Simple HPLC-PDA Analysis to Determine Illegal Synthetic Dyes in Herbal Medicines
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Herbal Medicines and Synthetic Dyes
2.2. Chemicals and Reagents
2.3. Preparation of the Standard Solution
2.4. Sample Preparations
2.5. HPLC-PDA Analysis Conditions
2.6. Method Validation
2.7. LC-MS/MS Analysis
2.8. Cross-Validation
2.9. Application of the Proposed Method to Real Samples
3. Results and Discussion
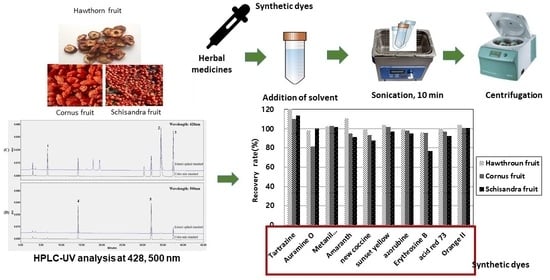
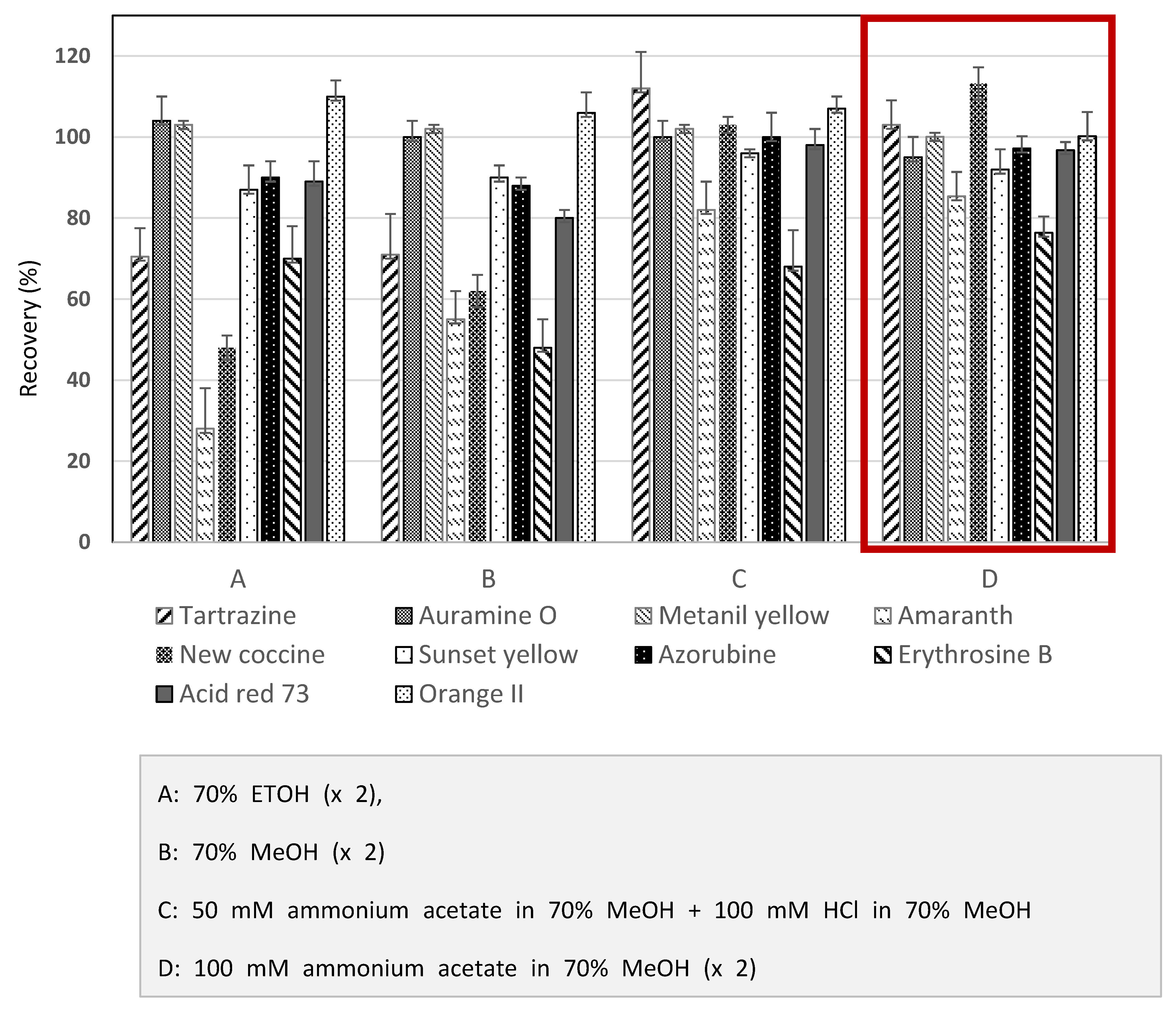
3.1. Optimization of Extraction
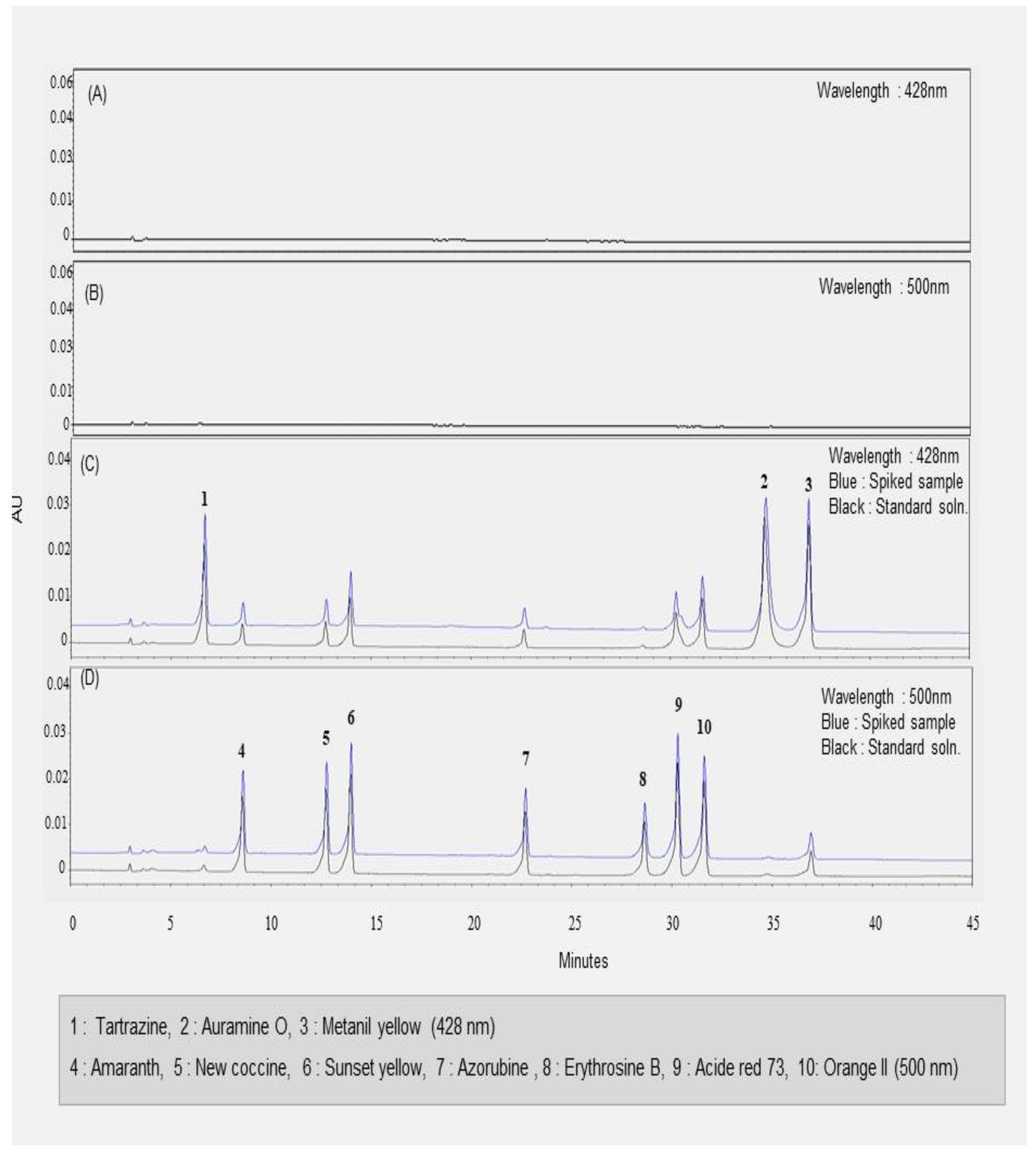
3.2. Selectivity of the Proposed Method
3.3. Linearity and Sensitivity
3.4. Accuracy and Precision
3.5. Confirmation by LC-MS/MS Analysis
3.6. Cross-Validation
3.7. Application of the Proposed Method to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esen, B.; Oymak, T.; Dural, E. Determination of food colorings in pharmaceutical preparations and food additives by a validated HPLC method. Int. J. Sci. Eng. Res. 2018, 9, 72–76. [Google Scholar]
- Pérez-Ibarbia, L.; Majdanski, T.; Schubert, S.; Windhab, N.; Schubert, U.S. Safety and regulatory review of dyes commonly used as excipients in pharmaceutical and nutraceutical applications. Eur. J. Pharm. Sci. 2016, 10, 264–273. [Google Scholar] [CrossRef] [PubMed]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behavior in 3-year-old and 8/9-year-old children in the community: A randomized, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Xu, M.; Huang, B.; Gao, F.; Zhai, C.; Yang, Y.; Li, L.; Wang, W.; Shi, L. Assessment of adulterated traditional Chinese medicines in China:2003–2017. Front. Pharmacol. 2019, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, K.; Xing, S.; Sun, H.; Shi, F.; Zhang, G.; Sun, H. Application of quadrupole-Orbitrap high-resolution mass spectrometry in rapid screening and identification of synthetic dyes in herbal medicines. Eur. J. Mass Spectrom. 2019, 25, 419–427. [Google Scholar] [CrossRef]
