Study of Different Crystal Habits of Aprepitant: Dissolution and Material Attributes
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solvent Screening and Solubility Determination
2.3. Recrystallization Experiments
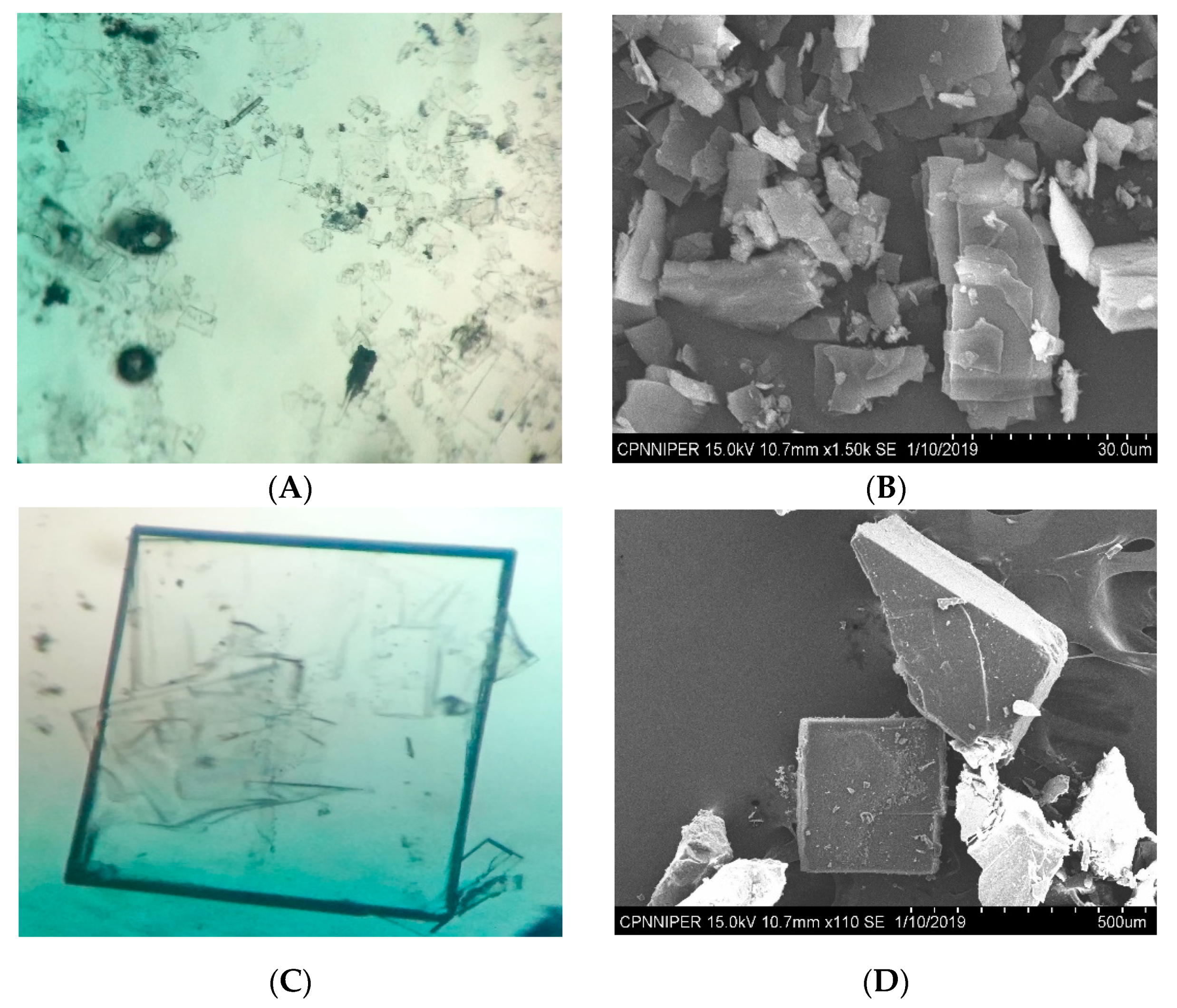
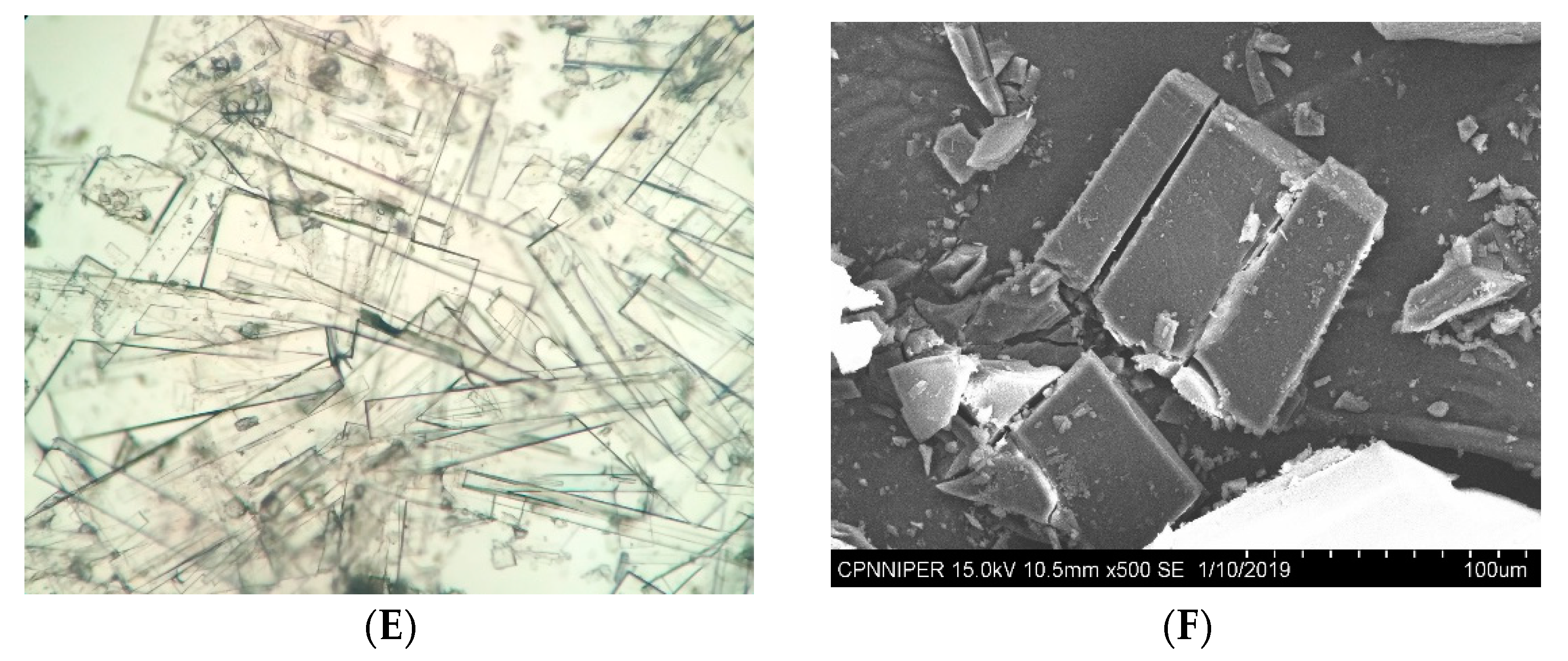
2.4. Optical Microscopy
2.5. Scanning Electron Microscopy