Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Materials
2.3. Microwave-Assisted Extraction (MAE)
2.4. Total Polyphenol Content (TPC)
2.5. Acquisition of Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Spectra
2.6. HPLC Analysis
2.7. Microencapsulation Process: Liquid Feed Preparation and Spray-Drying Conditions
2.8. Powder Characterization
2.8.1. Production Yield and Encapsulation Efficiency
2.8.2. Quantitative Analysis
2.8.3. Differential Scanning Calorimetry (DSC)
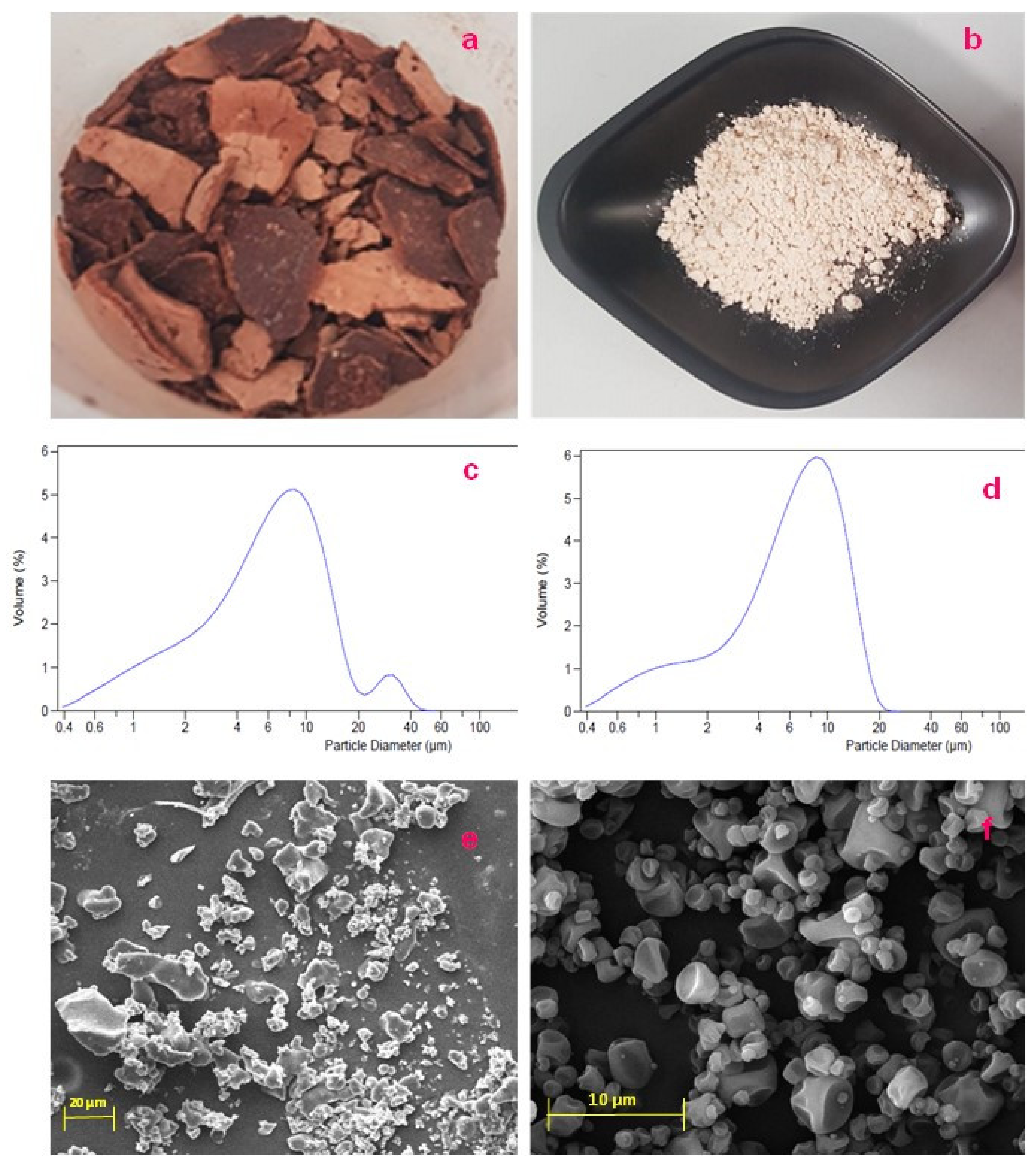
2.8.4. Dimensional Distribution (LLS)
2.8.5. Morphological Analysis of Materials
2.8.6. Powder Flowability Test
2.8.7. Dissolution/Release Test in Water
2.8.8. Free Radical Scavenging Activity
2.8.9. Stability Study
2.9. Statistical Analysis
3. Results and Discussion
3.1. Microwave-Assisted Extraction
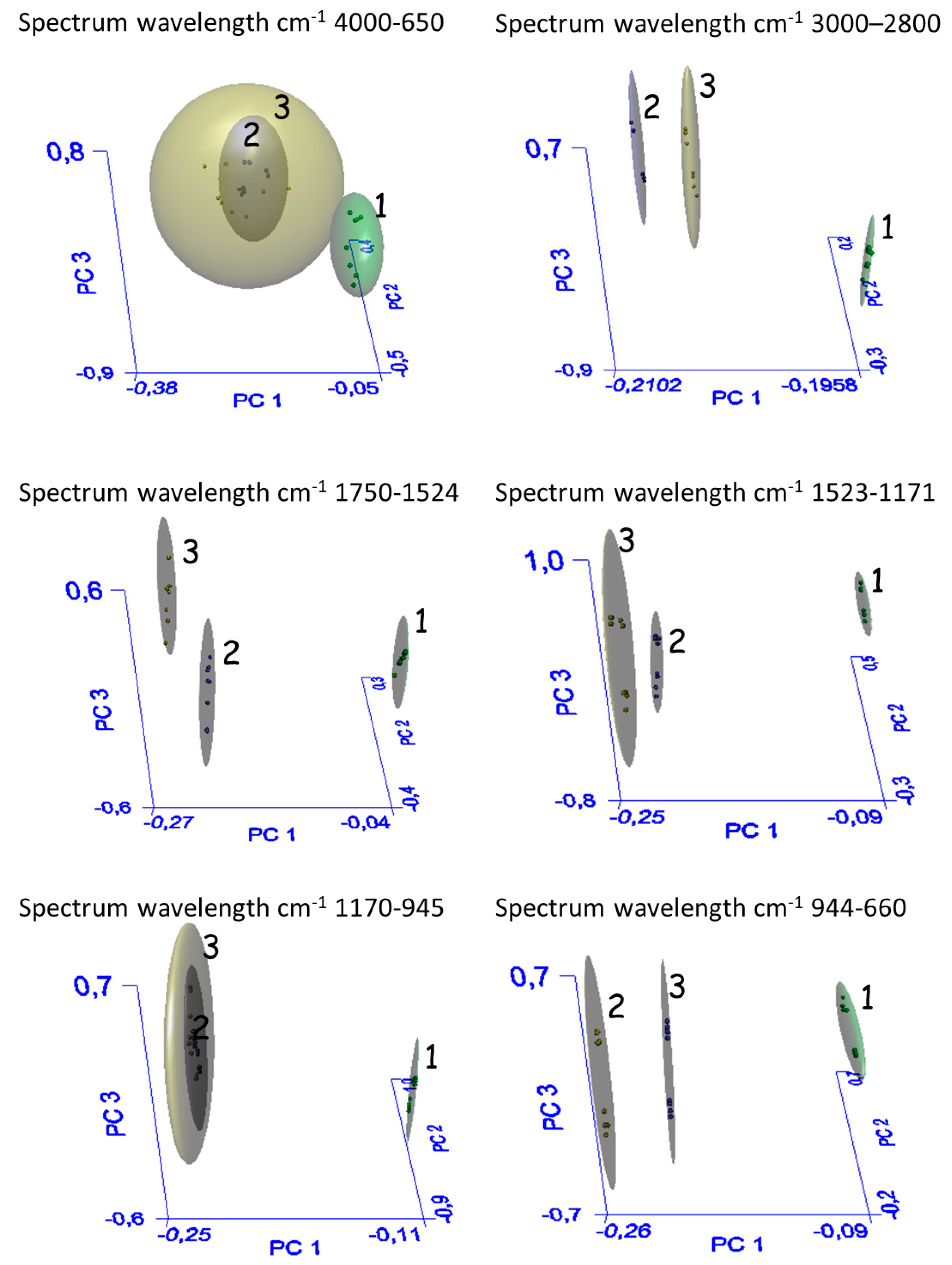
3.2. Spectroscopic Profile by ATR-FTIR
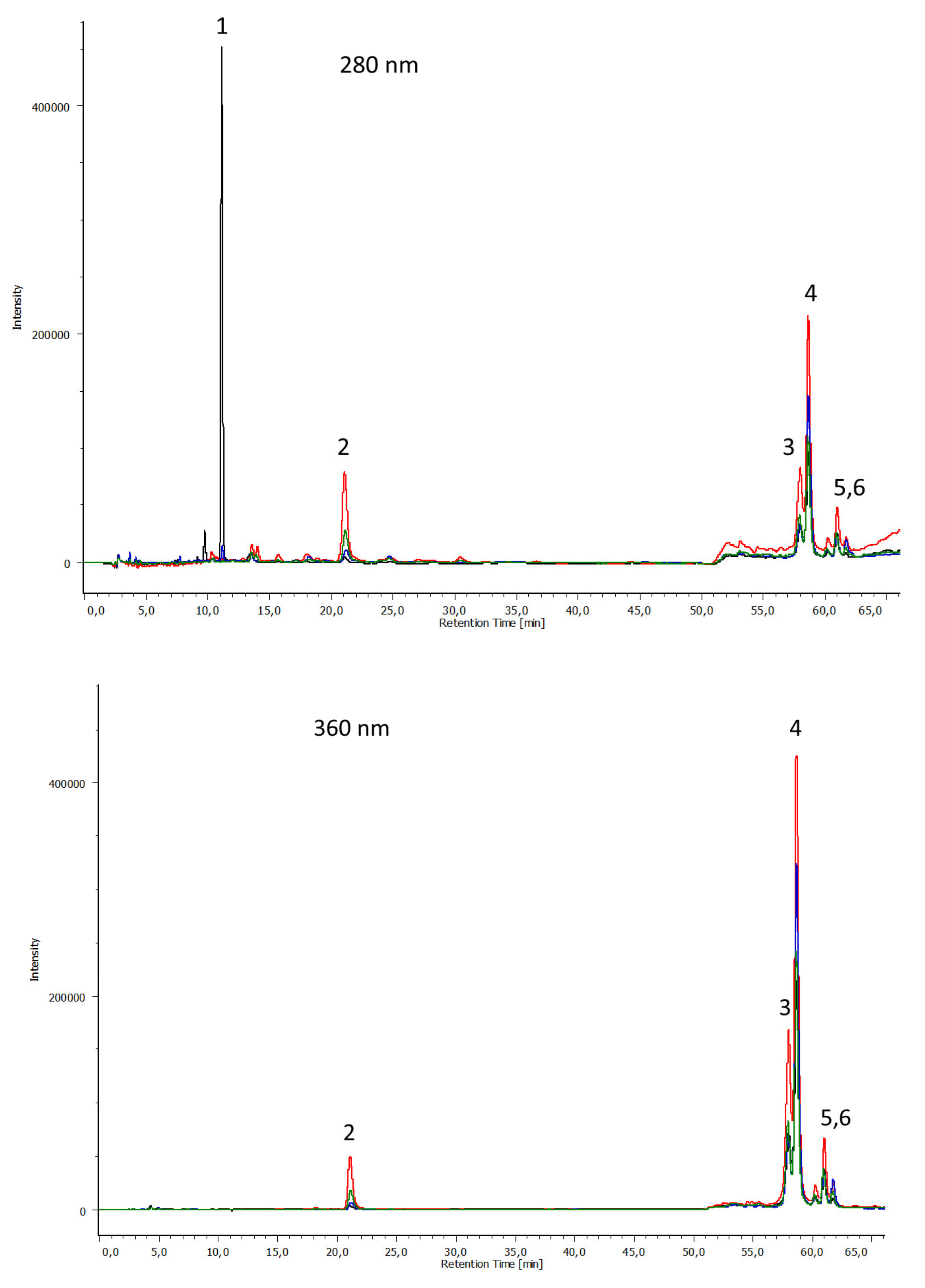
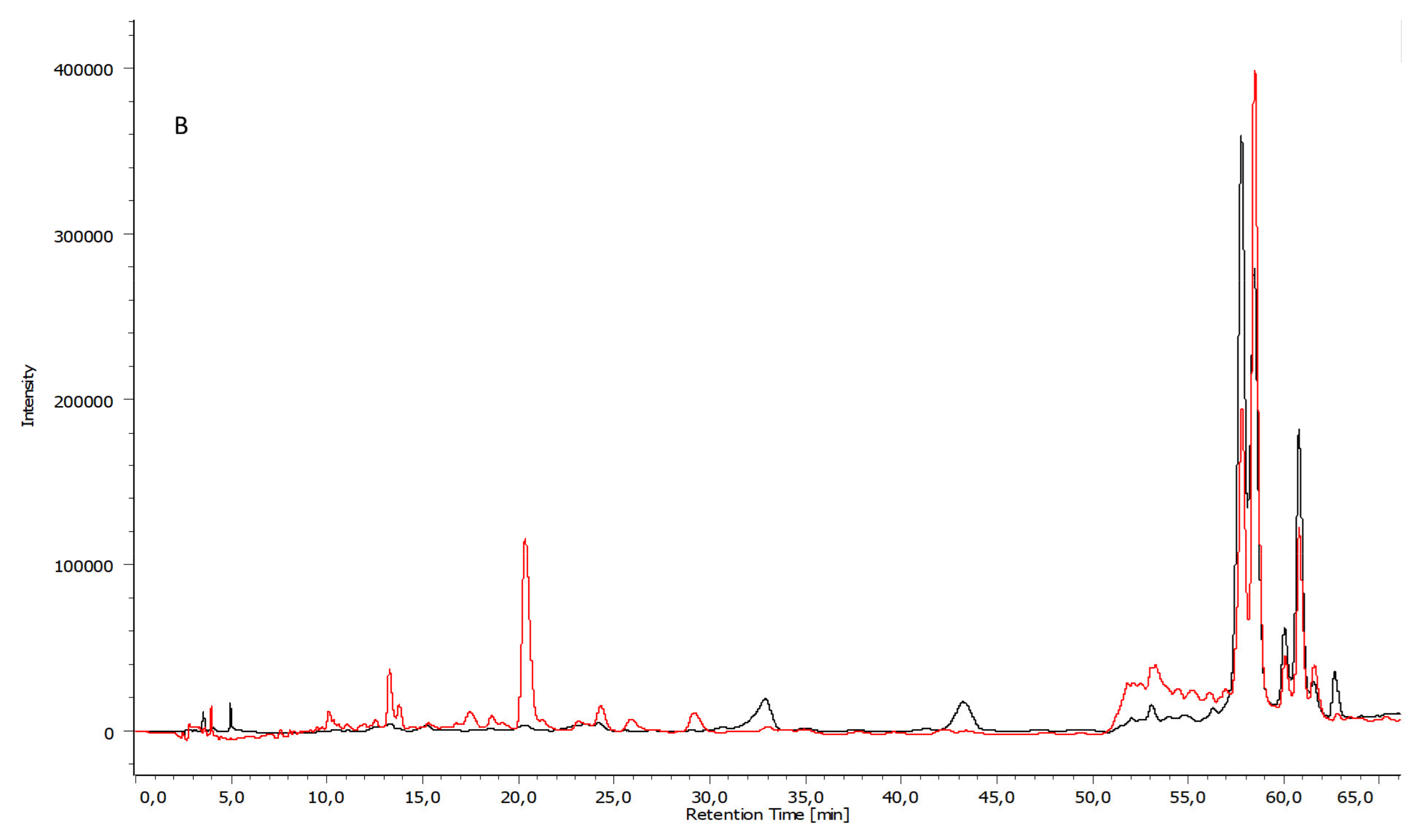
3.3. Chemical Composition by HPLC
3.4. Microencapsulation Process and Evaluation of Process Efficiency
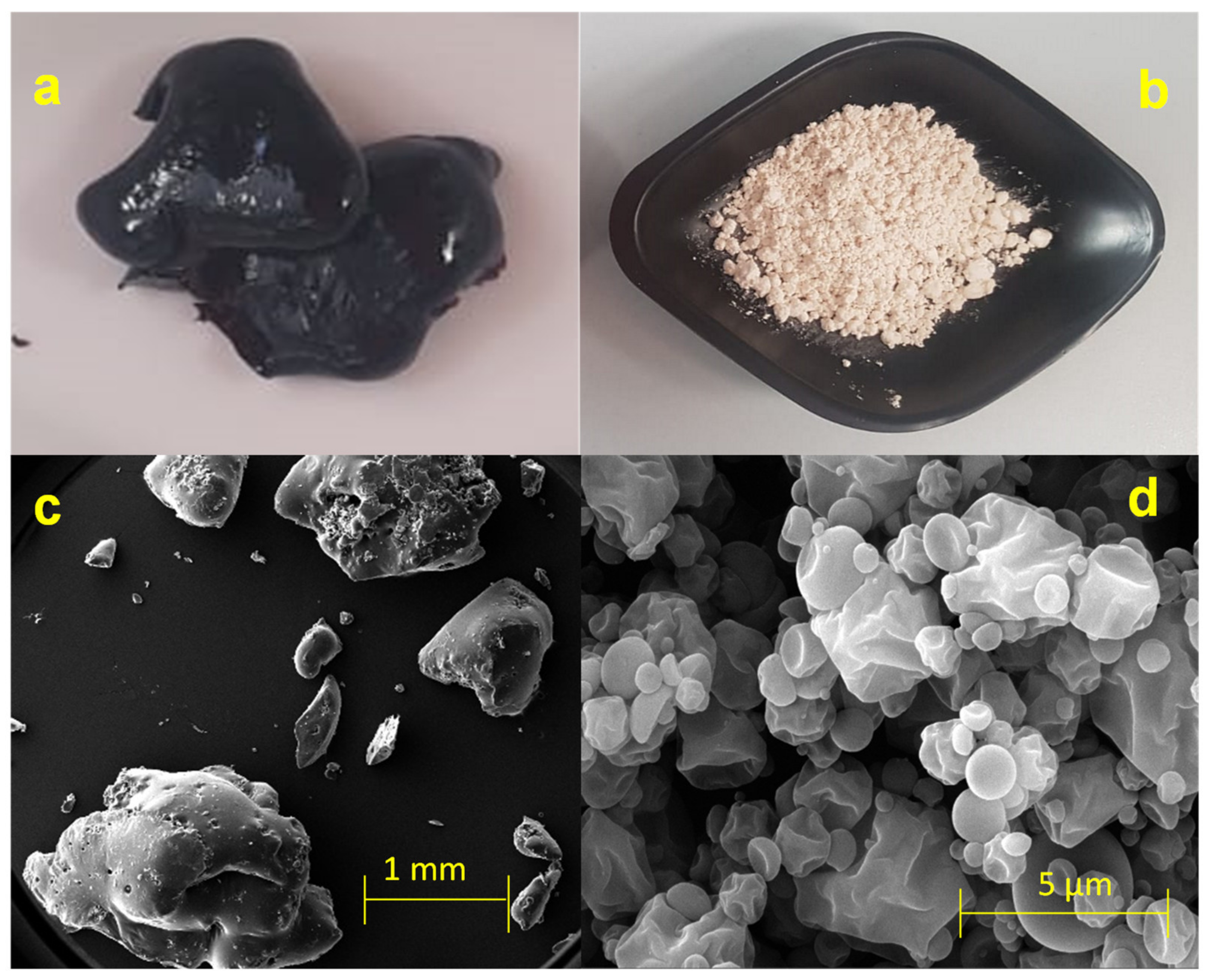