- YunNan Institute for Food and Drug Control (IFDC); SiChuan IFDC; JiLin IFDC. Supplementary Inspection Method for Lemon Yellow, Acid Yellow 36 and Goldamine O Test Items in Typhae Pollen of Chinese Herbal Medicine and Beverage Tablets. 2018. 03. National Institutes for Food and Drug Control (NIFDC), China. Available online: https://www.nifdc.org.cn/nifdc/bzhchx/ypjyfaxm/index.html (accessed on 27 June 2021).
- Tikhomirova, T.I.; Ramazanova, G.R.; Apyari, V.V. A hybrid sorption–spectrometric method for determination of synthetic anionic dyes in foodstuffs. Food Chem. 2017, 221, 351–355. [Google Scholar] [CrossRef]
- de Andrade, F.I.; Guedes, M.I.F.; Vieira, Í.G.P.; Mendes, F.N.P.; Rodrigues, P.A.S.; Maia, C.S.C.; Ávila, M.M.M.; de Matos Ribeiro, L. Determination of synthetic food dyes in commercial soft drinks by TLC and ion-pair HPLC. Food Chem. 2014, 157, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Benmassaoud, Y.; Villaseñor, M.J.; Salghi, R.; Jodeh, S.; Algarra, M.; Zougagh, M.; Ríos, Á. Magnetic/Non-Magnetic Argan Press Cake Nanocellulose for the Selective Extraction of Sudan Dyes in Food Samples before the Determination by Capillary Liquid Chromatograpy. Talanta 2017, 166, 63–69. [Google Scholar] [CrossRef]
- Oplatowska, M.; Elliott, C.T. Development and validation of rapid disequilibrium enzyme-linked immunosorbent assays for the detection of Methyl Yellow and Rhodamine B dyes in foods. Analyst 2011, 136, 2403–2410. [Google Scholar] [CrossRef]
- Lipskikh, O.; Korotkova, E.; Khristunova, Y.P.; Barek, J.; Kratochvil, B. Sensors for voltammetric determination of food azo dyes-a critical review. Electrochim. Acta 2018, 260, 974–985. [Google Scholar] [CrossRef]
- Karanikolopoulos, G.; Gerakis, A.; Papadopoulou, K.; Mastrantoni, I. Determination of synthetic food colorants in fish products by an HPLC-DAD method. Food Chem. 2015, 177, 197–203. [Google Scholar] [CrossRef]
