Abstract
Aim: Study aimed to test whether implants inserted in posterior mandible sites augmented with screw-guided bone regeneration (S-GBR) technique differ from implants placed in non-grafted sites regarding the success and survival rate. Materials and Methods: 20 edentulous patients (mean age 59.45 ± 15.220) were divided in a test group (S-GBR) (immediate implants placed simultaneously with grafting procedures) and control group (implants placed in naturally healed sites). Primary outcomes (implants success; implants survival) and secondary outcomes (clinical parameters of soft tissues: mPI; mGI; probing depth; keratinized mucosa; marginal-bone-level) were evaluated at 24-months follow-up. Results: Plaque levels were higher (p = 0.046) in S-GBR group (0.97 ± 0.882 mm) when compared with control (0.66 ± 0.695 mm). Keratinized mucosa width was higher in S-GBR group (4.13 ± 1.033 mm) than control (3.34 ± 0.821 mm) (p = 0.000) Probing depth (PD) width was higher in S-GBR group (3.50 ± 1.372 mm) than control (2.56 ± 1.332 mm) (p = 0.000). mGI was higher among implants placed in S-GBR group (0.90 ± 1.020 mm) than control (0.56 ± 0.794 mm) (p = 0.061). The difference between the average bone loss (MBL) for implants placed in grafted sites (Group S-GBR: 2.20 ± 1.867 mm) and for those placed in naturally healed sites (Group B: 1.09 ± 1.678 mm) was statistically significant (p = 0.000). The overall implant success rate after 24-month follow-up was 76.7% in S-GBR group and 90.6% in control group (p = 0.001). The survival rate after 24-month follow-up was 86.7% in S-GBR group and 93.8% in control group (p = 0.182). The reconstruction of the alveolar bone using S-GBR technique and immediate implant placement is a valid guided bone regeneration strategy for mandibular alveolar bone with severe horizontal resorption. The choice of S-GBR technique should be based on specific indications as implants placed in grafted sites recorded worse marginal success rate, survival rate and bone resorption than those placed in non-grafted sites.
1. Introduction
The implant-prosthetic treatment of partially edentulous patients has become in last 20 years a routine treatment procedure with reliable functional and esthetic results. Despite the evolution of implant dentistry, severely resorbed posterior mandible, due to periodontal disease or natural atrophy, remains a challenge even for experienced clinicians. A functional and stable mandibular implant-prosthetic restoration in posterior area can be compromised in the long term due to shape and orientation of the residual alveolar bone, improper sagittal intermaxillary relationships as well as the presence of mandibular inferior nerves. These clinical situations require the enhancement of the alveolar bone volume and quality for long-term success of the implant-prosthetic therapy. However, the high density and low vascularization of the mandibular cortical bone can hinder an effective grafting on the mandible body or regeneration processes of some bone chambers [1].
A various range of bone regenerative bone have been proposed to create conditions for long term success of the implant-prosthetic restorations in mandible posterior areas (guided bone regeneration (GBR), distraction osteogenesis, alveolar ridge splitting techniques, bone expansion techniques, bone grafting techniques) [2,3]. Every surgical technique has advantages and disadvantages, but specialists should give priority to those procedures which are easy-to-use (according to the practical experience of the clinician), less invasive, have lower risk of complications, and allow osteointegration of dental implants within the shortest post-operatory time [4]. Data are controversial regarding the success rate on medium and long term due to low quality of methodology (poor sample size, undefined success criteria, short-term follow-up) [4]. A scientifically validated opinion on which is the best technique and grafting materials is hard to obtain, especially in posterior mandible area. Due to vertical and horizontal bone resorption, augmentation procedures require a minimal surgical technique aiming to avoid soft tissue dehiscence above the bone regeneration compartment. The use of minimally invasive tunneling technique in the reconstruction of horizontally bone defects is associated with difficulty in the positioning and maintenance of the grafted bone in coronal position to increase the area of the peri-implant soft tissues [5]. The success of the lateral ridge augmentation depends on the experience of operator and by ability to stabilize and maintain the graft at the alveolar