3.5. Powder Characterization: Macroscopic Analysis and Morphology
3.6. Derived Powder Properties: Powder Flowability
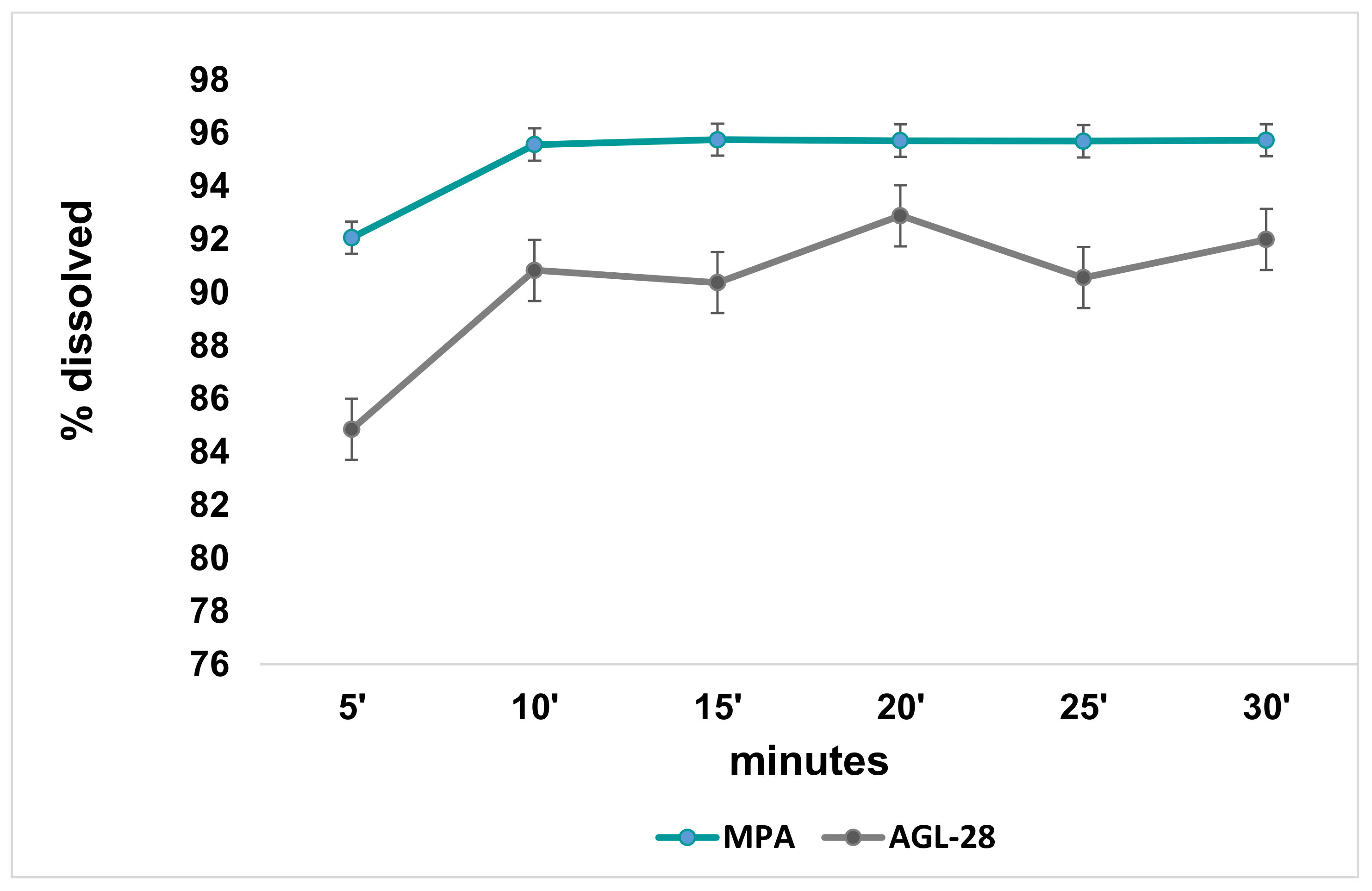
3.7. Dissolution/Release Test
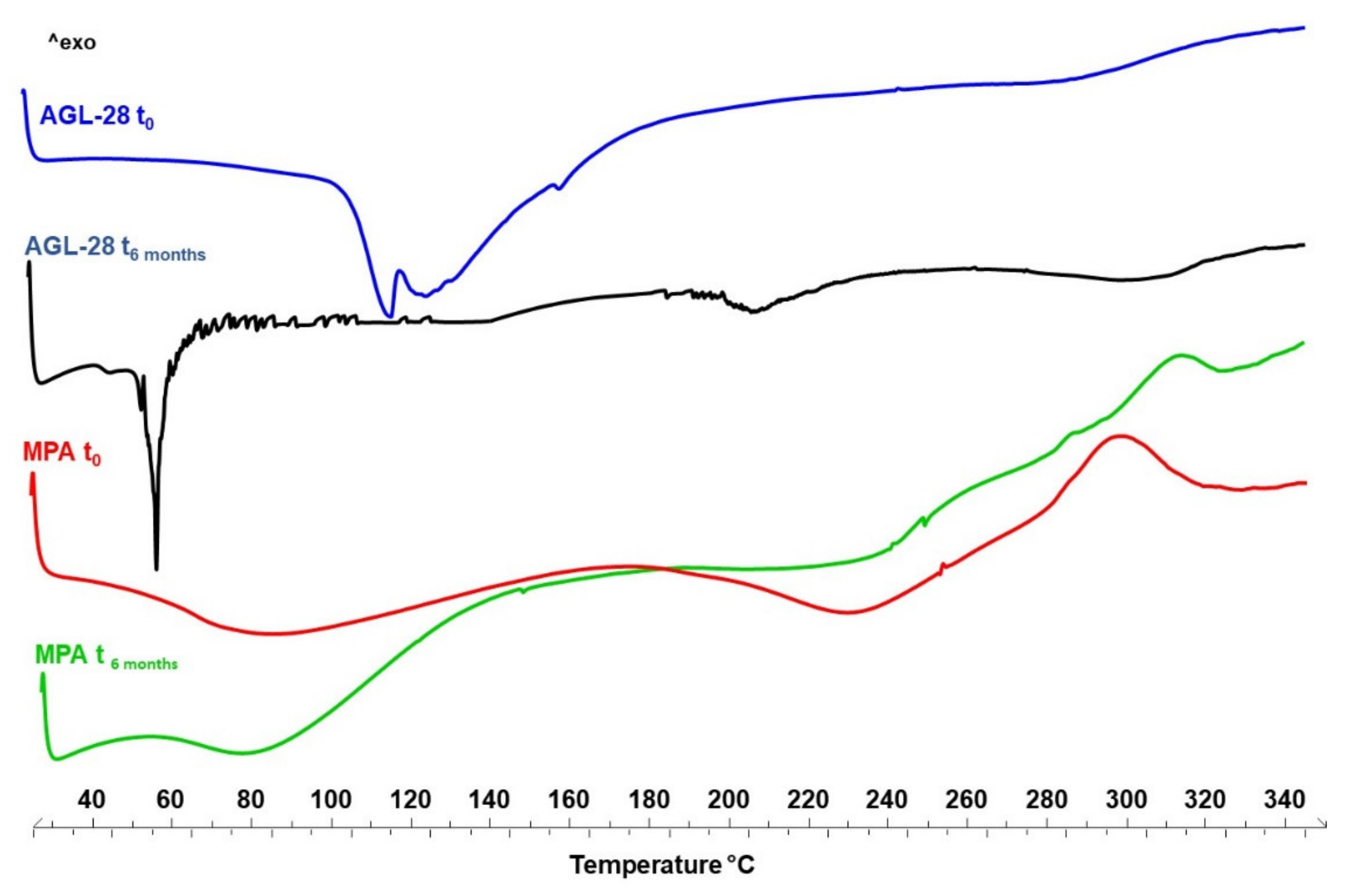
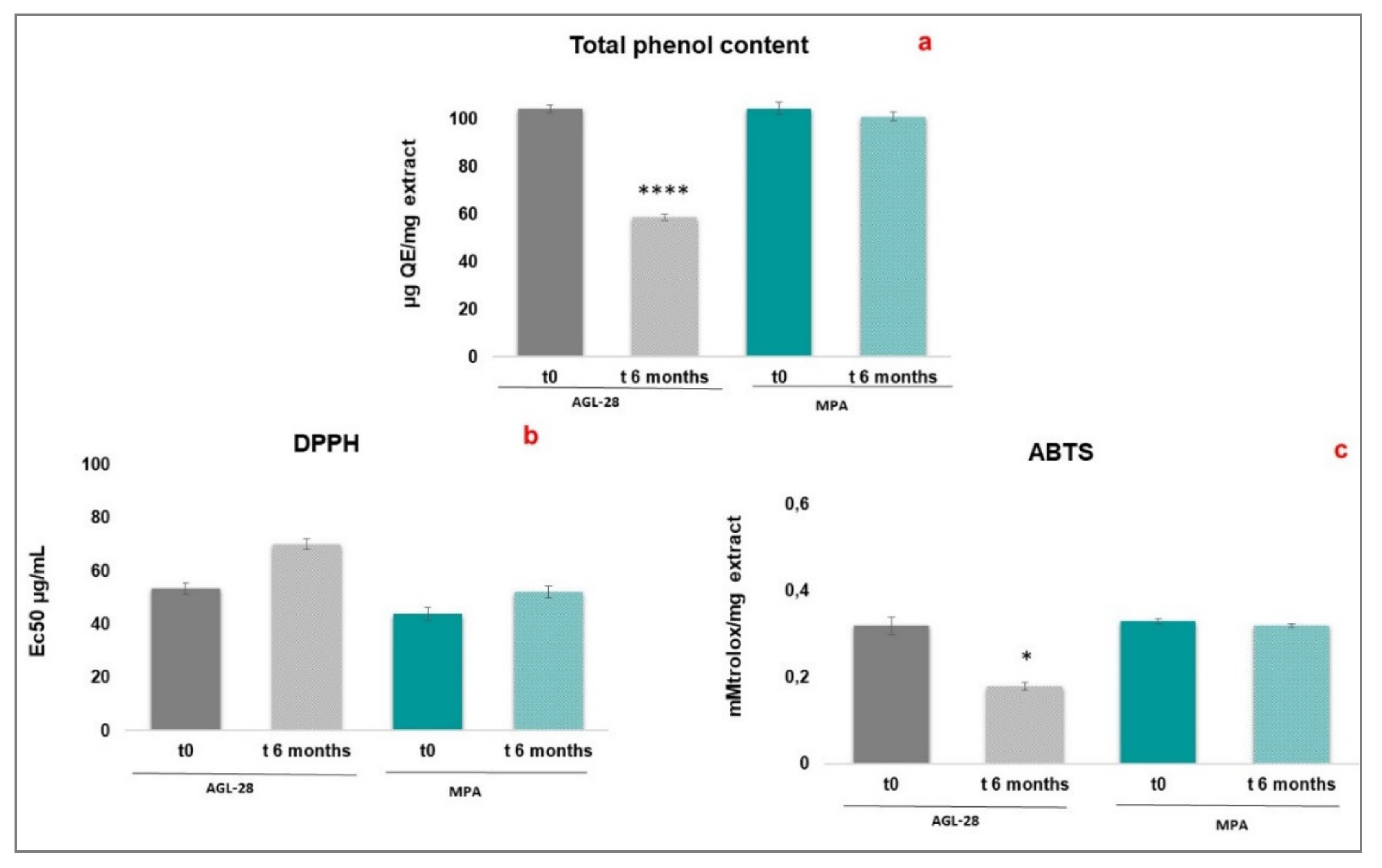
3.8. Evaluation of Physical-Chemical and Functional Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Tristantini, D.; Setiawan, H.; Santoso, L.L. Feasibility assessment of an encapsulated longevity spinach (Gynura procumbens L.) extract plant in Indonesia. Appl. Sci. 2021, 11, 4093. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Dresch, R.R.; Dresch, M.K.; Guerreiro, A.F.; Biegelmeyer, R.; Holzschuh, M.H.; Rambo, D.F.; Henriques, A.T. Phenolic Compounds from the Leaves of Vitis labrusca and Vitis vinifera L. as a Source of Waste Byproducts: Development and Validation of LC Method and Antichemotactic Activity. Food Anal. Methods 2014, 7, 527–539. [Google Scholar] [CrossRef]
- Tedesco, I.; Moccia, S.; Volpe, S.; Alfieri, G.; Strollo, D.; Bilotto, S.; Spagnuolo, C.; Di Renzo, M.; Aquino, R.P.; Russo, G.L. Red wine activates plasma membrane redox system in human erythrocytes. Free Radic. Res. 2016, 50, 557–569. