Effects of External Heat Flux and Exhaust Flow Rate on CO and Soot Yields of Acrylic in a Cone Calorimeter
Abstract
1. Introduction
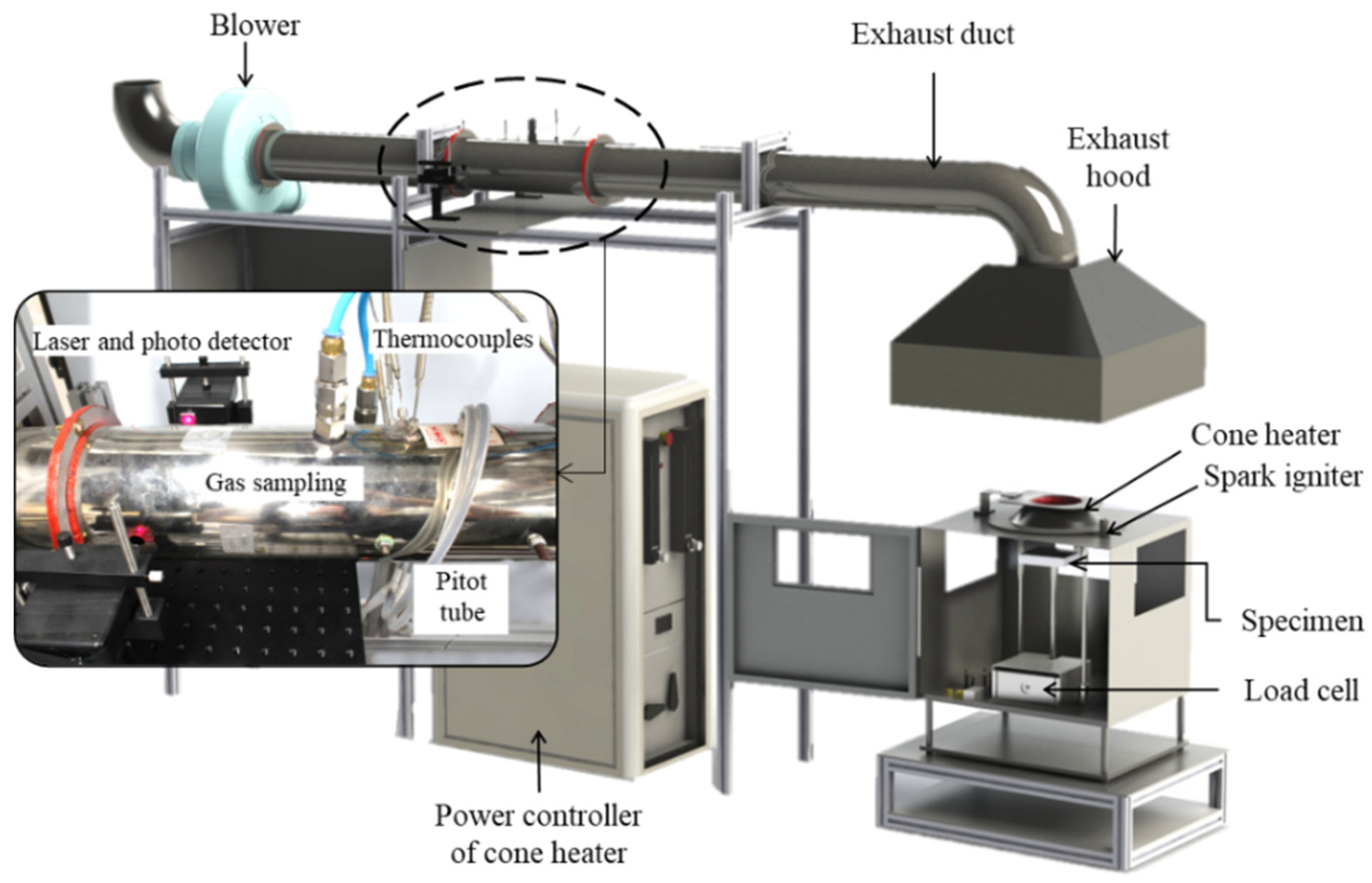
2. Experimental Method and Conditions
2.1. Material
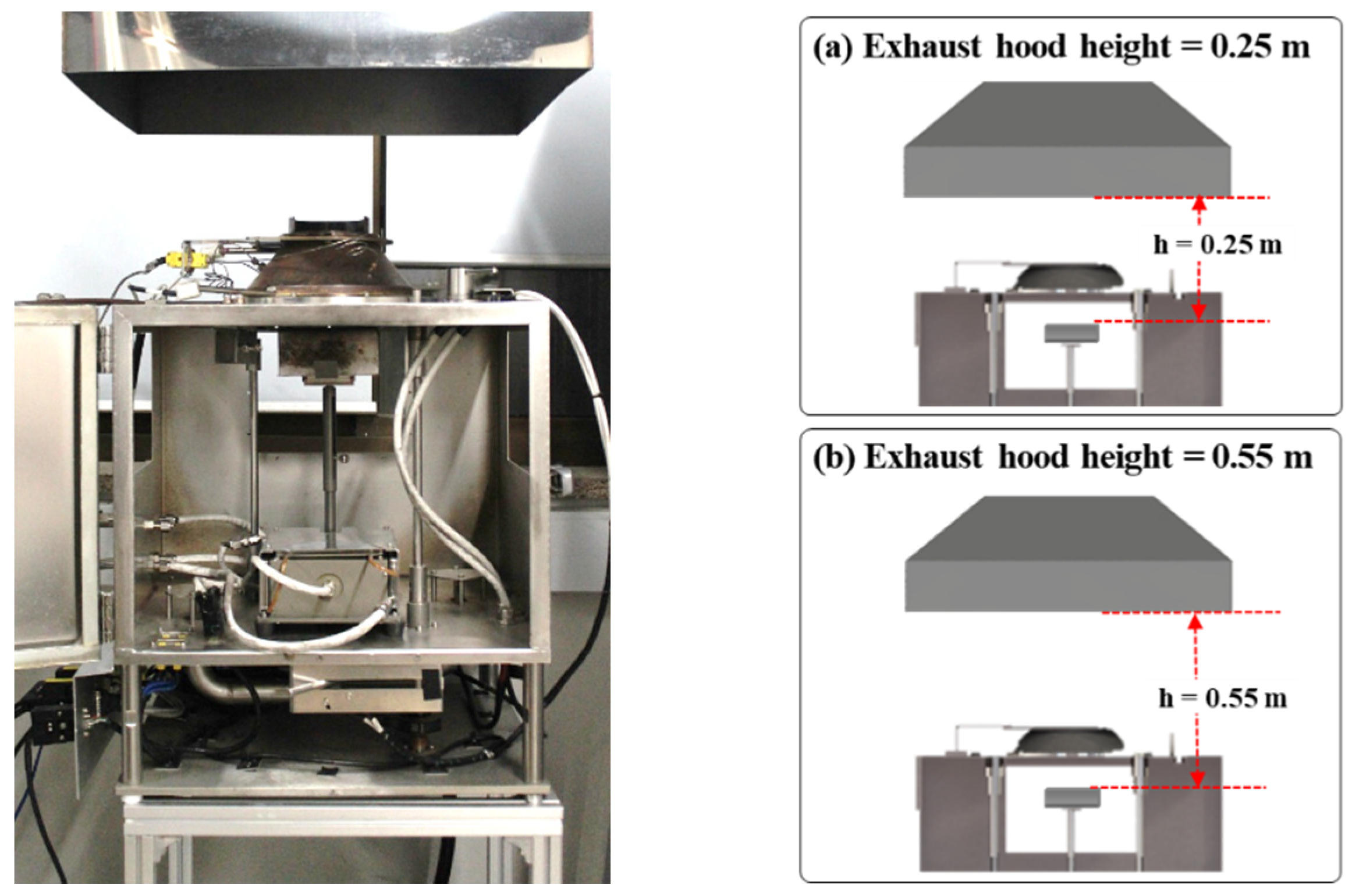
2.2. Experimental Conditions
2.3. Calculation of CO and Soot Yields
3. Results and Discussion
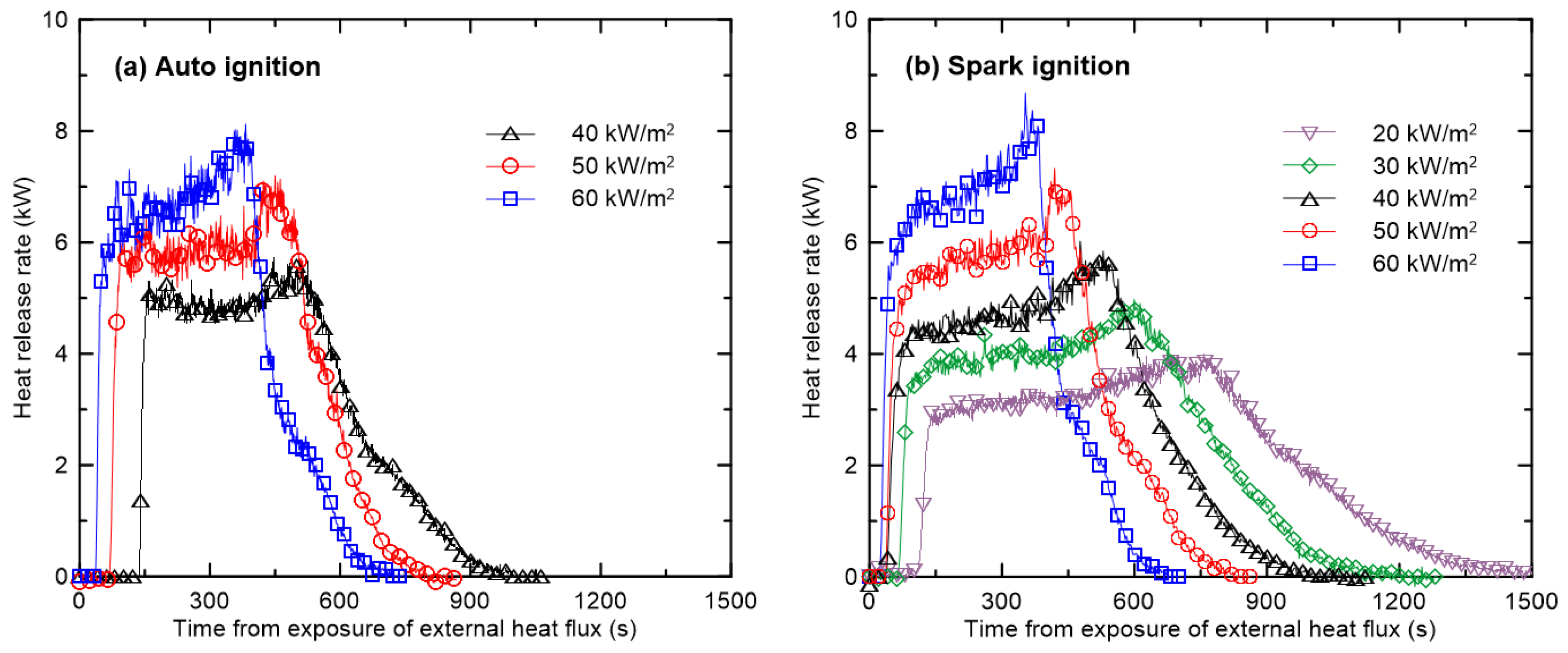
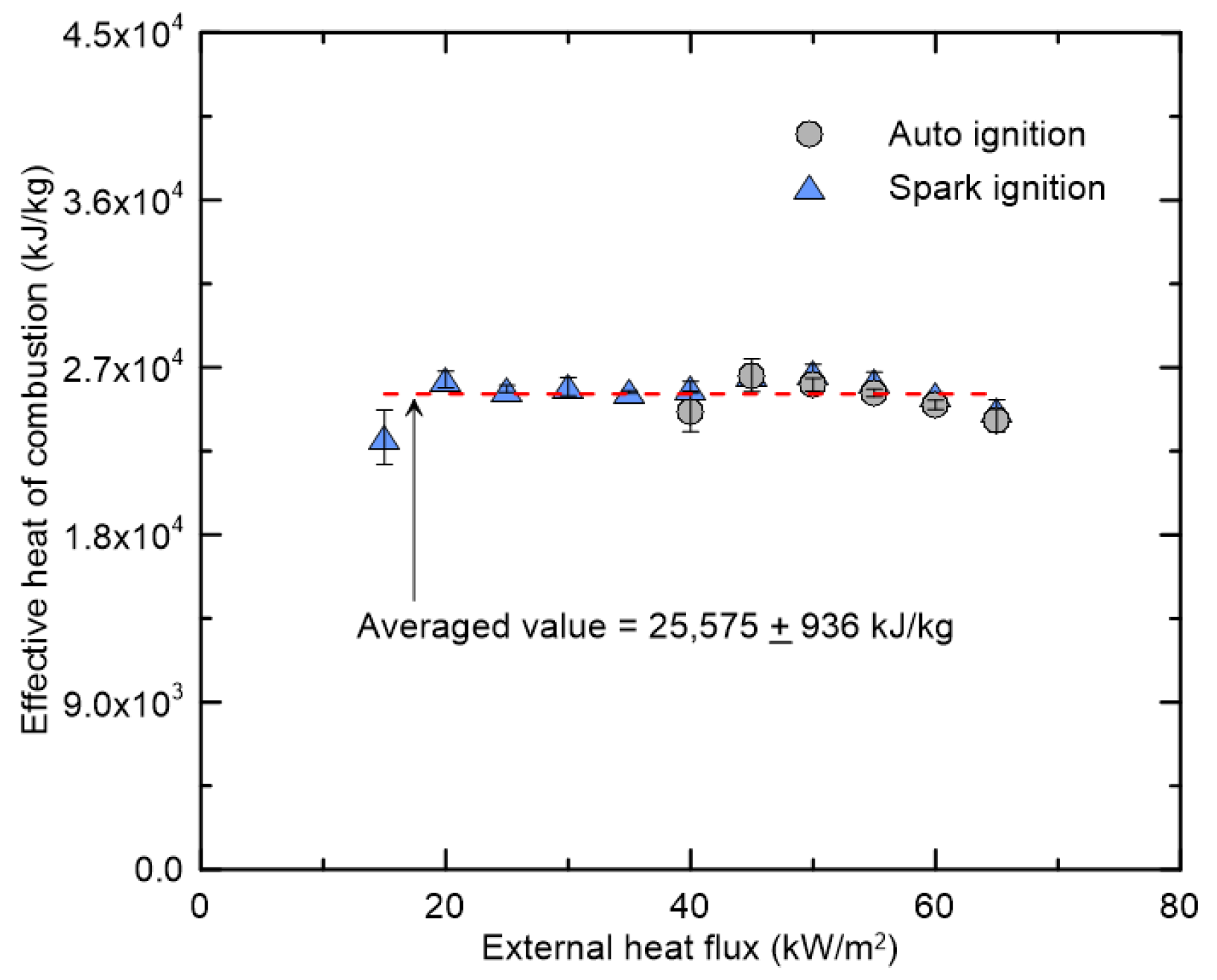
3.1. Burning Characteristics of Black Acrylic According to External Heat Flux
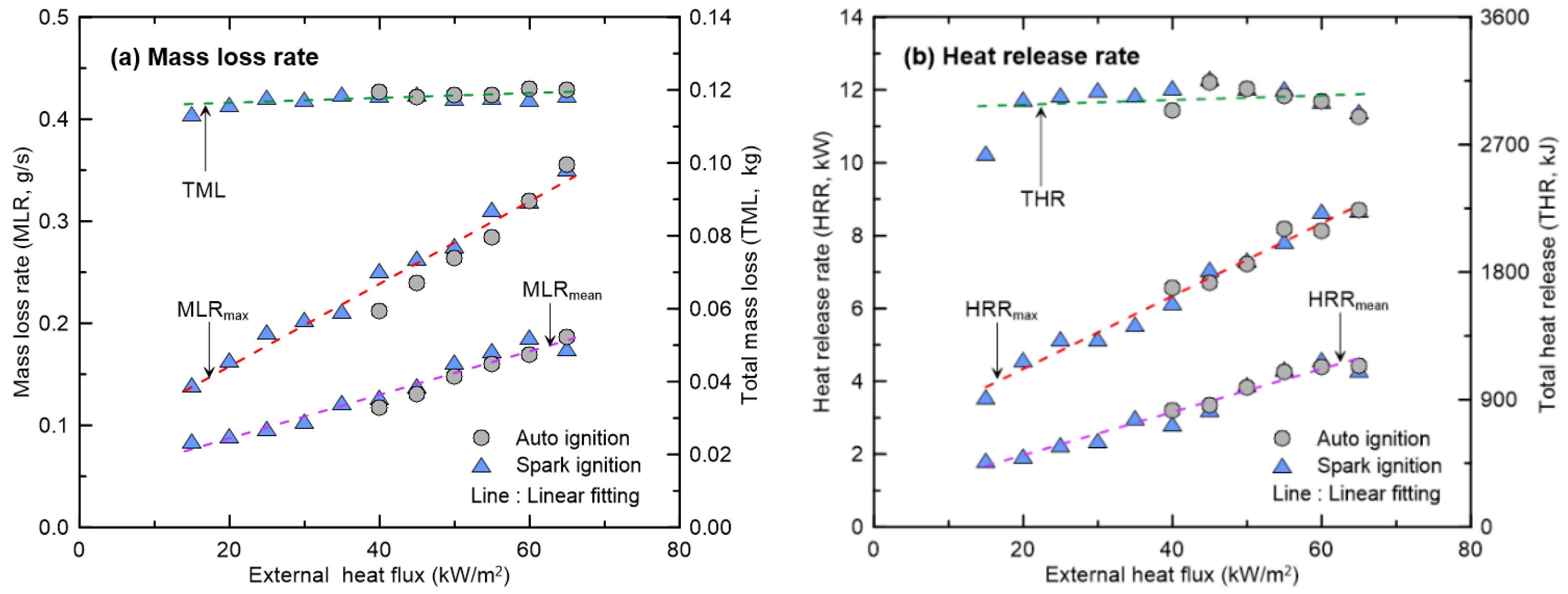
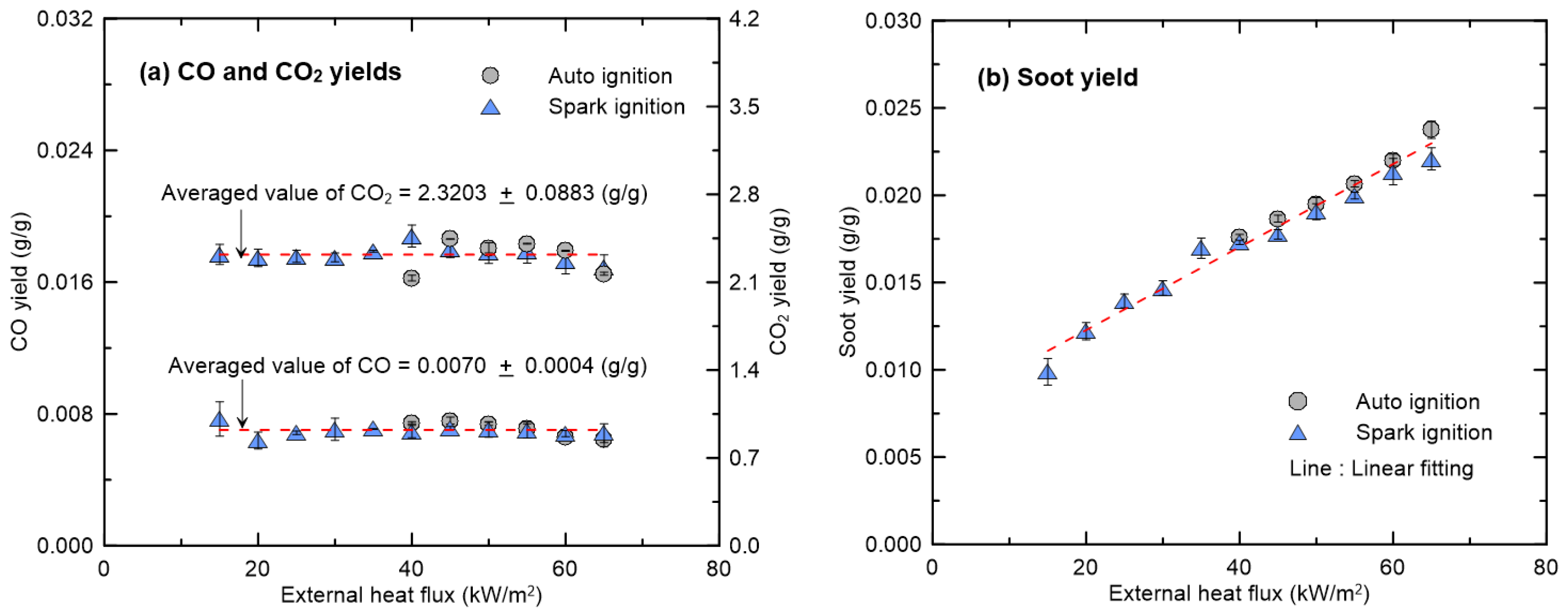
3.2. Effect of External Heat Flux on CO and Soot Yields
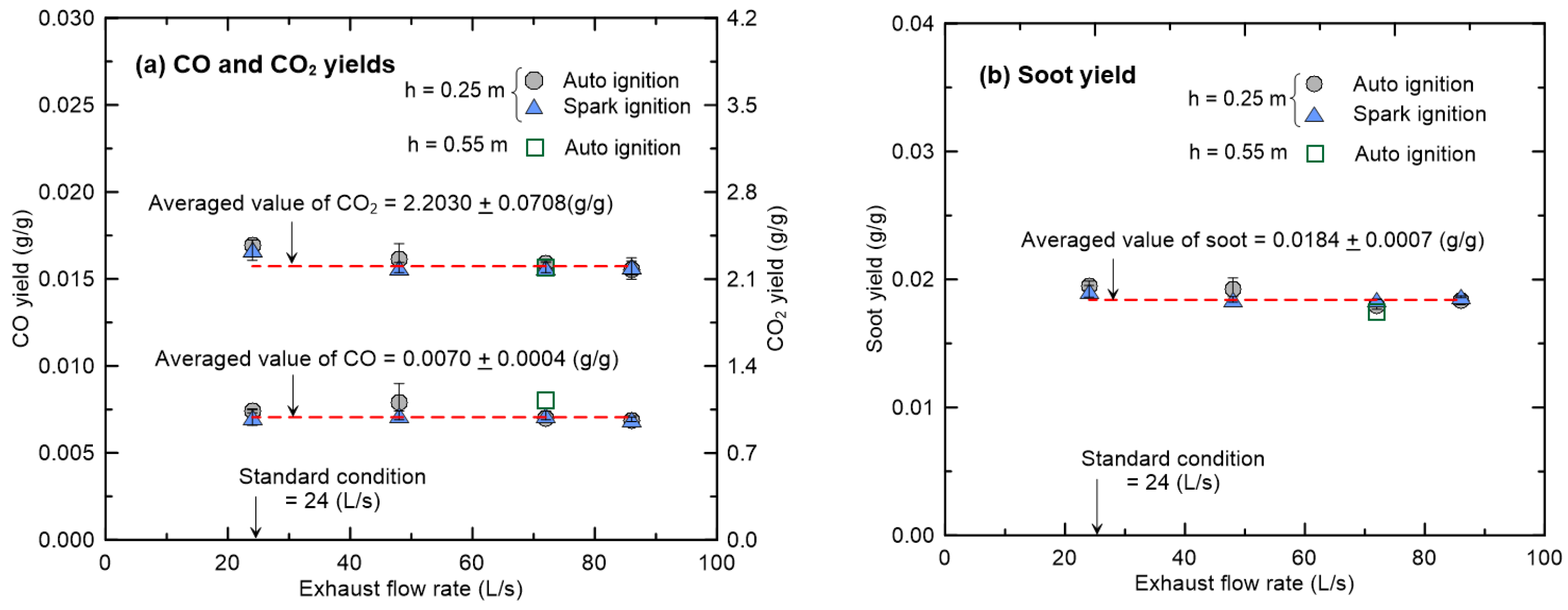
3.3. Effects of Exhaust Flow Rate and Hood Height on CO and Soot Yields
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most important variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- ISO. Reaction-to-Fire Tests-Heat