that developing a prognostic screening tool involving emotional, behavioral, social, and functional factors is necessary to complement the risk assessment by EuroSCORE [67].
Although it seems that the BDI-II scale represents a sound path for detecting depression in CABG patients, the need for careful adjustment of cut-off points and evidence-based interpretation of score values should be addressed before its usage in clinical decision-making [68].
Our study has several strengths. We examined patients undergoing CABG at a single hospital and removed the influence of inter-hospital variations in discharge policy. The study’s design allowed for a temporal relationship between depression, preoperative, and postoperative CRP levels, and length of stay to be analyzed. In addition, the repeated assessment of CRP levels allowed us to test the contribution of both preoperative and postoperative inflammation to the postoperative recovery of CABG patients. There were also some important limitations to the present study. Firstly, the study encompasses a too-small group of patients to observe the impact on operative risk and patient mortality. Furthermore, due to the relatively small cohort of CABG patients analyzed in this study, the 95% confidence intervals used to present the results of logistic regression analyses were too wide to reach a firm conclusion. Secondly, other non-medical factors, such as social housing constraints, also likely have a role in determining hospital LOS; such confounders were not able to be considered in our analyses.
5. Conclusions
Contrary to the previous reports, the present study revealed no significant association between elevated preoperative BDI-II (>13) depression symptom scores and the extent of postoperative hospital LOS in patients subjected to CABG. Additionally, the patient’s preoperative CRP levels and subsequent early (1–3 days postoperatively) and persistent (4–6 days post-operatively) CRP responses to CABG surgery were not associated with preoperative depressive symptoms measured by the BDI-II questionary. Nevertheless, higher persistent CRP responses were significantly associated with a prolonged postoperative hospital LOS; notably, when the male and female patient subgroups were analyzed separately that remained significant only for the male patient subgroup. Significant contributors to early and persistent CRP response in our cohort of CABG patients were smoking status and postoperative atrial fibrillation and, in the case of the persistent CRP change, concomitant (post)operative infection. Further work on a larger cohort of CABG patients is needed to explore the extent and pathways through which depression might influence the postoperative recovery of CABG patients.
Author Contributions
S.I., V.C., A.M.P., T.S. and B.B. made substantial contributions to the conception, design, and acquisition of data. S.I., F.P. and T.S. contributed to the analysis and interpretation of data. S.I., F.P., V.K. and M.P. were involved in drafting the manuscript and revising it critically for important intellectual content. V.C. and B.B. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present study was a prospective observational (non-interventional) study. All participants signed the informed consent. The study was conducted by the Declaration of Helsinki. The Ethics Committee of the University Hospital Centre (UHC) Zagreb, Croatia, approved the study protocol (Approval number of the Ethics Committee UHC Zagreb, Class: 8.1-11/84-3).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Most of the data generated and/or analyzed during the current study is included in the paper. The demographic and clinical characteristics of CABG patients and statistical datasets used and/or analyzed during the present study is available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Diodato, M.; Chedrawy, E.G. Coronary artery bypass graft surgery: The past, present, and future of myocardial revascularization. Surg Res. Pract. 2014, 2014, 726158. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Khawaja, I.S.; Westermeyer, J.J.; Gajwani, P.; Feinstein, R.E. Depression and coronary artery disease: The association, mechanisms, and therapeutic implications. Psychiatry 2009, 6, 38–51. [Google Scholar] [PubMed]
