Fluorescence Correlation Spectroscopy Measurement Based on Fiber Optics for Biological Materials
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
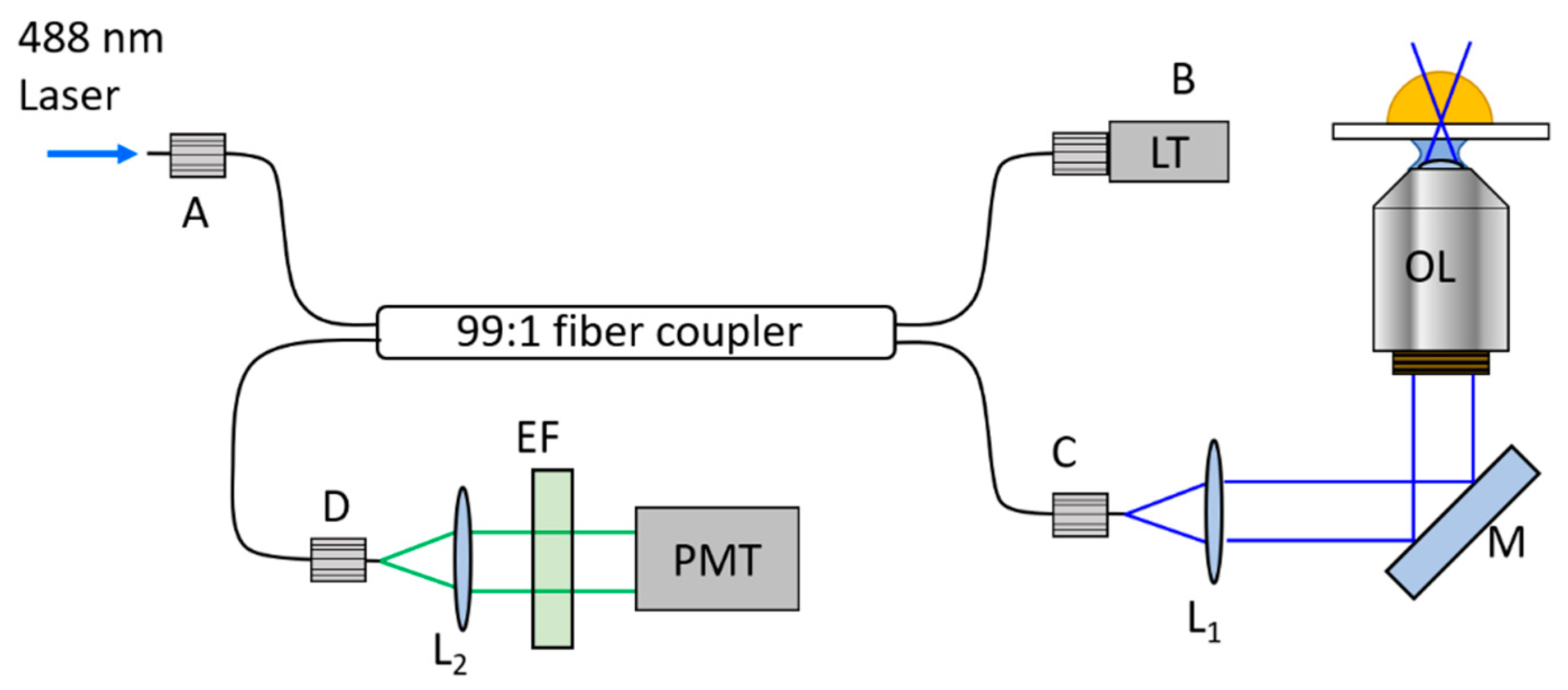
2.1. Fiber-Optic Based Fluorescence Correlation Spectroscopy (FB-FCS)
2.2. Background Intensity Correction
2.3. Fitting Analysis
3. Results and Discussion
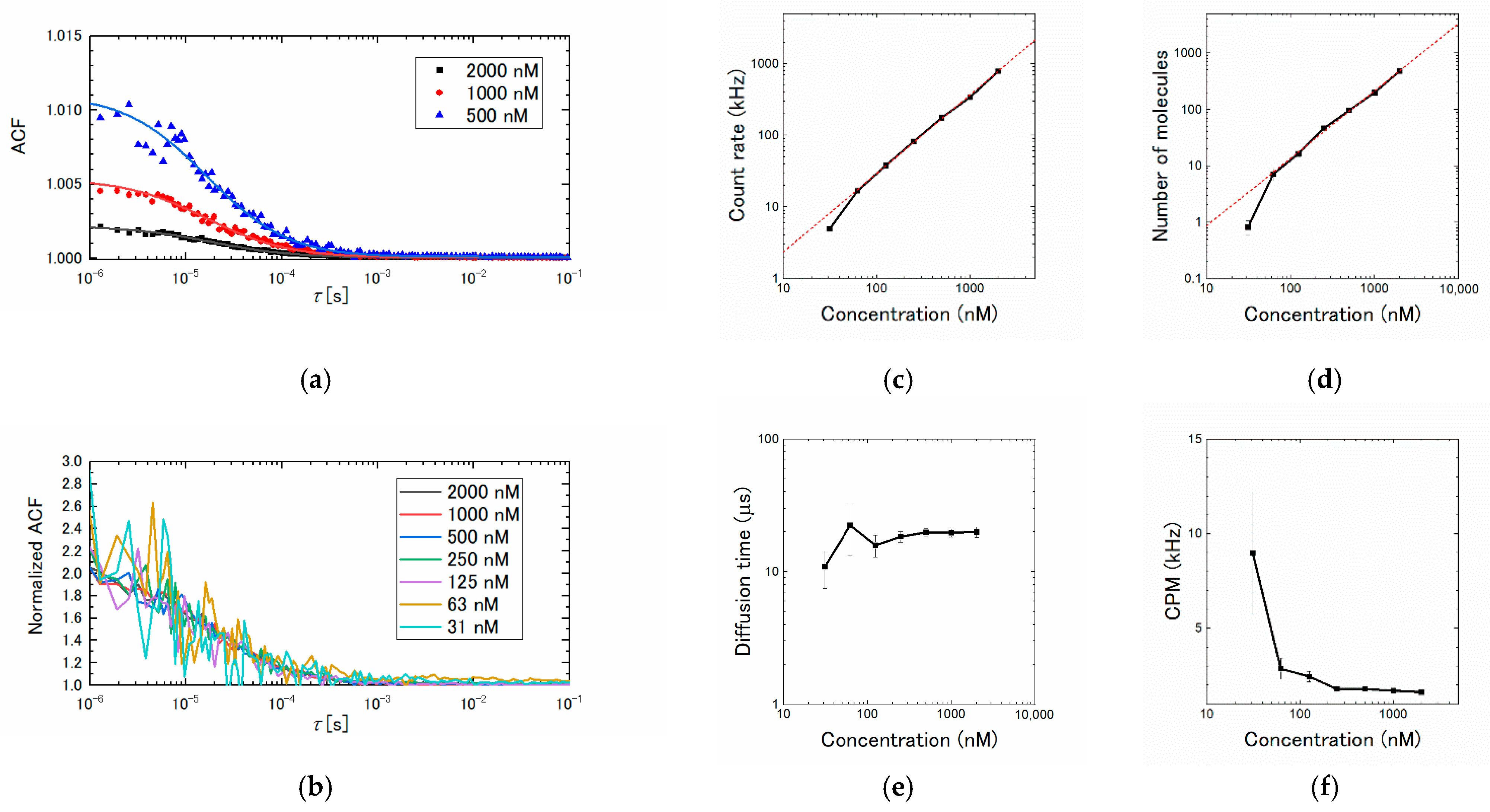
3.1. Concentration Dependence
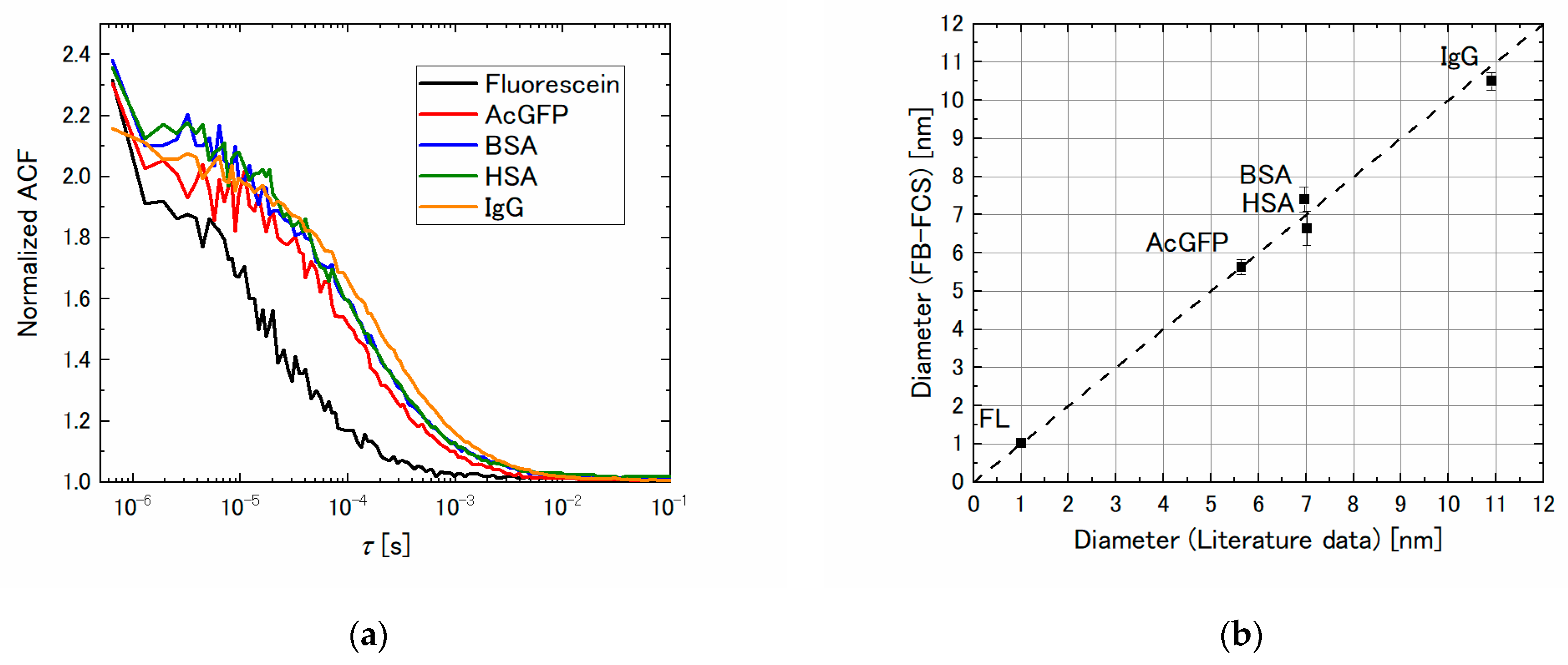
3.2. Molecular Size Dependence
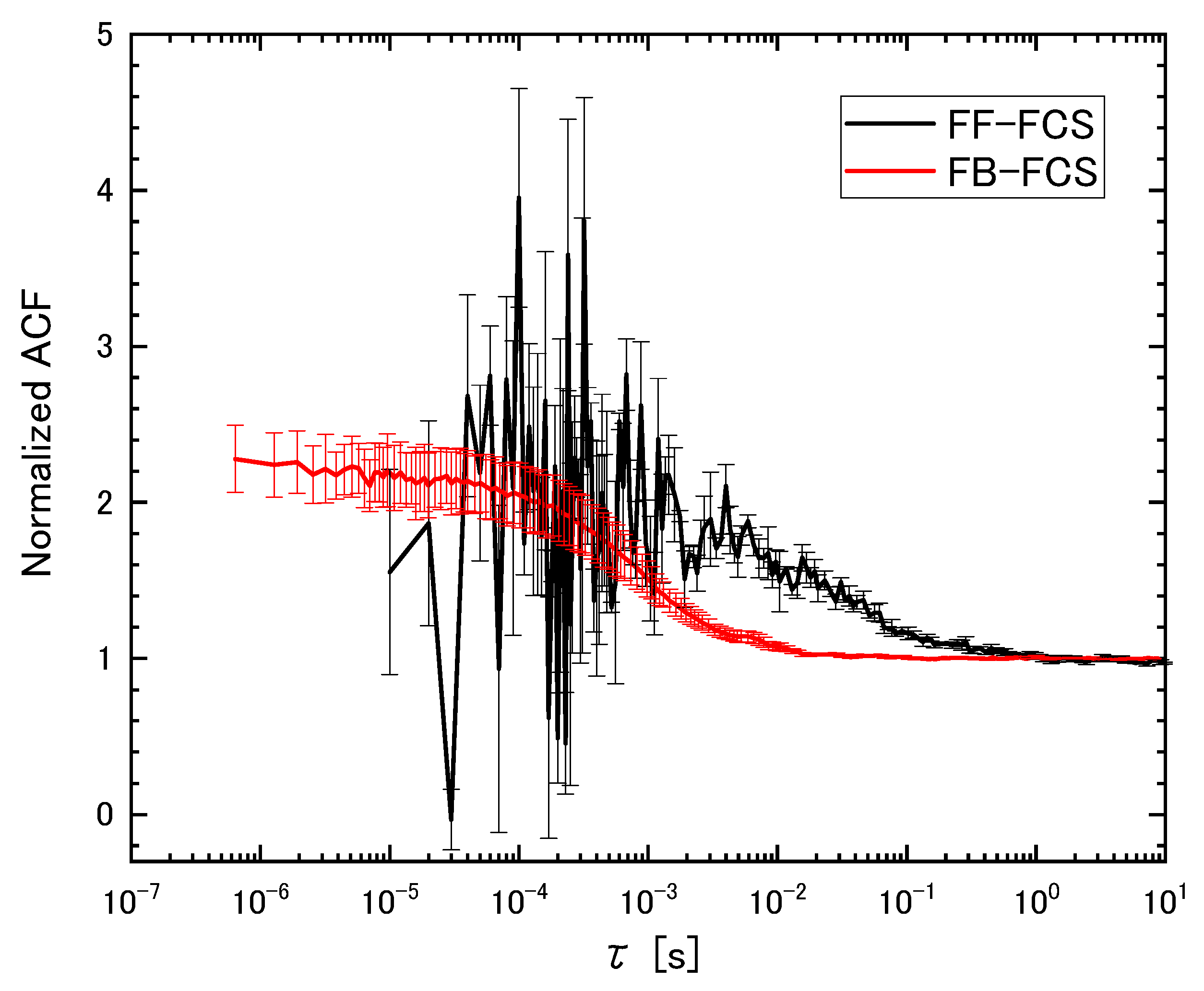
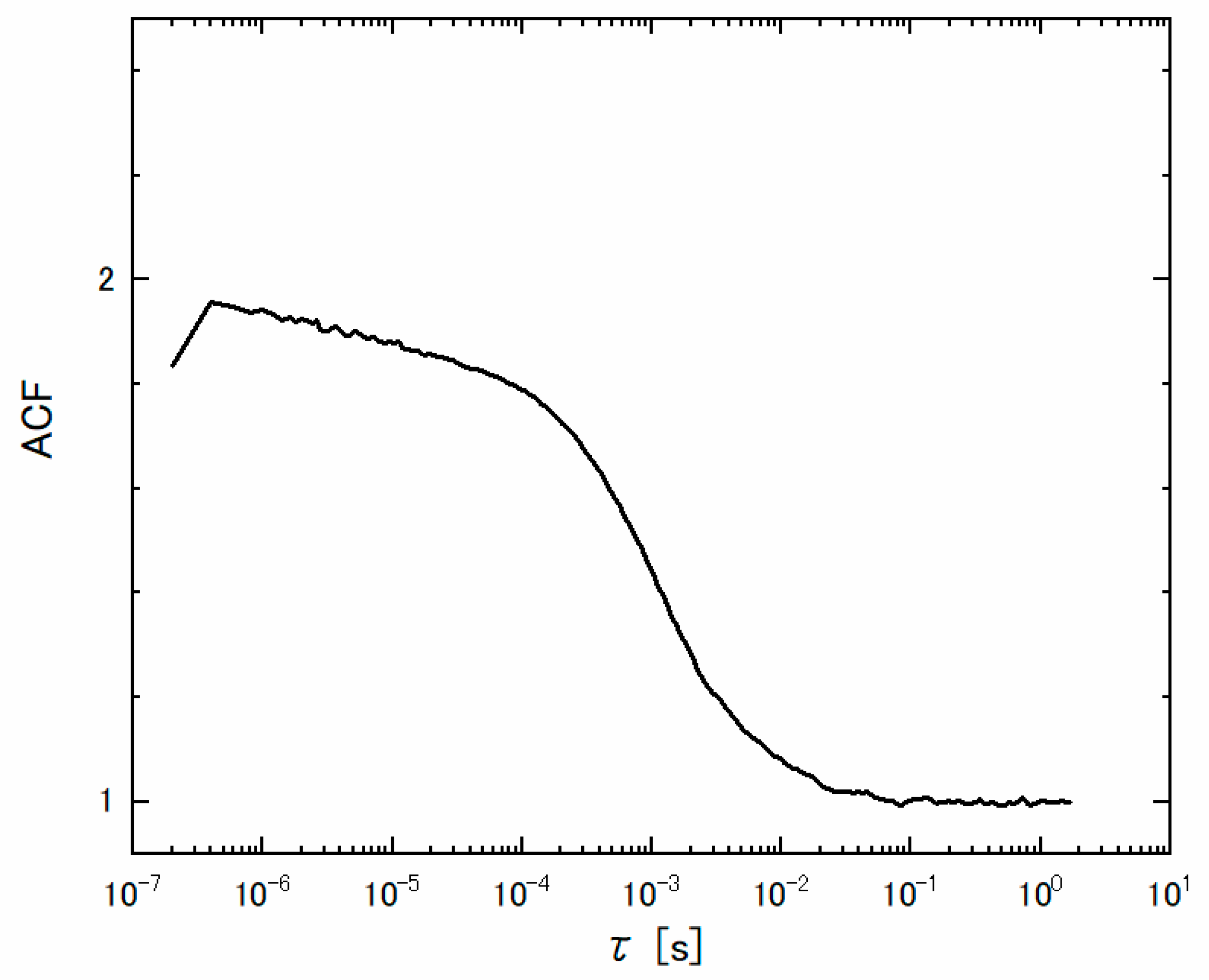
3.3. Robustness
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| τD [ms] | CPM [kHz] | CPM/LP [kHz/μW] | |
|---|---|---|---|
| FF-FCS | 18.26 ± 1.95 | 0.17 ± 0.01 | 0.023 ± 0.002 |
| FB-FCS | 0.57 ± 0.02 | 80.16 ± 2.42 | 27.35 ± 0.82 |
| Conv. FCS | 1.07 (n = 1) | 65.57 (n = 1) | 43.71 (n = 1) |
References
- Elson, E.L.; Magde, D. Fluorescence correlation spectroscopy. I. Conceptual basis and theory. Biopolymers 1974, 13, 1–27. [Google Scholar] [CrossRef]