and Morphology Generation
2.6. Differential Scanning Calorimetry
2.7. Thermogravimetric Analysis
2.8. Powder X-ray Diffraction
2.9. Fourier Transform Infrared Spectroscopy
2.10. Particle Size Distribution and Surface Area
2.11. Aspect Ratio Determination
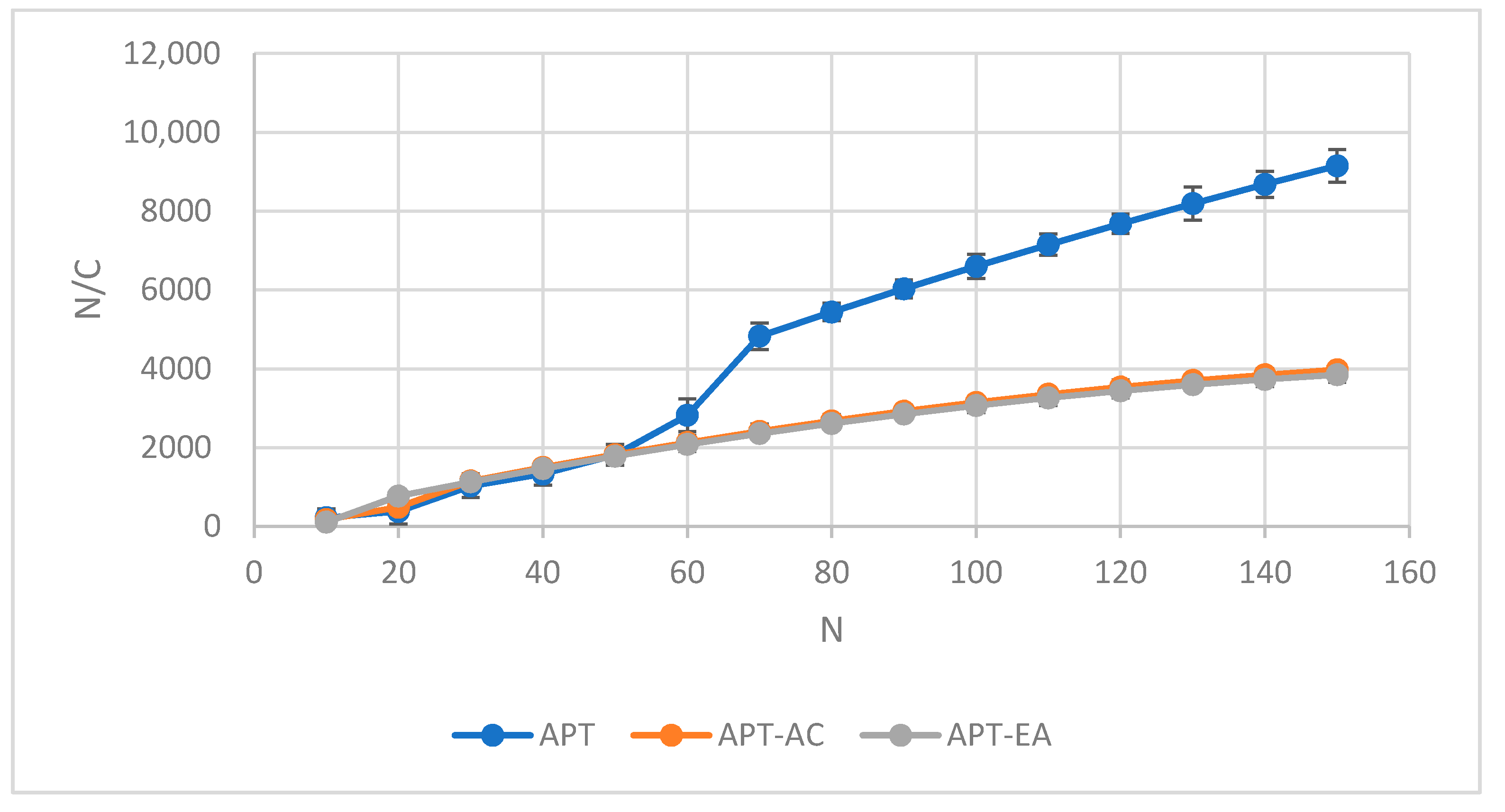
2.12. Kawakita Plots Analysis
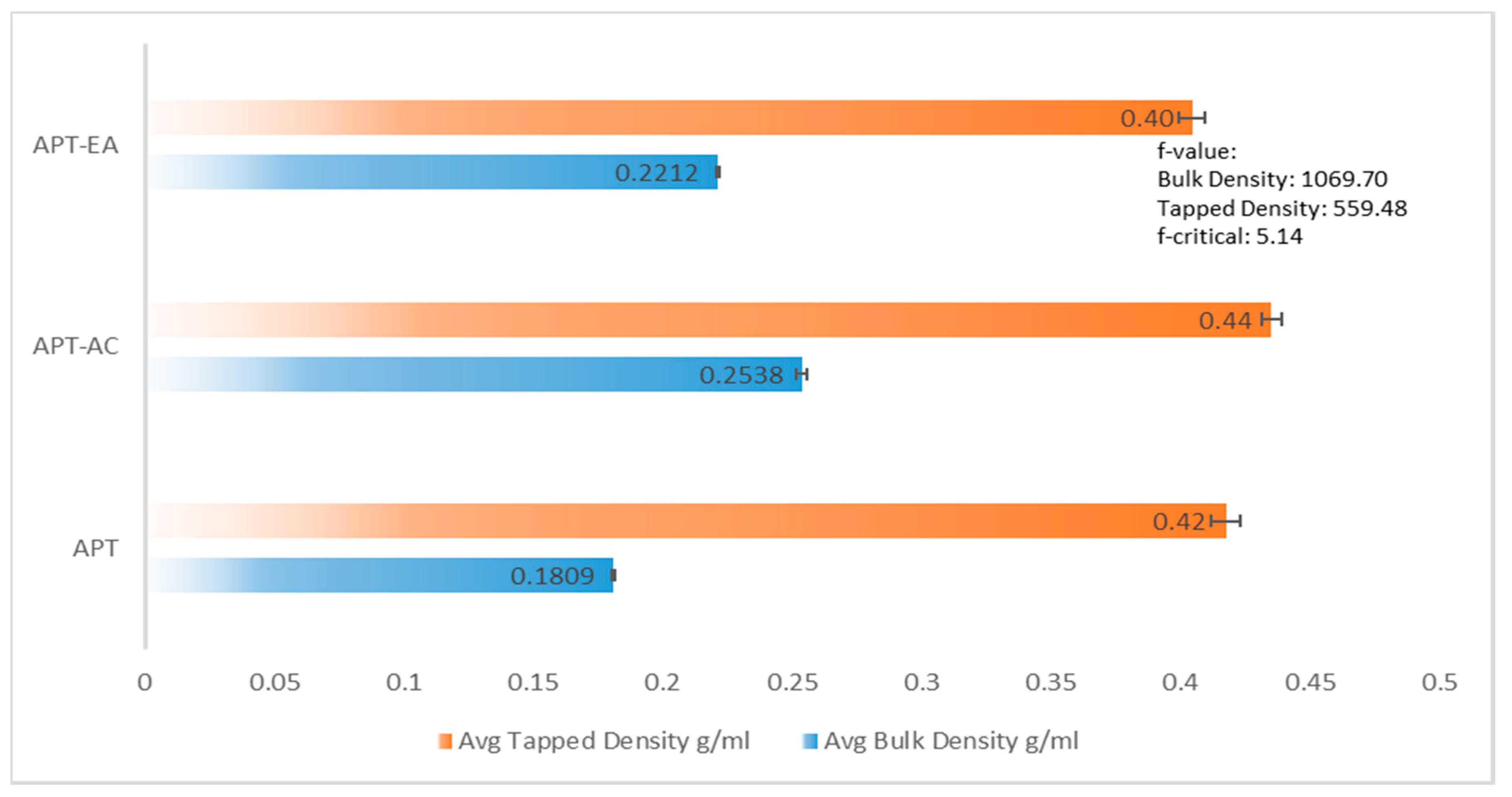