crest. In large alveolar defects the maintaining of the stability of the grafted areas becomes even more difficult when using this technique [5]. The success of GBR techniques is more predictable when used to patients with mild to moderate horizontal alveolar bone resorption and small edentulous span that allows simultaneous placement of implants. Variants of GBR techniques that use tenting mechanisms can prevent collapse of the soft tissues and bone resorption [5]. Considering these issues, we focused our research on a particular technique used in our dental practice that allows us to obtain excellent results for mandibular edentulous patients with mild-to-moderate atrophy of alveolar bone [6,7]. Screw-guided bone regeneration (S-GBR) is a technique derived from GBR that uses a membrane that delimitates the regenerative bone compartment supported by osteosynthesis screws or dental implants [7]. S-GBR is mostly recommended to the lateral augmentation of the mandibular alveolar bone with moderate or severe horizontal resorption, by using a combination of autograft, xenograft, resorbable or non-resorbable membranes. The significant difference between S-GBR technique and other techniques used in the horizontal augmentation of the alveolar crest is the system used for the maintenance of the regenerative bone space (osteosynthesis screws as space holders, pericardium membrane to protect the graft material from soft tissue invasion), with lower rate of complications. The other techniques use non-resorbable titanium-, zirconium-, or titanium-reinforced membranes (with potential for wound infection following the exposure of e-PTFE membranes) or resorbable membranes (poor space-making ability, fast degradation) [8].
Study aimed to test whether implants inserted in posterior mandible sites augmented with screw-guided bone regeneration (S-GBR) technique differ from implants placed in non-grafted sites regarding success rate, survival rate, interproximal marginal bone level (MBL), and clinical parameters of peri-implant soft tissues.
2. Materials and Method
2.1. Patient Selection and Study Design
The prospective cohort study was conducted at Implant Institute Török (Nuernberg, Germany) between December 2017 and December 2020. The study adhered to the ethical values of the Declaration of Helsinki and received approval of ethics committee of U.M.F. “Grigore T.Popa” Iasi (Romania) (Nr. 10833) Among the patients who visited the dental clinic, 20 edentulous patients (mean age 59.45 ± 15.220) were selected (Table 1). All patients involved in study received information about the objectives of the research and gave informed consent. Inclusion criteria (test group) were age >18 years; partially edentulous posterior mandible; severe horizontal resorption of ridges; treatment with fixed implant-supported prosthesis. Additional inclusion criteria for control group: alveolar bone of minimum 10 mm length and minimum 3.8 mm diameter. Exclusion criteria were: insufficient alveolar bone for implant-prosthetic therapy; history of untreated periodontal disease; history of smoking; decompensated metabolic diseases; pregnancy; history of bisphosphonates therapy; severe bruxism; non-collaborative patients.

Table 1.
Characteristics of test and control group.
The selected patients were grouped as follows:
- Test group (n = 10; 30 implants): simultaneous implant placement with ridge augmentation by S-GBR technique.
- Control group (n = 10; 32 implants): implant placement in naturally healed sites (non-grafted).
The components of the study design (population, intervention, comparison, outcomes) are presented in Table 2.

Table 2.
PICO study design.
2.2. Surgical Procedures
The same surgeon (T.R.), with over 20 years of experience, performed alveolar augmentation and implant procedures. For all patients, systemic antibiotics were given prophylactic preoperatively and at 4 days postoperatively. The procedures of dental implants insertion for test group and control were as follows:
- (a)
- Dental implants placement simultaneously with alveolar augmentation by SGBR technique (xenograft, or a mixture with autogenous bone; porcine collagen membrane) (test group);
- (b)
- Standard dental implants placement (control group).
The surgical protocol of S-GBR technique and immediate implant placement is described in Table 3.

Table 3.
Operative protocol in S-GBR technique and immediate implant placement.
Prosthetic loading was carried out after 14–16 weeks following implants placement in the mandible. Each patient was included in a maintenance program consisting of oral hygiene and recall visit every 6 months.