- Aragón, S.R.; Pecora, R. Fluorescence correlation spectroscopy as a probe of molecular dynamics. J. Chem. Phys. 1976, 64, 1791–1803. [Google Scholar] [CrossRef]
- Rigler, R.; Mets, Ü.; Widengren, J.; Kask, P. Fluorescence correlation spectroscopy with high count rate and low background: Analysis of translational diffusion. Eur. Biophys. J. 1993, 22, 169–175. [Google Scholar] [CrossRef]
- Rauer, B.; Neumann, E.; Widengren, J.; Rigler, R. Fluorescence correlation spectrometry of the interaction kinetics of tetramethylrhodamin α-bungarotoxin with Torpedo californica acetylcholine receptor. Biophys. Chem. 1996, 58. [Google Scholar] [CrossRef]
- Oasa, S.; Sasaki, A.; Yamamoto, J.; Mikuni, S.; Kinjo, M. Homodimerization of glucocorticoid receptor from single cells investigated using fluorescence correlation spectroscopy and microwells. FEBS Lett. 2015, 589, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Knapp, M.; Puchinger, M.G.; Shahzad, A.; Gaubitzer, E.; Shen, A.D.; Koehler, G. Using fluorescence correlation spectroscopy (FCS) for IFN-g detection: A preliminary study. J. Immunol. Methods 2014, 407, 35–39. [Google Scholar] [CrossRef]