- Martin, F.; Oberson, J.M.; Meschiari, M.; Munari, C. Determination of 18 water-soluble artificial dyes by LC-MS in selected matrices. Food Chem. 2016, 197, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- He, F.; He, Y.; Zheng, X.; Wang, R.; Lu, J.; Dai, Z.; Ma, S. Screening of Chemical Dyes in Traditional Chinese Medicine by HPTLC-MS. J. AOAC Int. 2018, 101, 686–694. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Cheng, X.; Wei, F.; Ma, S. Study on Major Problems Affecting the Quality of Typhae Pollen and Quality Standard. Chin. Pharm. Affairs. 2018, 32, 463–468. [Google Scholar]
- Tang, T.; Yang, S.; Zhao, H.; Tan, Y.; Feng, J.; Xia, M.; Li, T. Analysis of traditional Chinese medicine components by high performance liquid chromatography with diode array detection based on double qualitative principles. Se Pu 2018, 36, 766–771. [Google Scholar] [CrossRef]
- Zhai, H.-Y.; Liu, Z.-P.; Yu, X.; Su, Z.-H.; Wen, F.-R. Simultaneous determination of five kinds of unallowable yellow industrial dyes in traditional Chinese medicine by HPLC. Chem. Res. Appl. 2014, 26, 135. [Google Scholar]
- Feng, F.; Zhao, Y.; Yong, W.; Sun, L.; Jiang, G.; Chu, X. Highly sensitive and accurate screening of 40 dyes in soft drinks by liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B. 2011, 879, 1813–1818. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.L.; Li, J.H.; Li, X.L.; Li, J.; Lu, X.Y.; Shen, J.Z.; Wang, Y.W.; Zhang, Z.H. Analysis of water-soluble azo dyes in soft drinks by high-resolution UPLC-MS. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 2011, 28, 1315–1323. [Google Scholar] [CrossRef]
- Xu, M.; Dai, S.; Wu, Z.; Shi, X.; Qiao, Y. Rapid analysis of dyed safflowers by color objectification and pattern recognition methods. J. Tradit. Chin. Med. Sci. 2016, 3, 234–241. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; Zhu, Q.; Wu, M.; Li, H.; Lu, F. Functional paper-based SERS substrate for rapid and sensitive detection of Sudan dyes in herbal medicine. Spectrochim. Acta Part A Mole Biomol. Spectrochim. 2018, 196, 110–116. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chen, B.Q.; Yuan, S.S.; Yang, B.B.; Yang, T.; Shi, M.H.; Lyu, G.-H. Determination of common dyes in dyed safflower by near-infrared spectroscopy. China J. Chin. Mater. Med. 2019, 44, 1537–1544. [Google Scholar]