Clinical and CBCT aspects of patient from the test group, describing the stages of S-GBR technique and implant-prosthetic stage, are presented in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11. CBCT exam allows the evaluation of the horizontal alveolar bone defect and the position of the mandibular alveolar nerf (Figure 1A,B). Figure 1C,D show cross-sectional CBCT aspects of the implant sites. Figure 2 shows the narrowed mandibular alveolar bone with horizontal resorption. Figure 3 shows the exposed buccal surface of the alveolar ridge with severe horizontal resorption, after flap opening. The inserted implants (4.5 mm diameter, 11.5 mm length) and osteosynthesis screws (45° from the occlusal plan) are shown in Figure 4A. The placement of graft (autologous bone and xenograft) and collagen membrane is shown in Figure 4B. Figure 5A shows tension-free sutures, due to periosteal incisions alveolar ridge. Figure 5B shows clinical aspect at 7 months after surgery, with gingival tissue adherent on the reconstructed alveolar ridge. Figure 6 shows OPG aspect at follow-up of 7 months, with osseointegration of the dental implants. Figure 7A shows clinical aspect before osteosynthesis screws removal. Figure 7B shows clinical aspect after osteosynthesis screws removal. Figure 8A shows healthy peri-implant soft tissues. Figure 8B shows repositioning key for perfect position of abutments. Figure 10A,B show clinical aspects of implant-supported prosthetic restoration. Figure 11A,B show CBCT aspects of Osseo integrated dental implants at 24 months follow-up.
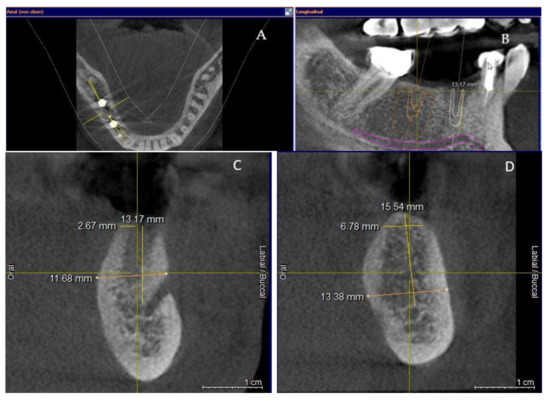
Figure 1.
(A,B) Pre-operative CBCT aspects. (A) Narrow residual alveolar ridge. (B) Analysis of the mandibular nerve position. (C,D) Pre-operative CBCT aspects (cross-sectional).
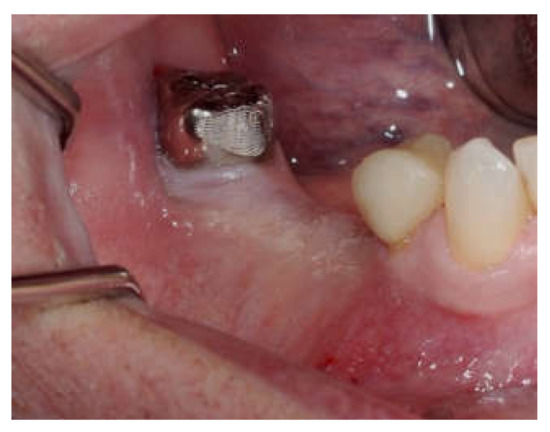
Figure 2.
Pre-operative clinical aspect of the narrow residual ridge.
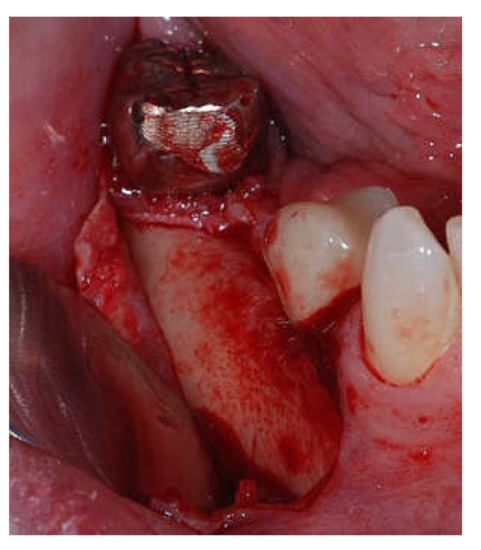
Figure 3.