Release, Smoke Production and Mass Loss Rate-Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement); International Organization for Standardization: Geneva, Switzerland, 2015; p. 5660. Available online: https://www.iso.org/standard/57957.html (accessed on 3 February 2021).
- ASTM E1354-17, Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter; ASTM International: West Conshohocken, PA, USA, July 2017. [CrossRef]
- Babrauskas, V. Development of the Cone Calorimeter—A Bench-Scale Heat Release Rate Apparatus Based on Oxygen Consumption; NBSIR 82-2611; National Bureau of Standards: Washington, DC, USA, 1982. [Google Scholar]
- Redfern, J.P. Rate of heat release measurement using the cone calorimeter. J. Therm. Anal. 1989, 35, 1861–1877. [Google Scholar] [CrossRef]
- Paul, K.T. Cone calorimeter: Initial experiences of calibration and use. Fire Saf. J. 1994, 22, 67–87. [Google Scholar] [CrossRef]
- Kuang-Chung, T.; Drysdale, D. Using cone calorimeter data for the prediction of fire hazards. Fire Saf. J. 2002, 37, 696–706. [Google Scholar] [CrossRef]
- Modak, A.T.; Croce, P.A. Plastic pool fires. Combust. Flame. 1977, 30, 251–265. [Google Scholar] [CrossRef]
- Babrauskas, V. Cone Calorimeter Annotated Bibliography 1982–1991; Technical Note 1296; National Institute of Standard and Technology: Gaithersburg, MD, USA, 1992. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the use of cone calorimeter data. Polym. Degrad. Stab. 2005, 88, 540–547. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 65–84. [Google Scholar] [CrossRef]
- Chen, R.; Lu, S.; Li, C.; Ding, Y.; Zhang, B.; Lo, S. Correlation analysis of heat flux and cone calorimeter test data of commercial flame-retardant ethylene-propylene-diene monomer (EPDM) rubber. J. Therm. Anal. Calorim. 2015, 123, 545–556. [Google Scholar] [CrossRef]