- Serrano, C.V., Jr.; Setani, K.T.; Andrei, A.M.; Fraguas, R. Association between depression and development of coronary artery disease: Pathophysiologic and diagnostic implications. Vasc. Health Risk Manag. 2011, 7, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Parissis, J.T.; Fountoulaki, K.; Filippatos, G.; Adamopoulos, S.; Paraskevaidis, I.; Kremastinos, D. Depression in coronary artery disease: Novel pathophysiologic mechanisms and therapeutic implications. Int. J. Cardiol. 2007, 116, 153–160. [Google Scholar] [CrossRef]
- Celano, C.M.; Huffman, J.C. Depression and cardiac disease: A review. Cardiol. Rev. 2011, 19, 130–142. [Google Scholar] [CrossRef]
- Rudisch, B.; Nemeroff, C.B. Epidemiology of comorbid coronary artery disease and depression. Biol. Psychiatry 2003, 54, 227–240. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Abu Ejheisheh, M.; Suleiman-Martos, N.; Membrive-Jiménez, M.J.; Velando-Soriano, A.; Schmidt-RioValle, J.; Gómez-Urquiza, J.L. Prevalence of Depression in Coronary Artery Bypass Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 909. [Google Scholar] [CrossRef]
- Stenman, M.; Holzmann, M.J.; Sartipy, U. Association between preoperative depression and long-term survival following coronary artery bypass surgery—A systematic review and meta-analysis. Int. J. Cardiol. 2016, 222, 462–466. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Kollia, N.; Tousoulis, D. The link between depression and atherosclerosis through the pathways of inflammation and endothelium dysfunction. Maturitas 2018, 109, 1–5. [Google Scholar] [CrossRef]
- Baghai, T.C.; Varallo-Bedarida, G.; Born, C.; Häfner, S.; Schüle, C.; Eser, D.; Rupprecht, R. Classical Risk Factors and Inflammatory Biomarkers: One of the Missing Biological Links between Cardiovascular Disease and Major Depressive Disorder. Int. J. Mol. Sci. 2018, 19, 1740. [Google Scholar] [CrossRef]
- Morris, G.; Puri, B.K.; Olive, L.; Carvalho, A.; Berk, M.; Walder, K.; Gustad, L.T.; Maes, M. Endothelial dysfunction in neuroprogressive disorders—Causes and suggested treatments. BMC Med. 2020, 18, 305. [Google Scholar] [CrossRef]
- Ain, Q.U.; Sarfraz, M.; Prasesti, G.K.; Dewi, T.I.; Kurniati, N.F. Confounders in Identification and Analysis of Inflammatory Biomarkers in Cardiovascular Diseases. Biomolecules 2021, 11, 1464. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Tsetsekou, E.; Papageorgiou, C.; Christodoulou, G.; Stefanadis, C. Inflammation, coagulation, and depressive symptomatology in cardio-vascular disease-free people; the ATTICA study. Eur. Heart J. 2004, 25, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef]
- Chávez-Castillo, M.; Nava, M.; Ortega, Á.; Rojas, M.; Núñez, V.; Salazar, J.; Rojas-Quintero, J. Depression as an Immunometabolic Disorder: Exploring Shared Pharma-cotherapeutics with Cardiovascular Disease. Curr. Neuropharmacol. 2020, 18, 1138–1153. [Google Scholar] [CrossRef]
- Strawbridge, R.; Arnone, D.; Danese, A.; Papadopoulos, A.; Vives, A.H.; Cleare, A.J. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Bin Wei, Y.; Strawbridge, R.; Bao, Y.; Chang, S.; Shi, L.; Que, J.; Gadad, B.S.; Trivedi, M.H.; Kelsoe, J.R.; et al. Peripheral cytokine levels and response to antidepressant treatment in depression: A systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; De Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; De Andrade, N.Q.; Morris, G.; Fernandes, B.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.F.; Watkins, L.R. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 1998, 105, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Schott, L.L.; Bromberger, J.T.; Cyranowski, J.M.; Everson-Rose, S.A.; Sowers, M. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav. Immun. 2010, 24, 96–101. [Google Scholar] [CrossRef]
- Shimbo, D.; Chaplin, W.; Crossman, D.; Haas, D.; Davidson, K.W. Role of Depression and Inflammation in Incident Coronary Heart Disease Events. Am. J. Cardiol. 2005, 96, 1016–1021. [Google Scholar] [CrossRef]