Intra-operative clinical aspect after flap opening.
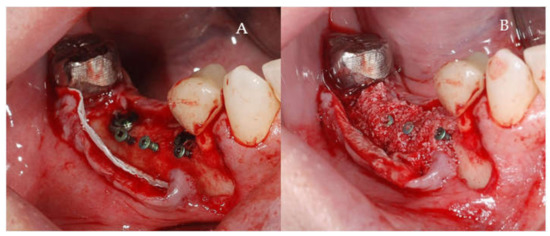
Figure 4.
(A,B) Intra-operative clinical aspects. (A) Clinical aspect of the inserted implants and osteosynthesis screws. (B) Clinical aspect of the graft material and collagen membrane placed to delimit the future bone regeneration compartment.
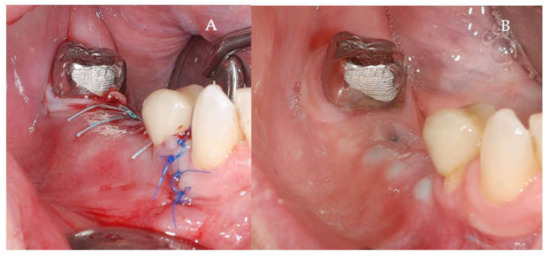
Figure 5.
(A,B) Follow-up of the S-GBR surgery. (A) Tension-free sutures. (B) Clinical aspect at 7 months post-surgery.
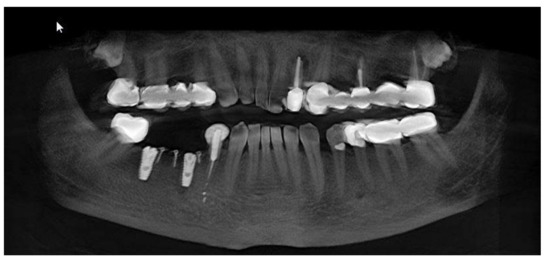
Figure 6.
OPG aspect at follow-up of 7-months.
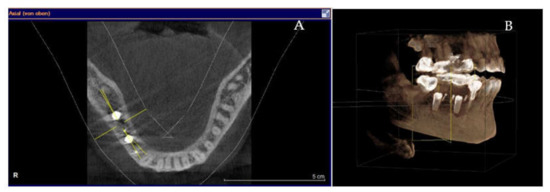
Figure 7.
(A,B) 7-months follow-up of S-GBR surgery. (A) CBCT shows 2 mm bone around implants. (B) CBCT shows reduced bone resorption in the coronal area of implants.
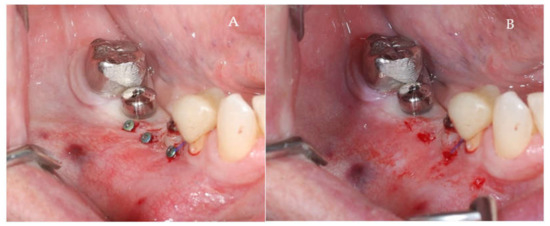
Figure 8.
(A,B) Clinical aspects at the second stage surgical session. (A) Aspect before screw removal. (B) Aspect after screw removal.
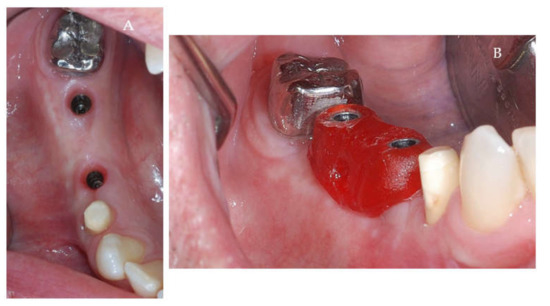
Figure 9.
(A,B) Post-operative clinical aspects 3 weeks after second stage surgery. (A) Healthy peri-implant soft tissues (B) The repositioning key of abutments.