2.13. Bulk Density and Tapped Density
2.14. Compressibility Index and Hausner’s Ratio
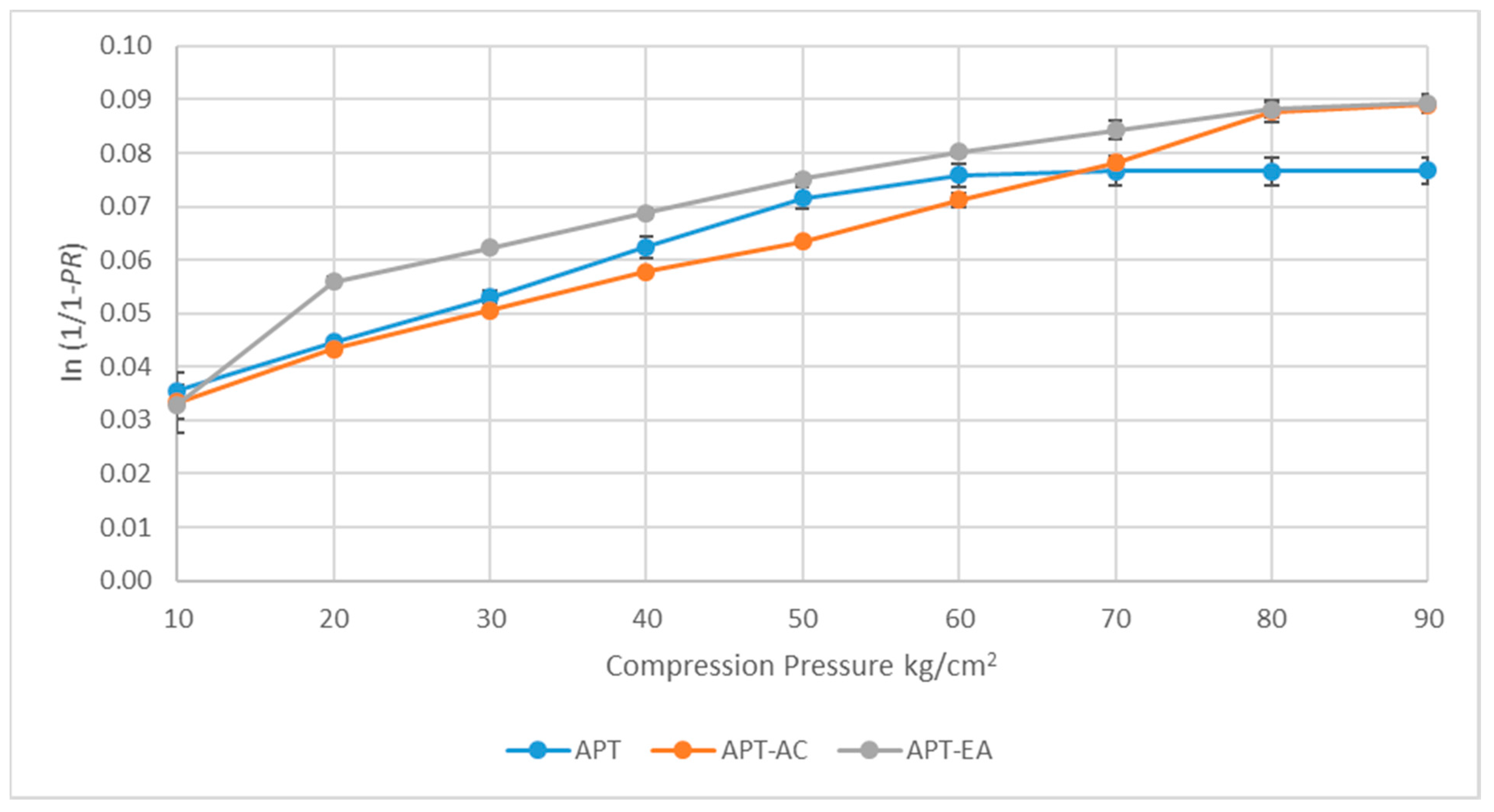
2.15. Heckel Plot Analysis