- AOAC. Guidelines for Single-Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. In Official Methods of Analysis, 19th ed.; Appendix K; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. A review of extraction and analytical methods for the determination of Tartrazine(E102) in foodstuffs. Crit. Rev. Analy. Chem. 2017, 47, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the analysis of azo dyes employed in the food industry—A review. Food Chem. 2015, 192, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, J. Identification and Quantitation of Water-Soluble Synthetic Colors in Foods by Liquid Chromatography/Ultraviolet–Visible Method Development and Validation. ACS Omega 2018, 3, 6577–6586. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.G.; Gong, W.J.; Zhao, Y.G. Rapid method for quantification of seven synthetic pigments in colored Chinese steamed buns using UFLC-MS/MS without SPE. Anal. Sci. 2015, 31, 205–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Che, F.; Lu, Y.; Xi, X.; Lan, T.; Wei, Y. Identification of the impurity in auramine O by high-performance liquid chromatography-ion trap-time of flight mass spectrometry and preparation of the auramine O reference standard by preparative high-performance liquid chromatography. Chin. J. Chromatogr. 2019, 37, 299–304. [Google Scholar] [CrossRef]


| Pigments | Chemical Structure | Mole. Formula (M.W) | CAS No. | Pigments | Chemical Structure | Mole. Formula (M.W) | CAS No. |
|---|---|---|---|---|---|---|---|
| Tartrazine |  | C16H9N4Na3O9S2 (534.3) | 1934-21-0 | Acid red 73 | 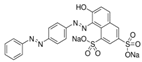 | C22H14N4Na2O7S2 (556.48) | 5413-75-2 |
| Sunset yellow |  | C16H10N2Na2O7S2 (452.37) | 2783-94-0 | Amaranth | 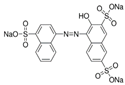 | C20H11N2Na3O10S (604.5) | 915-67-3 |
| Metanil yellow | 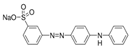 | C18H14N3NaO3S (375.4) | 587-98-4 | New Coccine |  | C20H11N2Na3O10S3 (604.47) | 2611-82-7 |
| Auramine O | 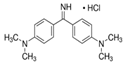 | C17H22CIN3 (303.8) | 2465-27-2 | Azorubine |  | C20H12N2Na2O7S2 (502.4) | 3567-69-9 |