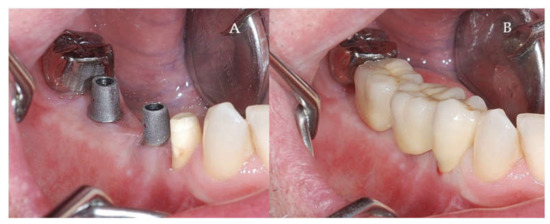
Figure 10.
(A,B) Final prosthetic stage. (A) Titanium Abutment (B) Cemented metal-ceramic crowns.
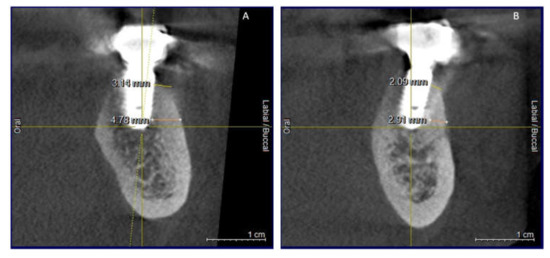
Figure 11.
(A,B) CBCT aspects (cross-sectional) of osseointegrated implants at 24-months follow-up.
2.3. Results Analysis
The clinical and radiographic analysis of the parameters related to the primary outcomes and secondary outcomes was performed by one investigator (T.B) at 24-months post-loading. Criteria for the success of S-GBR grafting procedure were: absence of fistula, flow out from mucosal dehiscence of the particles of the graft material, or the chronic inflammation. Buser criteria, used to define the implant success, are as follows: the absence of pain, the absence of implant mobility, the absence of recurrent peri-implant infection, the absence of peri-implant radiolucency at 6 months and 24 months post-loading [9].
Diagnostic of peri-implant mucositis [10,11]:
- (1)
- Clinical inflammation signs (erythema, swelling, bleeding on probing at force not overpassing 0.25 N, and/or suppuration) [12]
- (2)
- Mild increase of probing depth.
- (3)
- Absence of peri-implant bone loss (after post-loading stage of bone remodeling).
Diagnostic of peri-implantitis [10,11]:
- (1)
- Clinical inflammation signs (erythema, swelling, bleeding on probing and/or suppuration);
- (2)
- Probing depth >3 mm, and/or recession of the mucosal margin.
- (3)
- Loss of peri-implant bone >3 mm (from implant shoulder).
Indices mPI and mGI were used to evaluate the status of the peri-implant soft tissues (Table 4 and Table 5) [13,14,15]. Probing Depth (PD) (mm) was evaluated with periodontal probe (Click-Probe®, Kerr, Bioggio, Switzerland).

Table 4.
mPI Indice (peri-implant bacterial plaque accumulation).

Table 5.
mGI Indice (peri-implant soft tissues status).
Keratinized Mucosa (KM) (mm) was measured with periodontal probe (Click-Probe®, Kerr) as the distance between the mucogingival junction and the most coronal point of the keratinized mucosa in the center of the implant-prosthetic restoration
The marginal bone loss at buccal area (MBL) at 24-months follow-up was measured on CBCT as the distance between the connection implant-abutment and the peri-implant MBL.
2.4. Statistical Analysis
Using the statistical software G*Power (Version 3.1.9, Heinrich-Heine-University, Düsseldorf, Germany), a sample of 9 subjects in each group was calculated as minimum number required to determine a significant statistical difference in marginal bone level loss, success rate and survival rate of implants (80% power, 5% confidence level). Considering a dropout rate of 10% during the study period, 10 subjects were included in each group.
The frequencies distributions were calculated for qualitative variables, while the averages and standard deviations for the quantitative ones. The normality of data distribution was checked by Shapiro–Wilks test. The comparison of the quantitative variables between test group and control was performed by using t-test and Mann-Whitney test. The comparison of the qualitative variables between test group and control was performed by using Chi-squared test. The significance level was set at p < 0.05. Statistical analysis was performed using SPSS version 27.0 for Windows (IBM, Armonk, NY. USA).