2.16. Compactibility Assessment
2.17. Dissolution Study
3. Results and Discussion
3.1. Solubility Study
3.2. Recrystallization Experiments
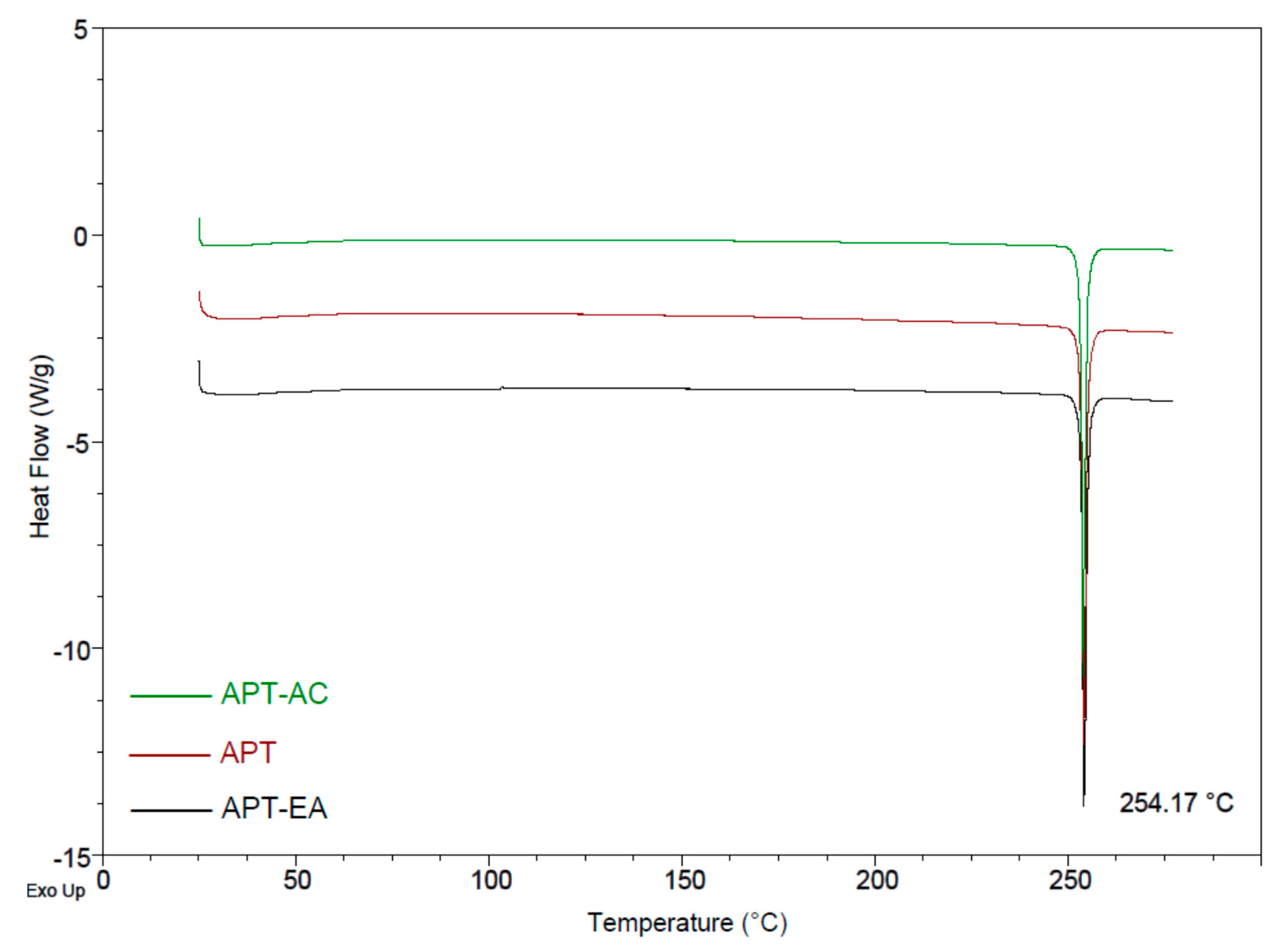