| Orange II | 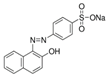 | C16H11N2NaO4S (350.32) | 633-96-5 | Erythrosine B | 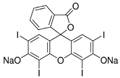 | C20H6I4Na2O5 (879.9) | 16423-68-0 |
| Colorants | Wavelength (nm) | Retention Time (min) | Equation of Calibration Plot | Linearity (r2) | Hawthorn Fruit (µg/g) | Cornus Fruit(µg/g) | Schisandra Fruit (µg/g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| LOD | LOQ | LOD | LOQ | LOD | LOQ | |||||
| Tartrazine | 428 | 6.5 | Y = 19,762x − 615 | 0.999 | 5.6 | 16.8 | 1.8 | 5.6 | 8.0 | 24.4 |
| Auramine O | 438 | 34.6 | Y = 73,712x − 3496 | 0.999 | 1.4 | 4.0 | 2.4 | 7.2 | 1.6 | 5.0 |
| Metanil yellow | 428 | 37.5 | Y = 41,191x − 8884 | 0.999 | 2.2 | 7.0 | 1.4 | 4.0 | 2.4 | 7.4 |
| Amaranth | 500 | 8.5 | Y = 19,636x − 1522 | 0.999 | 5.0 | 15.0 | 2.8 | 8.4 | 6.6 | 19.8 |
| New coccine | 500 | 12.9 | Y = 20,368x − 3070 | 0.999 | 0.8 | 2.4 | 1.0 | 3.0 | 4.0 | 12.0 |
| Sunset yellow | 500 | 14.0 | Y = 26,844x − 3877 | 0.999 | 1.4 | 4.0 | 1.8 | 5.8 | 4.6 | 14.0 |
| Azorubine | 500 | 22.9 | Y = 18,108x − 3446 | 0.999 | 1.4 | 4.2 | 2.6 | 8.0 | 4.2 | 12.6 |
| Erythrosine B | 500 | 28.9 | Y = 16,748x − 5497 | 0.999 | 1.4 | 4.4 | 2.4 | 7.4 | 8.4 | 25.4 |
| Acid red 73 | 500 | 30.7 | Y = 35,988x − 6893 | 0.999 | 1.0 | 3.0 | 1.0 | 3.2 | 4.0 | 12.4 |
| Orange II | 500 | 32.0 | Y = 31,493x − 7246 | 0.999 | 0.8 | 2.2 | 2.2 | 6.4 | 4.4 | 13.4 |
| Colorants | Spiked Conc. (μg/mL) | Hawthorn Fruit (%) | Cornus Fruit(%) | Schisandra Fruit (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery | RSD | RSDr | Recovery | RSD | RSDr | Recovery | RSD | RSDr | ||
| Tartrazine | 5 | 132.1 | 0.9 | 0.7 | 109.6 | 0.4 | 0.3 | 113.3 | 0.5 | 0.5 |
| 10 | 128.8 | 1.4 | 1.1 | 109.7 | 1.0 | 0.9 | 111.8 | 1.8 | 1.6 | |
| 25 | 122.6 | 6.9 | 5.7 | 111.2 | 0.7 | 0.6 | 115.0 | 1.5 | 1.3 | |
| Auramine O | 5 | 98.6 | 0.3 | 0.3 | 80.9 | 2.7 | 3.4 | 99.0 | 0.3 | 0.3 |
| 10 | 97.3 | 0.8 | 0.8 | 78.3 | 2.5 | 3.2 | 99.1 | 1.6 | 1.6 | |
| 25 | 98.8 | 2.3 | 2.3 | 85.0 | 1.3 | 1.5 | 102.4 | 0.2 | 0.2 | |