- Li, Z.; Xie, X.; Zhang, L.; Guo, X.; Tu, R. Heat transfer blockage effect of small-scale PMMA flame under exterior heat radiation. Int. J. Therm. Sci. 2007, 122, 26–32. [Google Scholar] [CrossRef]
- Scudamore, M.J.; Briggs, P.J.; Prager, F.H. Cone calorimetry—A review of tests carried out on plastics for the association of plastic manufactures in europe. Fire Mater. 1991, 15, 65–84. [Google Scholar] [CrossRef]
- Bryner, N.P.; Johnsson, E.L.; Pitts, W.M. Carbon Monoxide Production in Compartment Fires—Reduced-Scale Enclosure Test Facility; NISTIR 5568; National Institute of Standard and Technology: Gaithersburg, MD, USA, 1994. [Google Scholar] [CrossRef]
- Ukleja, S.; Delichatsios, M.A.; Delichatsios, M.M.; Lee, Y.P. Carbon monoxide and smoke production downstream of a compartment for underventilated fires. Fire Saf. Sci. 2008, 9, 849–860. [Google Scholar] [CrossRef][Green Version]
- Köylü, Ü.Ö.; Sivathanu, Y.R.; Faeth, G.M. Carbon monoxide and soot emissions from buoyant turbulent diffusion flames. Fire Saf. Sci. 1991, 3, 625–634. [Google Scholar] [CrossRef][Green Version]
- Turns, S.R. An Introduction to Combustion, 2nd ed.; McGraw-Hill Companies: New York, NY, USA, 2000; pp. 158–167, 553–559, 584–586. [Google Scholar]
- Pitts, W.M. The global equivalence ratio concept and the formation mechanisms of carbon monoxide in enclosure fires. Prog. Energy Combust. Sci. 1995, 21, 197–237. [Google Scholar] [CrossRef]
- Smoke, M.D.; Long, M.B.; Connelly, B.C.; Colket, M.B.; Hall, R.J. Soot formation in laminar diffusion flames. Combust. Flame. 2005, 143, 613–628. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.H. Soot formation in laminar counterflow flames. Prog. Energy Combust. Sci. 2019, 74, 152–238. [Google Scholar] [CrossRef]
- Shaddix, C.R.; Williams, T.C. Soot: Giver and Taker of light. Am. Sci. 2007, 95, 232–239. [Google Scholar] [CrossRef]