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Ventura-Sobrevilla, J.M.; González-Hernández, M.D.; Rojas, R.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Martínez-Ávila, G.C.G. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef]
- Karami, Z.; Emam-Djomeh, Z.; Mirzaee, H.A.; Khomeiri, M.; Mahoonak, A.S.; Aydani, E. Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root. J. Food Sci. Technol. 2015, 52, 3242–3253. [Google Scholar] [CrossRef] [Green Version]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New insights into the exploitation of Vitis vinifera L. Cv. aglianico leaf extracts for nutraceutical purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef]
- Sansone, F.; Esposito, T.; Mencherini, T.; Piccinelli, A.L.; Gazzerro, P.; Picerno, P.; Russo, P.; Del Gaudio, P.; Essolito, M.; Campiglia, P.; et al. Annurca peel extract: From the chemical composition, through the functional activity, to the formulation and characterisation of a topical oil-in-water emulsion. Nat. Prod. Res. 2016, 30, 1398–1403. [Google Scholar] [CrossRef]
- Menezes Oliveira, D.; Clemente, E.; Afonso, M.R.A.; da Costa, J.M.C. Hygroscopic behavior of lyophilized powder of grugru palm (Acrocomia aculeata). Am. J. Anal. Chem. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Trindade, E.P.; Regis, F.T.; da Silva, G.A.; Aguillar, B.N.; de Paiva Amorim, M.V.; Ramos Ferreira, G.L.; Soares Aragão, C.F.; da Silva Solon, L.G. Laboratory-scale preparation and characterization of dried extract of Muirapuama (Ptychopetalumolacoides Benth) by green analytical techniques. Molecules 2020, 25, 1095. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Sansone, F.; Auriemma, G.; Franceschelli, S.; Pecoraro, M.; Picerno, P.; Aquino, R.P.; Mencherini, T. Study on ajuga reptans extract: A natural antioxidant in microencapsulated powder form as an active ingredient for nutraceutical or pharmaceutical purposes. Pharmaceutics 2020, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Mencherini, T.; Picerno, P.; D’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Picerno, P.; Sansone, F.; Mencherini, T.; Prota, L.; Aquino, R.P.; Rastrelli, L.; Lauro, M.R. Citrus bergamia juice: Phytochemical and technological studies. Nat. Prod. Commun. 