- Stewart, J.C.; Rand, K.L.; Muldoon, M.F.; Kamarck, T.W. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav. Immun. 2009, 23, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, D.; Kivimaki, M.; Brunner, E.; Elovainio, M.; De Vogli, R.; Steptoe, A.; Kumari, M.; Lowe, G.D.; Rumley, A.; Marmot, M.; et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009, 39, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Duivis, H.E.; De Jonge, P.; Penninx, B.W.; Na, B.Y.; Cohen, B.E.; Whooley, M.A. Depressive Symptoms, Health Behaviors, and Subsequent Inflammation in Patients with Coronary Heart Disease: Prospective Findings from the Heart and Soul Study. Am. J. Psychiatry 2011, 168, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, L.; Pariante, C.M.; Palacios, J.E.; Tylee, A.; Carvalho, L.A.; Viganò, C.A.; Nikkheslat, N. Inflammation associated with coronary heart disease predicts onset of depression in a three-year prospective follow-up: A preliminary study. Brain Behav. Immun. 2019, 81, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.F.; LeDue, T.B.; Winyard, P.G.; Willoughby, D.A. Laboratory Assessment of the Acute Phase Response Using CRP as a Model. Methods Mol Biol. 2003, 225, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Moutachakkir, M.; Baraou, A.; Boukhira, A.; Chellak, S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann. Biol. Clin. 2017, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, C.; De Loecker, I.; Donadello, K.; Moussa, M.D.; Markowicz, S.; Gullo, A.; Vincent, J.-L. C-Reactive Protein Kinetics after Major Surgery. Anesth. Analg. 2014, 119, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nielsen, S.F.; Nordestgaard, B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry 2013, 70, 176–184. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Pearson, R.M.; Zammit, S.; Lewis, G.; Jones, P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psychiatry 2014, 71, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Zalli, A.; Jovanova, O.; Hoogendijk, W.J.G.; Tiemeier, H.; de Carvalho, L.A. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology 2016, 233, 1669–1678. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Zuber, V.; Rees, J.; Carvalho, L.; Mason, A.M.; Foley, C.N.; Burgess, S. Shared mechanisms between coronary heart disease and depression: Findings from a large UK general population-based cohort. Mol. Psychiatry 2020, 25, 1477–1486. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Abolhasani, S.; Shahbazloo, S.V.; Saadati, H.M.; Mahmoodi, N.; Khanbabaei, N. Evaluation of Serum Levels of Inflammation, Fibrinolysis and Oxidative Stress Markers in Coronary Artery Disease Prediction: A Cross-Sectional Study. Arq. Bras. Cardiol. 2019, 113, 667–674. [Google Scholar] [CrossRef]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2004, 350, 1387–1397. [Google Scholar] [CrossRef]
- Van Wijk, D.F.; Boekholdt, S.M.; Wareham, N.J.; Ahmadi-Abhari, S.; Kastelein, J.J.; Stroes, E.S.; Khaw, K.T. C-reactive protein, fatal and nonfatal coronary artery disease, stroke, and peripheral artery disease in the prospective EPIC-Norfolk cohort study. Arterioscler. Thromb. Vasc. Biology. 2013, 33, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, D.; Zhao, Y.; Liu, D.; Li, Q.; Guo, C.; Tian, G.; Han, M.; Qie, R.; Huang, S.; et al. Association between serum level of C-reactive protein and risk of cardiovascular events based on cohort studies. J. Hum. Hypertens. 2021, 35, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Strang, F.; Schunkert, H. C-reactive protein and coronary heart disease: All said—Is not it? Mediators Inflamm. 2014, 2014, 757123. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, Y.; Liu, E. C-reactive protein and cardiovascular disease: From animal studies to the clinic (Review). Exp. Ther. Med. 2020, 20, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- AbuRuz, M.E.; Momani, A.; Shajrawi, A. The Association Between Depressive Symptoms and Length of Hospital Stay Following Coronary Artery Bypass Graft is Moderated by Perceived Control. Risk Manag. Health Policy 2021, 14, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- AbuRuz, M.E. Pre-operative depression predicted longer hospital length of stay among patients undergoing coronary artery bypass graft surgery. Risk Manag. Healthc. Policy 2019, 12, 75–83. [Google Scholar] [CrossRef]
- Oxlad, M.; Stubberfield, J.; Stuklis, R.; Edwards, J.; Wade, T.D. Psychological Risk Factors for Increased Post-Operative Length of Hospital Stay Following Coronary Artery Bypass Graft Surgery. J. Behav. Med. 2006, 29, 179–190. [Google Scholar] [CrossRef]