3. Results
Plaque levels (mPI) were significantly higher (p = 0.046) in S-GBR group (0.97 ± 0.882 mm) when compared with control (0.66 ± 0.695 mm) (Table 6).

Table 6.
Clinical parameters and radiographic results at 24-months follow-up.
Keratinized mucosa width (KM) was higher in S-GBR group (4.13 ± 1.033 mm) than control (3.34 ± 0.821 mm), with statistically significant differences (p = 0.000) (Table 6).
Probing depth (PD) width was higher in S-GBR group (3.50 ± 1.372 mm) than control (2.56 ± 1.332 mm), with statistically significant differences (p = 0.000) (Table 6).
Modified gingival index (mGI) was higher among implants placed in S-GBR group (0.90 ± 1.020 mm) than control (0.56 ± 0.794 mm), although differences were not statistically significant between groups (p-0.061) (Table 6).
The difference between the average bone loss (MBL) for implants in Group S-GBR (2.20 ± 1.867 mm) and for implants placed in non-grafted sites (Group B: 1.09 ± 1.678 mm) was statistically significant (p = 0.000) (Table 6).
The success rate and the survival rate were higher for control when compared with S-GBR group after 24-months follow-up (Table 7).

Table 7.
Success rate and survival rate at 24-months follow-up.
The overall implant success rate at 24-month follow-up was 76.7% in S-GBR group and 90.6% in control group, with significant differences between groups (p = 0.001).
The survival rate after 24-month follow-up was 86.7% in S-GBR group and 93.8% in control group, without statistically significant differences although differences between groups (p = 0.182).
4. Discussion
This study compared clinical and radiographic parameters of the implant-supported fixed restorations, following immediate implants placement in sites grafted by the S-GBR technique.
A condition of the implants success rate when placed in grafted sites is the absence of post-operatory early complications due to exposure or improper stabilization of bone graft as well as to post-operatory infection. In our study, post-grafting complications, that could interfere with implants osseointegration processes, were absent. The absence of the risk factors (due to the selection criteria of patients) could contribute significantly in maximizing the rate of the grafting procedures success [16]. However, Dastaran et al. found that the implant survival rate is not influenced by alveolar bone augmentation, despite higher complications rate for implants inserted in sites with vertical and horizontal resorption [17]. The S-GBR technique was performed using mixed grafts (autologous bone and bovine bone). Based on reviewed studies, with a follow-up of minimum 3–5 years, Ellakia et al. recommend the replacement of allografts and xenografts with autologous bone in the implant sites with medium and severe bone resorption [18]. Xenografts can delay bone formation in comparison to the naturally healed sites, but also preserve the alveolar ridge and stimulate bone formation as to enable placement of implants with high success rate in mandibular implant-prosthetic restorations [19]. Our study investigated the success rate and survival rate of immediate implants placed in alveolar bone reconstructed by S-GBR technique. Immediate implant placement simultaneously with alveolar bone grafting had been well investigated in some systematic review. One study reported, at 20-months follow-up, the absence of statistically significant differences regarding various clinical parameters of the peri-implant soft tissue and peri-implant marginal bone loss, between the immediate implants and delayed implants [20]. In our study we found higher probing depth (p = 0.000), mGI index (p = 0.061), and average bone loss (p = 0.000) for immediate implants placed in grafted sites, when compared with naturally healed sites at 24-month follow-up. However, implant placement simultaneous with horizontal ridge augmentation is recommended when possible as implant survival rates are similar to subsequent implant placement [21]. We found after 24-months follow-up an implant success rate of 76.7% in S-GBR group, while the survival rate was 86.7%. The statistical analysis revealed the lack of significant statistical differences regarding the survival rate, but statistically significant differences for success rate when comparing S-GBR sites and non-grafted sites. According to a comprehensive review of studies, implants placed with GBR techniques had a mean 95.5% rate of survival for follow-ups of 5–74 months [22]. The survival rate of the implants inserted in augmented sites, irrespective of the surgical technique, ranged from 91.7% to 100%, while survival rates for non-grafted sites ranged between 93.2% and 100% at follow-up periods of 1–5 years [23]. A review of studies with 1–3 years follow-up, found non-significant changes of peri-implant soft tissues clinical parameters and marginal bone levels, when lateral bone augmentation techniques are used [24]. The implant survival rate for the alveolar bone submitted to lateral ridge augmentation was 97% to 100% at 6–12 months of follow-up [25].