2011, 6, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Righi da Rosa, J.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; Ragagnin de Menezes, C.; Severo da Rosa, C. Microencapsulation of anthocyanin compounds extracted from blueberry (Vaccinium spp.) by spray drying: Characterization, stability and simulated gastrointestinal conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Rutz, J.K.; Zambiazi, R.C.; Borges, C.D.; Krumreich, F.D.; Da Luz, S.R.; Hartwig, N.; Da Rosa, C.G. Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydr. Polym. 2013, 98, 1256–1265. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; Esposito, T.; Del Gaudio, P.; Russo, P.; Pepe, G.; Lauro, M.R.; Aquino, R.P. Microencapsulation by spray drying of Lannea microcarpa extract: Technological characteristics and antioxidant activity. J. Pharm. Pharmacogn. Res. 2014, 2, 100–109. [Google Scholar]
- Picariello, G.; De Vito, V.; Ferranti, P.; Paolucci, M.; Volpe, M.G. Species- and cultivar-dependent traits of Prunus avium and Prunus cerasus polyphenols. J. Food Compos. Anal. 2016, 45, 50–57. [Google Scholar] [CrossRef]
- Volpe, M.G.; Costantini, S.; Coccia, E.; Parrillo, L.; Paolucci, M. Evaluation of metabolic changes induced by polyphenols in the crayfish Astacus leptodactylus by metabolomics using Fourier transformed infrared spectroscopy. J. Biosci. 2018, 43, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Esposito, T.; Lauro, M.R.; Picerno, P.; Mencherini, T.; Gasparri, F.; De Santis, S.; Chieppa, M.; Cirillo, C.; Aquino, R.P. Application of spray drying particle engineering to a high-functionality/low-solubility milk thistle extract: Powders production and characterization. Molecules 2018, 23, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquino, R.P.; Auriemma, G.; Conte, G.M.; Esposito, T.; Sommella, E.; Campiglia, P.; Sansone, F. Development of chitosan/mannitol microparticles as delivery system for the oral administration of a Spirulina bioactive peptide extract. Molecules 2020, 25, 2086. [Google Scholar] [CrossRef]
- Ministry of Labor, Health and Social Policies—Permanent Commission for Review and the Publication of the Official Pharmacopeia. Farmacopea Ufficiale Italiana, XII ed.; Istituto Poligrafico dello Stato: Roma, Italy, 2008.