- Roper, F.G. The prediction of laminar jet diffusion flame sizes: Part, I. Theoretical model. Combust. Flame. 1977, 29, 219–226. [Google Scholar] [CrossRef]
- Axelbaum, R.L.; Law, C.K. Soot formation and inert addition in diffusion flames. Proc. Combust. Inst. 1991, 23, 1517–1523. [Google Scholar] [CrossRef]
- Dai, Z.; Faeth, G.M. Hydrodynamic suppression of soot formation in laminar coflowing jet diffusion flames. Proc. Combust. Inst. 2000, 28, 2085–2092. [Google Scholar] [CrossRef]
- Botero, M.L.; Mosbach, S.; Kraft, M. Sooting tendency of paraffin components of diesel and gasoline in diffusion flames. Fuel 2014, 126, 8–15. [Google Scholar] [CrossRef]
- Peña, G.D.J.G.; Raj, A.; Stephen, S.; Anjana, T.; Hammid, Y.A.S.; Brito, J.L.; Shoaibi, A.A. Physicochemical properties of soot generated from toluene diffusion flames: Effects of fuel flow rate. Combust. Flame. 2017, 178, 286–296. [Google Scholar] [CrossRef]
- Peña, G.D.J.G.; Rahman, R.K.; Raj, A.; Stephen, S.; Anjana, T.; Brito, J.L. Effect of fuel flow rate on the characteristics of soot generated from unsubstituted and disubstituted aromatic hydrocarbon flames: Experimental and numerical study. Combust. Flame. 2018, 190, 224–239. [Google Scholar] [CrossRef]
- National Fire Protection Association; Society of Fire Protection Engineers. Generation of Heat and Chemical Compounds in Fires. In SFPE Handbook of Fire Protection Engineering, 3rd ed.; DiNenno, P.J., Drysdale, D., Eds.; Quincy, Mass.: Bethesda, MD, USA, 2002; pp. 2–35, 2-258–2-268, 3-111–3-115. [Google Scholar]
- McGrattan, K.; Hostikka, S.; McDermott, R.; Floyd, J.; Weinschenk, C.; Overholt, K. Fire Dynamics Simulator Technical Reference Guide Volume 1: Mathematical Model, 6th ed.; NIST Special Publication 1018-1; National Institute of Standard and Technology: Gaithersburg, MD, USA, 2020. Available online: https://pages.nist.gov/fds-smv/manuals.html (accessed on 14 January 2020).