- Chatterjee, M.; Nöding, B.; Willemse, E.A.J.; Koel-Simmelink, M.J.A.; van der Flier, W.M.; Schild, D.; Teunissen, C.E. Detection of contactin-2 in cerebrospinal fluid (CSF) of patients with Alzheimer’s disease using Fluorescence Correlation Spectroscopy (FCS). Clin. Biochem. 2017, 50, 1061–1066. [Google Scholar] [CrossRef]
- Fujii, F.; Horiuchi, M.; Ueno, M.; Sakata, H.; Nagao, I.; Tamura, M.; Kinjo, M. Detection of prion protein immune complex for bovine spongiform encephalopathy diagnosis using fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy. Anal. Biochem. 2007, 370, 131–141. [Google Scholar] [CrossRef]
- Sahoo, B.; Sil, T.B.; Karmakar, B.; Garai, K. A Fluorescence Correlation Spectrometer for Measurements in Cuvettes. Biophys. J. 2018, 115, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.K.; Chacko, J.V.; Sreenivasan, V.K.A.; Nag, S.; Maiti, S. Ultracompact alignment-free single molecule fluorescence device with a foldable light path. J. Biomed. Opt. 2011, 16, 025004. [Google Scholar] [CrossRef]
- Garai, K.; Muralidhar, M.; Maiti, S. Fiber-optic fluorescence correlation spectrometer. Appl. Opt. 2006, 45, 7538–7542. [Google Scholar] [CrossRef]