- Aïssaoui, H.; Mencherini, T.; Esposito, T.; De Tommasi, N.; Gazzerro, P.; Benayache, S.; Benayache, F.; Mekkiou, R. Heliotropium bacciferum Forssk. (Boraginaceae) extracts: Chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat. Prod. Res. 2019, 33, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Djemaa-Landri, K.; Hamri-Zeghichi, S.; Valls, J.; Cluzet, S.; Tristan, R.; Boulahbal, N.; Kadri, N.; Madani, K. Phenolic content and antioxidant activities of Vitis vinifera L. leaf extracts obtained by conventional solvent and microwave-assisted extractions. J. Food Meas. Charact. 2020, 14, 3551–3564. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Ezhilarasi, P.N.; Jena, B.S.; Anandharamakrishnan, C. A comparative study on conventional and microwave-assisted extraction for microencapsulation of Garcinia fruit extract. Food Bioprod. Process. 2013, 91, 103–110. [Google Scholar] [CrossRef]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. Varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Baranska, M. Optical Spectroscopy and Computational Methods in Biology and Medicine; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 9789400778320. [Google Scholar]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [Green Version]
- Wahyono, T.; Astuti, D.A.; Komang Gede Wiryawan, I.; Sugoro, I.; Jayanegara, A. Fourier Transform Mid-Infrared (FTIR) Spectroscopy to Identify Tannin Compounds in the Panicle of Sorghum Mutant Lines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042045. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Volpe, M.G.; Paolucci, M.; Pacifico, S. UHPLC-HR-MS/MS-Guided Recovery of Bioactive. Molecules 2019, 24, 3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackshaw, K.V.; Miller, J.S.; Aykas, D.P.; Rodriguez-Saona, L. Vibrational Spectroscopy for Identification of Metabolites in Biologic Samples. Molecules 2020, 25, 4725. [Google Scholar] [CrossRef]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Li, X.; Zhou, R.; Xu, K.; Xu, J.; Jin, J.; Fang, H.; He, Y. Rapid determination of chlorophyll and pheophytin in green tea using fourier transform infrared spectroscopy. Molecules 2018, 23, 1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranska, M.; Movasaghi, Z.; Rehman, S.; Rehman, I.U.; Kokalj Ladan, M.; Straus, J.; Tavčar Benković, E.; Kreft, S.; Samples, B.; Baker, M.J.; et al. FT-IR-based method for rutin, quercetin and quercitrin quantification in different buckwheat (Fagopyrum) species. Appl. Spectrosc. Rev. 2017, 9, 134–179. [Google Scholar]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- He, J.; Rodriguez-Saona, L.E.; Giusti, M.M. Midinfrared spectroscopy for juice authentication-rapid differentiation of commercial juices. J. Agric. Food Chem. 2007, 55, 4443–4452. [Google Scholar] [CrossRef]
- Zeb, A. A reversed phase HPLC-DAD method for the determination of phenolic compounds in plant leaves. Anal. Methods 2015, 7, 7753–7757. [Google Scholar] [CrossRef]
- Schneider, E.; Von Der Heydt, H.; Esperester, A. Evaluation of polyphenol composition in red leaves from different varieties of Vitis vinifera. Planta Med. 2008, 74, 565–572. [Google Scholar] [CrossRef]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; MacÊdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Portu, J.; López-Alfaro, I.; Gómez-Alonso, S.; López, R.; Garde-Cerdán, T. Changes on grape phenolic composition induced by grapevine foliar applications of phenylalanine and urea. Food Chem. 2015, 180, 171–180. [Google Scholar] [CrossRef]
- Caliskan, G.; Nur Dirim, S. The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying of sumac extract. Food Bioprod. Process. 2013, 91, 539–548. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, S.; Mahanta, C.L. Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of amla (Emblica officinalis) juice powder. Food Bioprod. Process. 2014, 92, 252–258. [Google Scholar] [CrossRef]


| Power (Watt) | Solvent | Extraction Time (min) | Solid:Liquid Ratio |
|---|---|---|---|
| 180 | EtOH | 5 | 1:10 |
| MeOH:H2O (70:30) | 5 | 1:10 | |
| H2O | 5 | 1:10 | |
| 300 | EtOH | 2 | 1:10 |
| MeOH:H2O (70:30) | 2 | 1:10 | |
| H2O | 2 | 1:10 |
| Compressibility Index | Flow Evaluation | Hausner Ratio |
|---|---|---|
| ≤10 | Excellent | 1.00–1.11 |
| 11–15 | Good | 1.12–1.18 |
| 16–20 | Fair | 1.19–1.25 |
| 21–25 | Passable | 1.26–1.34 |
| 26–31 | Poor | 1.35–1.45 |
| 32–37 | Very poor | 1.46–1.59 |
| >38 | Very, very poor | >1.60 |
| Polyphenols (mg/g) | |||
|---|---|---|---|
| 180 W 5 min | Extraction Solvent | ||
| EtOH | MeOH:H2O | H2O | |
| Aglianico October (AGL-1) | 24.92 ± 3.11 | 40.69 ± 3.76 | 75.39 ± 6.93 |
| Aglianico November (AGL-2) | 26.14 ± 1.98 | 54.99 ± 4.99 | 83.61 ± 7.45 |
| Greco di Tufo October (GRC-1) | 17.43 ± 2.12 | 45.34 ± 3.56 | 79.18 ± 6.43 |
| Greco di Tufo November (GRC-2) | 28.80 ± 3.71 | 25.76 ± 2.41 | 87.79 ± 7.51 |
| 300 W 2 min | |||
| EtOH | MeOH:H2O | H2O | |
| Aglianico October (AGL-18) | 16.00 ± 1.11 | 26.16 ± 2.05 | 83.99 ± 7.49 |
| Aglianico November (AGL-28) | 23.42 ± 1.57 | 43.64 ± 3.94 | 100.67 ± 9.29 |
| Greco di Tufo October (GRC-18) | 26.07 ± 1.99 | 35.47 ± 3.12 | 80.96 ± 7.50 |