At 24 months follow-up the probing depth and the peri-implant marginal bone loss are higher than control group. This result is correlated with higher resorption rate of the augmented alveolar area when compared with the implants placed in naturally healed alveolar bone. However, S-GBR is an augmentation technique with very low rate of complications [7]. The only complication could be the exposure of the osteosynthesis screw, managed by taking out the screw without any need of anesthesia.
An interesting research issue would be the comparison of the results obtained with S-GBR technique and immediate implantation when used in combination with different graft materials. Further studies will be published by our research group to report the influence of the graft material on the success and survival rate of dental implants placed in mandibular alveolar bone.
S-GBR technique is an easy method to regenerate the bone around implants and can be used in the daily work by every practitioner. The big advantage of this method is the low complication rate and the protection offered by the osteosynthesis screws to the augmented side against the compression forces in the oral cavity.
The comparison and interpretation of the results with those reported by literature must be done with caution as various factors impact the long-term success of the implant-prosthetic restorations [26,27,28,29].
Literature data is scarce in studies investigating the results of the implant-prosthetic treatments in simultaneous approach (augmentation technique combined with immediate implantation). Also, the success of the dental implants following immediate implantation in mandibular sites reconstructed with S-GBR technique, was not yet investigated.
Further studies can highlight the benefits and limits of this augmentation technique and can establish specific clinical situations when this technique can be recommended.
The limitations of this study are related to insufficient follow-up which is not enough to establish long-term survival rate of the implants, low sample size, and possible subjective bias of the investigators.
5. Conclusions
The reconstruction of the alveolar bone using S-GBR technique and immediate implant placement is a valid guided bone regeneration strategy for mandibular alveolar bone with severe horizontal resorption. The selection of S-GBR technique should be based on specific indications as implants placed in grafted sites recorded worse marginal success rate, survival rate and bone resorption than those placed in non-grafted sites.
Author Contributions
Conceptualization, B.T. and R.T.; data curation, B.T. and R.T.; formal analysis, N.C.F. and C.D.; methodology, B.T., D.M.D.E. and R.T.; resources, B.T. and R.T.; software, C.D.; supervision, N.C.F.; validation, N.C.F. and D.A.-F.; visualization, B.T. and R.T.; writing—original draft preparation, B.T., D.M.D.E. and R.T.; writing—review and editing, B.T., R.T., D.A.-F., C.D. and N.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was not funded by any grant.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Faculty of Dental Medicine, U.M.F., “Grigore T.Popa”, University Street no.16, 700115 Iasi, Romania (protocol code 10833).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sammartino, G.; Bernard, J.P. A clinical round table about the treatment of the severely resorbed posterior mandible. Part 1: Challenges, endeavor and perspectives. POSEIDO 2013, 1, 65–67. [Google Scholar]
- Tolstunov, L.; Hamrick, J.F.E.; Broumand, V.; Shilo, D.; Rachmiel, A. Bone Augmentation Techniques for Horizontal and Vertical Alveolar Ridge Deficiency in Oral Implantology. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Jindal, G.; Garg, S. Bone manipulation procedures in dental implants. Indian J. Dent. 2016, 7, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237–259. [Google Scholar]
- Le, B.; Burstein, J. Esthetic grafting for small volume hard and soft tissue contour defects for implant site development. Implant. Dent. 2008, 17, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Toeroek, R.; Mazor, Z.; Del Corso, M.; Dohan Ehrenfest, D.M. The concept of Screw-Guided Bone Regeneration (S-GBR). Part 1: From sinus-lift to general applications in the resorbed maxilla and mandible. POSEIDO 2013, 1, 69–84. [Google Scholar]