- Yamamoto, J.; Kinjo, M. Full fiber-optic fluorescence correlation spectroscopy. Opt. Express 2019, 27, 14835. [Google Scholar] [CrossRef]
- Krieger, J.W.; Singh, A.P.; Bag, N.; Garbe, C.S.; Saunders, T.E.; Langowski, J.; Wohland, T. Imaging fluorescence (cross-) correlation spectroscopy in live cells and organisms. Nat. Protoc. 2015, 10, 1948–1974. [Google Scholar] [CrossRef]
- Widengren, J.; Mets, U.; Rigler, R. Fluorescence correlation spectroscopy of triplet states in solution: A theoretical and experimental study. J. Phys. Chem. 1995, 99, 13368–13379. [Google Scholar] [CrossRef]
- Kim, S.A.; Heinze, K.G.; Schwille, P. Fluorescence correlation spectroscopy in living cells. Nat. Methods 2007, 4, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Saffarian, S.; Elson, E.L. Statistical Analysis of Fluorescence Correlation Spectroscopy: The Standard Deviation and Bias. Biophys. J. 2003, 84, 2030–2042. [Google Scholar] [CrossRef] [Green Version]
- Petrášek, Z.; Schwille, P. Precise Measurement of Diffusion Coefficients using Scanning Fluorescence Correlation Spectroscopy. Biophys. J. 2008, 94, 1437–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; Yamamoto, J.; Kinjo, M.; Noda, N. Absolute Quantification of RNA Molecules Using Fluorescence Correlation Spectroscopy with Certified Reference Materials. Anal. Chem. 2018, 90, 10865–10871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, M.B.; Tipton, D.L.; Barkley, M.D.; Russo, P.S.; Blum, F.D. Dye diffusion in isotropic and liquid-crystalline aqueous (hydroxypropyl)cellulose. Macromolecules 1993, 26, 370–378. [Google Scholar] [CrossRef]
- Terry, B.R.; Robards, A.W. Hydrodynamic radius alone governs the mobility of molecules through plasmodesmata. Planta 1987, 171, 145–157. [Google Scholar] [CrossRef]
- Ikeda, S.; Nishinari, K. Intermolecular Forces in Bovine Serum Albumin Solutions Exhibiting Solidlike Mechanical Behaviors. Biomacromolecules 2000, 1, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Amani, S. Deciphering Structural Intermediates and Genotoxic Fibrillar Aggregates of Albumins: A Molecular Mechanism Underlying for Degenerative Diseases. PLoS ONE 2013, 8, e54061. [Google Scholar] [CrossRef] [Green Version]
- Bermudez, O.; Forciniti, D. Aggregation and denaturation of antibodies: A capillary electrophoresis, dynamic light scattering, and aqueous two-phase partitioning study. J. Chromatogr. B 2004, 807, 17–24. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, J.; Sasaki, A. Fluorescence Correlation Spectroscopy Measurement Based on Fiber Optics for Biological Materials. Appl. Sci. 2021, 11, 6744. https://doi.org/10.3390/app11156744
Yamamoto J, Sasaki A. Fluorescence Correlation Spectroscopy Measurement Based on Fiber Optics for Biological Materials. Applied Sciences. 2021; 11(15):6744. https://doi.org/10.3390/app11156744
Chicago/Turabian StyleYamamoto, Johtaro, and Akira Sasaki. 2021. "Fluorescence Correlation Spectroscopy Measurement Based on Fiber Optics for Biological Materials" Applied Sciences 11, no. 15: 6744. https://doi.org/10.3390/app11156744
APA StyleYamamoto, J., & Sasaki, A. (2021). Fluorescence Correlation Spectroscopy Measurement Based on Fiber Optics for Biological Materials. Applied Sciences, 11(15), 6744. https://doi.org/10.3390/app11156744