| Greco di Tufo November (GRC-28) | 8.65 ± 0.75 | 22.36 ± 2.10 | 95.63 ± 9.51 |
| Spectral Ranges Analyzed with SIMCA | Peak Wavelength (cm−1) | Vibrational Mode | ||
|---|---|---|---|---|
| Solvent | ||||
| Ethanol | Methanol–Water (70:30) | Water | ||
| 3600–3000 | 3353- | 3322 | 3332 | OH and C-H stretch. |
| 2999–2800 | 2961- | CH2 and CH3 stretching vibrations | ||
| 2926- | 2932 | 2932 | ||
| 2855- | ||||
| 1750–1524 | 1721- | 1719 | 1720 | Carbonyl C=O streching |
| 1655- | Aromatic ring C=C stretching | |||
| 1608- | 1606 | 1606 | ||
| 1523–1171 | 1449- | |||
| 1398 | 1398 | C–O stretching vibrations | ||
| 1376 | ||||
| 1308 | 1308 | 1308 | ||
| 1251 | 1264 | 1260 | ||
| 1204 | 1202 | 1200 | ||
| 1170–945 | 1078 | 1075 | 1076 | Aromatic C–H in plane bend. C–O stretching vibrations |
| 944–660 | 920 | 920 | 92 | C–H deformation vibrations, out-of-plane bend |
| 895 | 895 | 893 | ||
| 823 | 820 | 820 | ||
| 778 | 778 | 779 | ||
| 712 | 712 | 712 | ||
| Spectrum Wavelength cm−1 4000–650 | ||||
| Groups | Recognition (%) a | Rejection (%) b | Interclass Distance c | |
| EtOH | 100(8/8) | 100(16/16) | EtOH-MeOH:H2O | 140 |
| MeOH: H2O | 100(8/8) | 93(15/16) | EtOH-H2O | 83 |
| H2O | 100(8/8) | 75(12/16) | MeOH: H2O-H2O | 2 |
| Spectrum wavelength cm−1 3000–2800 | ||||
| Groups | Recognition (%) a | Rejection (%) b | Interclass distance c | |
| EtOH | 100(8/8) | 100(16/16) | EtOH-MeOH:H2O | 23 |
| MeOH: H2O | 100(8/8) | 93(15/16) | EtOH-H2O | 24 |
| H2O | 100(8/8) | 50(8/16) | MeOH: H2O-H2O | 2 |
| Spectrum wavelength cm−1 1750–1524 | ||||
| Groups | Recognition (%) a | Rejection (%) b | Interclass distance c | |
| EtOH | 100(8/8) | 100(16/16) | EtOH-MeOH:H2O | 90 |
| MeOH: H2O | 100(8/8) | 87(14/16) | EtOH-H2O | 670 |
| H2O | 100(8/8) | 100(16/16) | MeOH: H2O-H2O | 4 |
| Spectrum wavelength cm−1 1523–1171 | ||||
| Groups | Recognition (%) a | Rejection (%) b | Interclass distance c | |
| EtOH | 100(8/8) | 100(16/16) | EtOH-MeOH:H2O | 18 |
| MeOH: H2O | 100(8/8) | 100(16/16) | EtOH-H2O | 30 |
| H2O | 100(8/8) | 87(14/16) | MeOH: H2O-H2O | 3 |
| Spectrum wavelength cm−1 1170–945 | ||||
| Groups | Recognition (%) a | Rejection (%) b | Interclass distance c | |
| EtOH | 100(8/8) | 100(16/16) | EtOH-MeOH:H2O | 7 |
| MeOH: H2O | 100(8/8) | 93(15/16) | EtOH-H2O | 5 |
| H2O | 100(8/8) | 50(8/16) | MeOH: H2O-H2O | 4 |
| Spectrum wavelength cm−1 944–660 | ||||
| Groups | Recognition (%) a | Rejection (%) b | Interclass distance c | |
| EtOH | 100(8/8) | 100(16/16) | EtOH-MeOH:H2O | 59 |
| MeOH: H2O | 100(8/8) | 81(13/16) | EtOH-H2O | 31 |
| H2O | 100(8/8) | 62(10/16) | MeOH: H2O-H2O | 2 |
| Vitis vinifera Varieties | Spectrum Wavelength cm−1 4000–650 | ||||
|---|---|---|---|---|---|
| Groups | Recognition (%) a | Rejection (%) b | Interclass Distance c | ||
| Aglianico | October | 100(8/8) | 12(2/16) | AO:AN | 1.35 |
| November | 100(8/8) | 6(15/16) | AN:GO | 3.38 | |
| AO:GO | 1.29 | ||||
| Greco di tufo | October | 100(8/8) | 100(0/16) | GO:GN | 3.40 |
| November | 100(8/8) | 31(5/16) | GN:AN | 1.97 | |
| GN:AO | 1.69 | ||||
| Sample | M/P g/100 mL | AGL-28 g/100 mL | Yield % | TEC a % | TAC b % | AEC c % | AAC d % | EE e % | d50 µm (Span) f |
|---|---|---|---|---|---|---|---|---|---|
| AGL-28 | - | - | - | - | - | - | 10.4 ± 1.5 d | 20.22 (8.13) | |
| MP0 | 10/0.1 | - | 61.84 ± 2.14 | - | - | - | - | - | n.d. |
| MP1 | 10/0.2 | - | 62.43 ± 3.36 | - | - | - | n.d. | ||
| MP2 | 10/0.3 | - | 69.84 ± 2.14 | ||||||
| MP3 | 10/0.4 | - | 77.88 ± 0.17 | - | - | - | - | - | 6.46 (1.74) |
| MP4 | 10/0.5 | - | 78.04 ± 1.21 | - | - | - | - | - | 5.34 (1.99) |
| MPA | 10/0.4 | 0.5 | 83.26 ± 0.34 | 4.8 ± 0.31 f | 0.48 ± 0.05 f | 4.51 ± 0.63 | 0.45 ± 0.08 | 93.96 | 6.17 (2.02) |
| MPA/1 | 10/0.5 | 0.5 | 77.57 ± 2.06 | n.d. g | n.d. | n.d. | n.d. | n.d. | 5.27 (1.47) |
| Sample | Bulk Density (g/cm3) ± S.D. * | Tap Density (g/cm3) ± S.D. | HR ± S.D. | CI ± S.D. | Flow Character |
|---|---|---|---|---|---|
| MPA | 245.70 ± 7.16 | 269.99 ± 9.19 | 1.10 ± 0.01 | 9.00 ± 0.01 | Excellent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, T.; Paolucci, M.; Sansone, F.; Mencherini, T.; Pacifico, S.; Volpe, M.G. Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form. Appl. Sci. 2021, 11, 5827. https://doi.org/10.3390/app11135827
Esposito T, Paolucci M, Sansone F, Mencherini T, Pacifico S, Volpe MG. Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form. Applied Sciences. 2021; 11(13):5827. https://doi.org/10.3390/app11135827
Chicago/Turabian StyleEsposito, Tiziana, Marina Paolucci, Francesca Sansone, Teresa Mencherini, Severina Pacifico, and Maria Grazia Volpe. 2021. "Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form" Applied Sciences 11, no. 13: 5827. https://doi.org/10.3390/app11135827
APA StyleEsposito, T., Paolucci, M., Sansone, F., Mencherini, T., Pacifico, S., & Volpe, M. G. (2021). Exploitation and Valorization of Agro-Food Wastes from Grape Harvesting: Production, Characterization of MAE-Extracts from Vitis vinifera Leaves and Stabilization in Microparticulate Powder Form. Applied Sciences, 11(13), 5827. https://doi.org/10.3390/app11135827