- Toeroek, R.; Dohan Ehrenfest, D.M. The concept of Screw-Guided Bone Regeneration (S-GBR). Part 2: S-GBR in the severely resorbed preimplant posterior mandible using bone xenograft and Leukocyte and Platelet-Rich Fibrin (L-PRF): A 5-year follow-up. POSEIDO 2013, 1, 85–92. [Google Scholar]
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Buser, D.; Mericske-Stern, R.; Dula, K.; Lang, N.P. Clinical Experience with One-Stage, Non-Submerged Dental Implants. Adv. Dent. Res. 1999, 13, 153–161. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Heitz, F.; Lang, N.P. Implant Disease Risk Assessment IDRA–a tool for preventing peri-implant disease. Clin. Oral Implant. Res. 2020, 31, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, N.P.; Berglundh, T.; Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: Where are we now?—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 178–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of peri-implantitis. Periodontol. 2000 1998, 17, 63–76. [Google Scholar] [CrossRef]
- Salvi, G.E.; Lang, N.P. Diagnostic parameters for monitoring peri-implant conditions. Int. J. Oral Maxillofac. Implant. 2004, 19, 116–127. [Google Scholar]
- Moy, P.K.; Aghaloo, T. Risk factors in bone augmentation procedures. Periodontol. 2000 2019, 81, 76–90. [Google Scholar] [CrossRef]
- Dastaran, M.; Bailey, D.; Austin, S.; Chandu, A.; Judge, R. Complications of augmentation procedures for dental implants in private practice, Victoria, Australia. Aust. Dent. J. 2019, 64, 223–228. [Google Scholar] [CrossRef]
- Elakkiya, S.; Ramesh, A.S.; Prabhu, K. Systematic analysis on the efficacy of bone enhancement methods used for success in dental implants. J. Indian Prosthodont. Soc. 2017, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xuan, F.; Choi, B.H.; Jeong, S.M. Minimally invasive ridge augmentation using xenogenous bone blocks in an atrophied posterior mandible: A clinical and histological study. Implant Dent. 2013, 22, 112–116. [Google Scholar] [CrossRef]
- Pellicer-Chover, H.; Peñarrocha-Oltra, D.; Bagán, L.; Fichy-Fernandez, A.J.; Canullo, L.; Peñarrocha-Diago, M. Single-blind randomized clinical trial to evaluate clinical and radiological outcomes after one year of immediate versus delayed implant placement supporting full-arch prostheses. Medicina Oral Patología Oral y Cirugia Bucal 2014, 19, e295–e301. [Google Scholar] [CrossRef] [PubMed]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Moy, P.K. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral Maxillofac. Implant. 2007, 22, 49–70. [Google Scholar]
- Donos, N.; Mardas, N.; Chadha, V. Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J. Clin. Periodontol. 2008, 35, 173–202. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Giannobile, W.V.; Jung, R.E.; Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 2-Effects of hard tissue augmentation procedures on the maintenance of peri-implant tissues. Clin. Oral Implant. Res. 2018, 29 (Suppl. 15), 11–13. [Google Scholar] [CrossRef] [Green Version]
- Elnayef, B.; Porta, C.; Suárez-López Del Amo, F.; Mordini, L.; Gargallo-Albiol, J.; Hernández-Alfaro, F. The Fate of Lateral Ridge Augmentation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 622–635. [Google Scholar] [CrossRef] [Green Version]
- Sakka, S.; Baroudi, K.; Nassani, M.Z. Factors associated with early and late failure of dental implants. J. Investig. Clin. Dent. 2012, 3, 258–261. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [Green Version]
- Topalo, E. Dental implants exposure prevention in their osseointegration period. Rom. J. Oral Rehabil. 2019, 11, 43–48. [Google Scholar]
- Ionescu, A.; Dodi, A.; Panagopoulos, V.; Nicolescu, M.I.; Mihai, A.T.; Tanase, G.; Vlasceanu, D. Biomechanical consequences of dental implants inserted in augmented alveolar ridges—A comparative study between tissue-level and bone-level implants: Finite elements analysis. Rom. J. Oral Rehabil. 2019, 11, 82–88. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).