| Metanil yellow | 5 | 102.8 | 0.5 | 0.5 | 103.4 | 0.3 | 0.3 | 101.3 | 0.3 | 0.3 |
| 10 | 101.8 | 0.7 | 0.7 | 102.2 | 0.2 | 0.2 | 100.3 | 1.1 | 1.1 | |
| 25 | 102.4 | 1.5 | 1.4 | 102.8 | 0.5 | 0.5 | 103.1 | 0.3 | 0.3 | |
| Amaranth | 5 | 111.5 | 0.6 | 0.5 | 93.6 | 0.9 | 0.9 | 90.6 | 1.0 | 1.1 |
| 10 | 109.7 | 0.9 | 0.8 | 93.8 | 0.3 | 0.3 | 90.0 | 2.3 | 2.5 | |
| 25 | 110.3 | 1.2 | 1.1 | 95.8 | 0.8 | 0.8 | 92.7 | 1.6 | 1.8 | |
| New coccine | 5 | 99.3 | 0.4 | 0.4 | 92.5 | 1.2 | 1.3 | 87.8 | 0.7 | 0.8 |
| 10 | 98.2 | 0.8 | 3.9 | 92.3 | 0.1 | 0.1 | 85.7 | 1.5 | 1.8 | |
| 25 | 99.6 | 1.2 | 2.9 | 94.0 | 0.8 | 0.9 | 88.3 | 1.4 | 1.6 | |
| Sunset yellow | 5 | 104.3 | 0.3 | 3.6 | 102.3 | 0.8 | 0.7 | 97.3 | 0.8 | 0.8 |
| 10 | 103.6 | 0.8 | 3.7 | 100.7 | 0.2 | 0.2 | 95.7 | 1.1 | 1.1 | |
| 25 | 103.3 | 2.0 | 1.2 | 101.9 | 0.7 | 0.7 | 98.4 | 0.4 | 0.4 | |
| Azorubine | 5 | 99.2 | 0.3 | 0.3 | 98.1 | 1.1 | 1.2 | 94.0 | 0.5 | 0.5 |
| 10 | 98.5 | 0.7 | 0.2 | 97.6 | 0.5 | 0.5 | 93.5 | 1.2 | 1.3 | |
| 25 | 100.4 | 1.1 | 0.7 | 99.0 | 0.7 | 0.7 | 96.7 | 0.7 | 0.7 | |
| Erythrosine B | 5 | 96.0 | 0.2 | 0.2 | 95.2 | 1.4 | 1.5 | 75.9 | 0.9 | 1.2 |
| 10 | 94.3 | 0.8 | 0.8 | 94.2 | 1.4 | 1.5 | 74.6 | 2.2 | 3.0 | |
| 25 | 97.6 | 2.1 | 2.1 | 97.0 | 0.9 | 1.0 | 79.8 | 1.3 | 1.6 | |
| Acid red 73 | 5 | 100.5 | 0.2 | 0.2 | 97.0 | 0.9 | 0.9 | 91.4 | 0.4 | 0.5 |
| 10 | 99.9 | 0.9 | 0.9 | 96.1 | 0.4 | 0.4 | 90.8 | 1.1 | 1.2 | |
| 25 | 101.0 | 1.2 | 1.2 | 98.1 | 0.7 | 0.7 | 94.0 | 0.7 | 0.7 | |
| Orange II | 5 | 104.1 | 0.4 | 0.4 | 100.5 | 0.5 | 0.5 | 100.1 | 0.7 | 0.7 |
| 10 | 103.3 | 1.0 | 1.0 | 99.9 | 0.2 | 0.2 | 99.9 | 1.1 | 1.1 | |
| 25 | 103.9 | 1.4 | 1.4 | 101.5 | 0.6 | 0.6 | 102.7 | 0.5 | 0.4 | |
| Colorants | Precursor Ion (m/z) | Product Ion (m/z) | Polarity | Collison Energy (V) | References | |
|---|---|---|---|---|---|---|
| Tartrazine | 467.1 | Target ion | 198.0 | - | 18 | [5,18,27] |
| Ref. ion 1 | 171.9 | - | 21 | |||
| Ref. ion 2 | 422.9 | - | 12 | |||
| Auramine O | 268.0 | Target ion | 147.1 | + | −29 | [5,28] |
| Ref. ion 1 | 131.0 | + | −50 | |||
| Ref. ion 2 | 252.1 | + | −34 | |||