- Luche, J.; Rogaume, T.; Richard, F.; Guillaume, E. Characterization of thermal properties and analysis of combustion behavior of PMMA in a cone calorimeter. Fire Saf. J. 2001, 46, 451–461. [Google Scholar] [CrossRef]
- Abu-Bakar, A.S.; Cran, M.J.; Moinuddin, K.A.M. Experimental investigation of effects of variation in heating rate, temperature and heat flux on fire properties of a non-charring polymer. J. Therm. Anal. Calorim. 2018, 137, 447–459. [Google Scholar] [CrossRef]
- Rhodes, B.T.; Quintiere, J.G. Burning rate and flame heat flux for PMMA in a cone calorimeter. Fire Saf. J. 1996, 26, 221–240. [Google Scholar] [CrossRef]
- Hopkins, D.; Quintiere, J.G. Material fire properties and predictions for thermoplastics. Fire Saf. J. 1996, 26, 241–268. [Google Scholar] [CrossRef]
- Lyon, R.; Quintiere, J. Criteria for piloted ignition of combustible solids. Combust. Flame 2007, 151, 551–559. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Li, M.-J.; Shih, I.-Y.; Jih, R.; Wong, S.-C. Experimental and Numerical Study of Autoignition and Pilot Ignition of PMMA Plates in a Cone Calorimeter. Combust. Flame 2001, 124, 466–480. [Google Scholar] [CrossRef]
- Shi, L.; Chew, M.Y.L. Fire behaviors of polymers under autoignition conditions in a cone calorimeter. Fire Saf. J. 2013, 61, 243–253. [Google Scholar] [CrossRef]
- Babrauskas, V.; Grayson, S.J. Heat Release in Fires; Interscience Communications Ltd.: London, UK, 1992; Chapter 8; pp. 251–255. [Google Scholar]
- Babrauskas, V.; Twilley, W.H.; Janssens, M.; Yusa, S. A cone calorimeter for controlled-atmosphere studies. Fire Mater. 1992, 16, 37–43. [Google Scholar] [CrossRef]
- Cho, J.H. An Experimental Study on the Ignition Characteristics of Solid Combustibles in Simulated Fire Environment. Master’s Thesis, Department of Fire and Disaster Prevention, Daejeon University, Daejeon, Korea, August 2016. [Google Scholar]
- Kim, B.J. Study on Ignition Characteristics of Solid Flammables Using Open and Controlled Atmosphere Cone Calorimeter. Master’s Thesis, Department of Fire and Disaster Prevention, Daejeon University, Daejeon, Korea, February 2017. [Google Scholar]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Parker, W.J. Calculations of the heat release rate by oxygen consumption for various applications. J. Fire Sci. 1984, 2, 380–395. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nam, D.G. Fire characteristics of flaming and smoldering combustion of wood combustibles considering Thickness. Fire Sci. Eng. 2015, 29, 67–72. [Google Scholar] [CrossRef][Green Version]
- Collier, P.C.R.; Whiting, P.N.; Wade, C.A. Fire Properties of Wall and Ceiling Linings: Investigation of Fire Test Methods for Use in NZBC Compliance Documents; BRANZ Study Report SR 160; Branz Ltd.: Judgeford, New Zealand, 2006. [Google Scholar]
- Kohse-Höinghaus, K.; Jeffries, J.B. Applied Combustion Diagnostics. In Combustion: An International Series; Taylor & Francis: New York, NY, USA, 2002; Chapter 20; pp. 218–233. [Google Scholar]
- Ko, G.H.; Park, C.H.; Hwang, C.H.; Park, S.H. A Study on the Combustion Efficiency Concept in Under-ventilated Compartment Fires. Fire Sci. Eng. 2010, 24, 145–152. [Google Scholar]
- Tsuchiya, Y. CO/CO2 Ratios in Fire. Fire Saf. Sci. 1994, 4, 515–526. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, S.-Y.; Cho, J.-H.; Hwang, C.-H. Effects of External Heat Flux and Exhaust Flow Rate on CO and Soot Yields of Acrylic in a Cone Calorimeter. Appl. Sci. 2021, 11, 5942. https://doi.org/10.3390/app11135942
Mun S-Y, Cho J-H, Hwang C-H. Effects of External Heat Flux and Exhaust Flow Rate on CO and Soot Yields of Acrylic in a Cone Calorimeter. Applied Sciences. 2021; 11(13):5942. https://doi.org/10.3390/app11135942
Chicago/Turabian StyleMun, Sun-Yeo, Jae-Ho Cho, and Cheol-Hong Hwang. 2021. "Effects of External Heat Flux and Exhaust Flow Rate on CO and Soot Yields of Acrylic in a Cone Calorimeter" Applied Sciences 11, no. 13: 5942. https://doi.org/10.3390/app11135942
APA StyleMun, S.-Y., Cho, J.-H., & Hwang, C.-H. (2021). Effects of External Heat Flux and Exhaust Flow Rate on CO and Soot Yields of Acrylic in a Cone Calorimeter. Applied Sciences, 11(13), 5942. https://doi.org/10.3390/app11135942