- Poole, L.; Kidd, T.; Leigh, E.; Ronaldson, A.; Jahangiri, M.; Steptoe, A. Depression, C-reactive protein and length of post-operative hospital stay in coronary artery bypass graft surgery patients. Brain Behav. Immun. 2014, 37, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.; Leigh, E.; Kidd, T.; Ronaldson, A.; Jahangiri, M.; Steptoe, A. The combined association of depression and socioeconomic status with length of post-operative hospital stay following coronary artery bypass graft surgery: Data from a prospective cohort study. J. Psychosom. Res. 2014, 76, 34–40. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Zhang, L.; Hou, J.; Yuan, X.; Hu, S.; Zheng, Z. Preoperative high-sensitivity C-reactive protein predicts depression in patients undergoing coronary artery bypass surgery: A single-center prospective observational study. J. Thorac. Cardiovasc. Surg. 2012, 144, 500–505. [Google Scholar] [CrossRef][Green Version]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Jakšić, N.; Ivezić, E.; Jokić-Begić, N.; Surányi, Z.; Stojanović-Špehar, S. Factorial and Diagnostic Validity of the Beck Depression Inventory-II (BDI-II) in Croatian Primary Health Care. J. Clin. Psychol. Med. Settings 2013, 20, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Galai, N.; Israeli, A.; Zitser-Gurevich, Y.; Simchen, E. Is discharge policy a balanced decision between clinical considerations and hospital ownership policy? The CABG example. J. Thorac. Cardiovasc. Surg. 2003, 126, 1018–1025. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744; discussion 44–45. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. Asymptotic and Resampling Strategies for Assessing and Comparing Indirect Effects in Multiple Mediator Models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Abu-Bader, S.; Jones, T.V. Statistical mediation analysis using the sobel test and hayes SPSS process macro. Int. J. Quant. Qual. Res. Methods 2021, 9, 42–61. [Google Scholar]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.; Ashbaugh, A.R. (Eds.) Simple and parallel mediation: A tutorial exploring anxiety sensitivity, sensation seeking, and gender. Quant. Methods Psychol. 2017, 13, 148–165. [Google Scholar] [CrossRef]
- Stenman, M.; Holzmann, M.J.; Sartipy, U. Relation of Major Depression to Survival After Coronary Artery Bypass Grafting. Am. J. Cardiol. 2014, 114, 698–703. [Google Scholar] [CrossRef]
- Gegenava, T.; Gegenava, M.; Kavtaradze, G. C-reactive protein level correlation with depression and anxiety among patients with coronary artery disease. Georgian Med. News 2011, 194, 34–37. [Google Scholar]
- Perry, T.E.; Muehlschlegel, J.D.; Liu, K.Y.; Fox, A.A.; Collard, C.D.; Body, S.C. Preoperative C-reactive protein predicts long-term mortality and hospital length of stay after primary, nonemergent coronary artery bypass grafting. Anesthesiology 2010, 112, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, L.B.; Wood, T.; Cheng, A.; Khan, A.R. Pre-existing psychological depression confers increased risk of adverse cardiovascular outcomes following cardiac surgery: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2017, 154, 1578–1586.e1. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.H.; Kassaian, S.E.; Sadeghian, S.; Karimi, A.; Saadat, S.; Peyvandi, F.; Rosendaal, F. Factors Associated with Depressive Symptoms in Young Adults with Coronary Artery Disease: Tehran Heart Center’s Premature Coronary Atherosclerosis Cohort (THC-PAC) Study. Iran J. Psychiatry 2016, 11, 214–223. [Google Scholar] [PubMed]
- Messerotti Benvenuti, S.; Palomba, D.; Zanatta, P.; Mazzarolo, A.P.; Valfrè, C. Biomedical and psychological risk in cardiac surgery: Is EuroSCORE a more comprehensive risk measure than Stroke Index? Eur. J. Cardiothorac. Surg. 2011, 39, e102–e106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cromhout, P.F.; Berg, S.K.; Moons, P.; Damgaard, S.; Nashef, S.; Thygesen, L. Updating EuroSCORE by including emotional, behavioural, social and functional factors to the risk assessment of patients undergoing cardiac surgery: A study protocol. BMJ Open 2019, 9, e026745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Gorenstein, C. Assessment of depression in medical patients: A systematic review of the utility of the Beck Depression Inventory-II. Clinics 2013, 68, 1274–1287. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).