| Metanil yellow | 352.2 | Target ion | 156.0 | - | 30 | [19] |
| Ref. ion 1 | 79.9 | - | 45 | |||
| Ref. ion 2 | 287.9 | - | 20 | |||
| Amaranth | 537.0 | Target ion | 316.8 | - | 31 | [18,27] |
| Ref. ion 1 | 236.9 | - | 43 | |||
| Ref. ion 2 | 194.0 | - | 52 | |||
| New Coccine | 268.3 | Target ion | 206.1 | - | 13 | [5,18] |
| Ref. ion 1 | 79.9 | - | 32 | |||
| Ref. ion 2 | 220.9 | - | 21 | |||
| Sunset yellow | 203.4 | Target ion | 171.0 | - | 15 | [5,18,27] |
| Ref. ion 1 | 181.1 | - | 21 | |||
| Ref. ion 2 | 114.0 | - | 30 | |||
| Erythrosin B | 834.5 | Target ion | 662.7 | - | 34 | [18,27] |
| Ref. ion 1 | 536.8 | - | 33 | |||
| Ref. ion 2 | 127.0 | - | 55 | |||
| Azorubine | 456.9 | Target ion | 377.0 | - | 20 | [5] |
| Ref. ion 1 | 220.9 | - | 32 | |||
| Ref. ion 2 | 170.0 | - | 43 | |||
| Acid red 73 | 255.0 | Target ion | 150.5 | - | 10 | [5,19] |
| Ref. ion 1 | 136.5 | - | 21 | |||
| Ref. ion 2 | 236.9 | - | 15 | |||
| Orange II | 327.0 | Target ion | 170.9 | - | 23 | [18,19] |
| Ref. ion 1 | 156.0 | - | 31 | |||
| Ref. ion 2 | 107.1 | - | 40 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, K.-Y.; Choi, E.-Y.; Jeong, S.-H.; Kim, S.; Lee, C.-K.; Lee, C.; Cho, S. Simple HPLC-PDA Analysis to Determine Illegal Synthetic Dyes in Herbal Medicines. Appl. Sci. 2021, 11, 6641. https://doi.org/10.3390/app11146641
Ko K-Y, Choi E-Y, Jeong S-H, Kim S, Lee C-K, Lee C, Cho S. Simple HPLC-PDA Analysis to Determine Illegal Synthetic Dyes in Herbal Medicines. Applied Sciences. 2021; 11(14):6641. https://doi.org/10.3390/app11146641
Chicago/Turabian StyleKo, Kyung-Yuk, Eun-Young Choi, Se-Hee Jeong, Sohwa Kim, Choon-Kil Lee, Chulhyun Lee, and Sooyeul Cho. 2021. "Simple HPLC-PDA Analysis to Determine Illegal Synthetic Dyes in Herbal Medicines" Applied Sciences 11, no. 14: 6641. https://doi.org/10.3390/app11146641
APA StyleKo, K.-Y., Choi, E.-Y., Jeong, S.-H., Kim, S., Lee, C.-K., Lee, C., & Cho, S. (2021). Simple HPLC-PDA Analysis to Determine Illegal Synthetic Dyes in Herbal Medicines. Applied Sciences, 11(14), 6641. https://doi.org/10.3390/app11146641