3.3. Differential Scanning Calorimetry
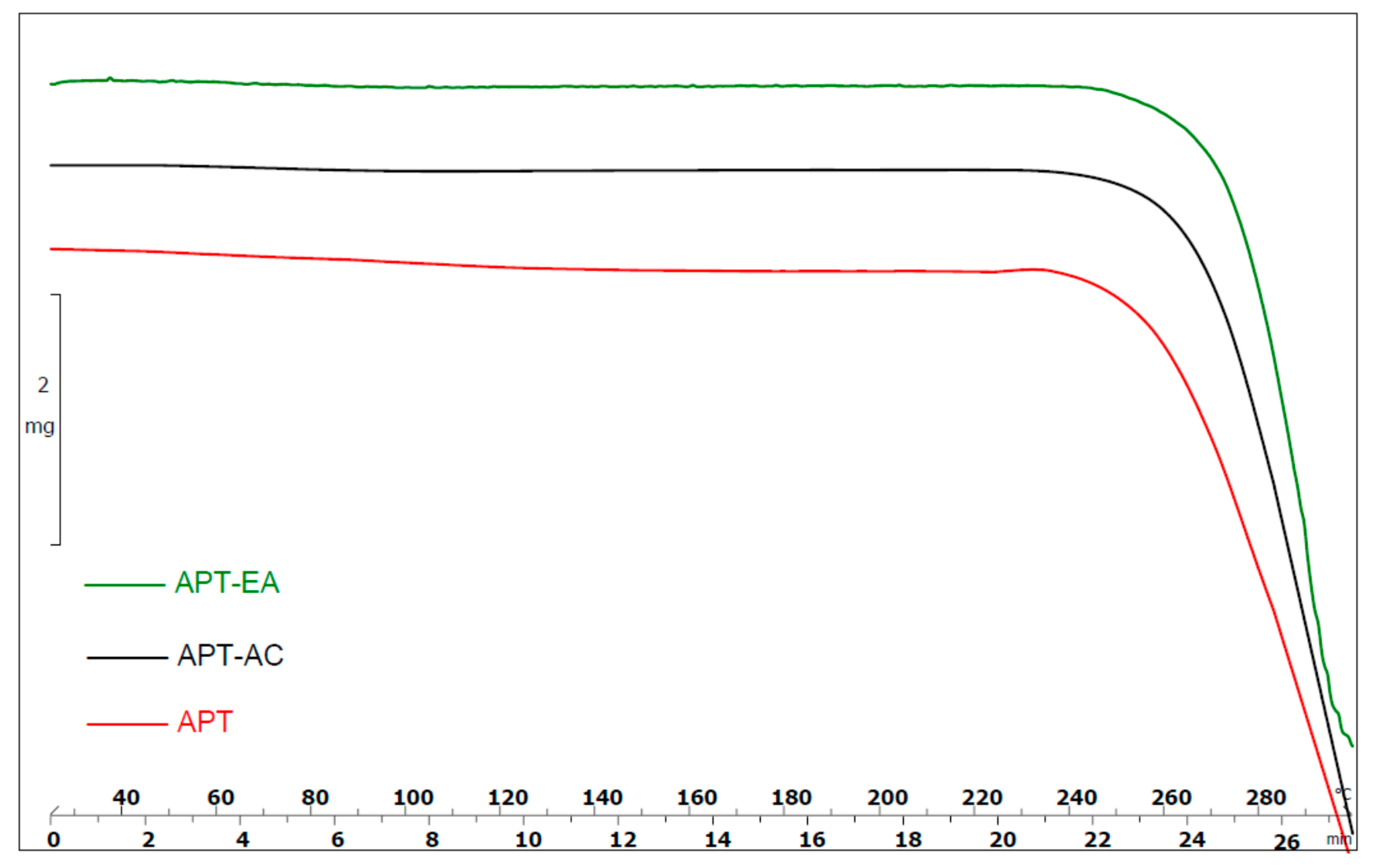
3.4. Thermogravimetric Analysis
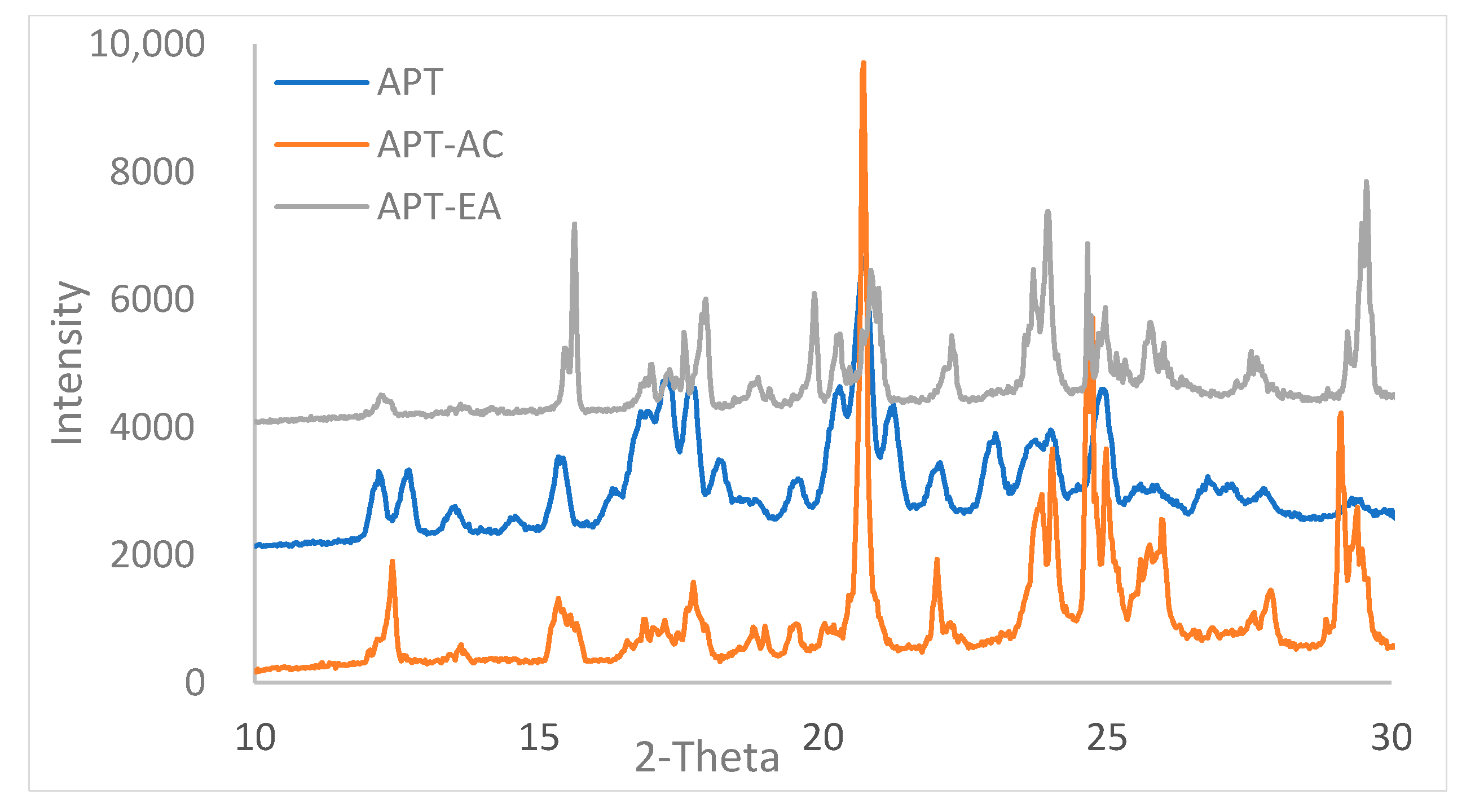
3.5. Powder X-ray Diffraction
3.6. Fourier Transform Infrared Spectroscopy
3.7. Particle Size Distribution
3.8. Flow Characterization
3.9. Kawakita Plots
3.10. Heckel Plots
3.11. Compactibility Assessment
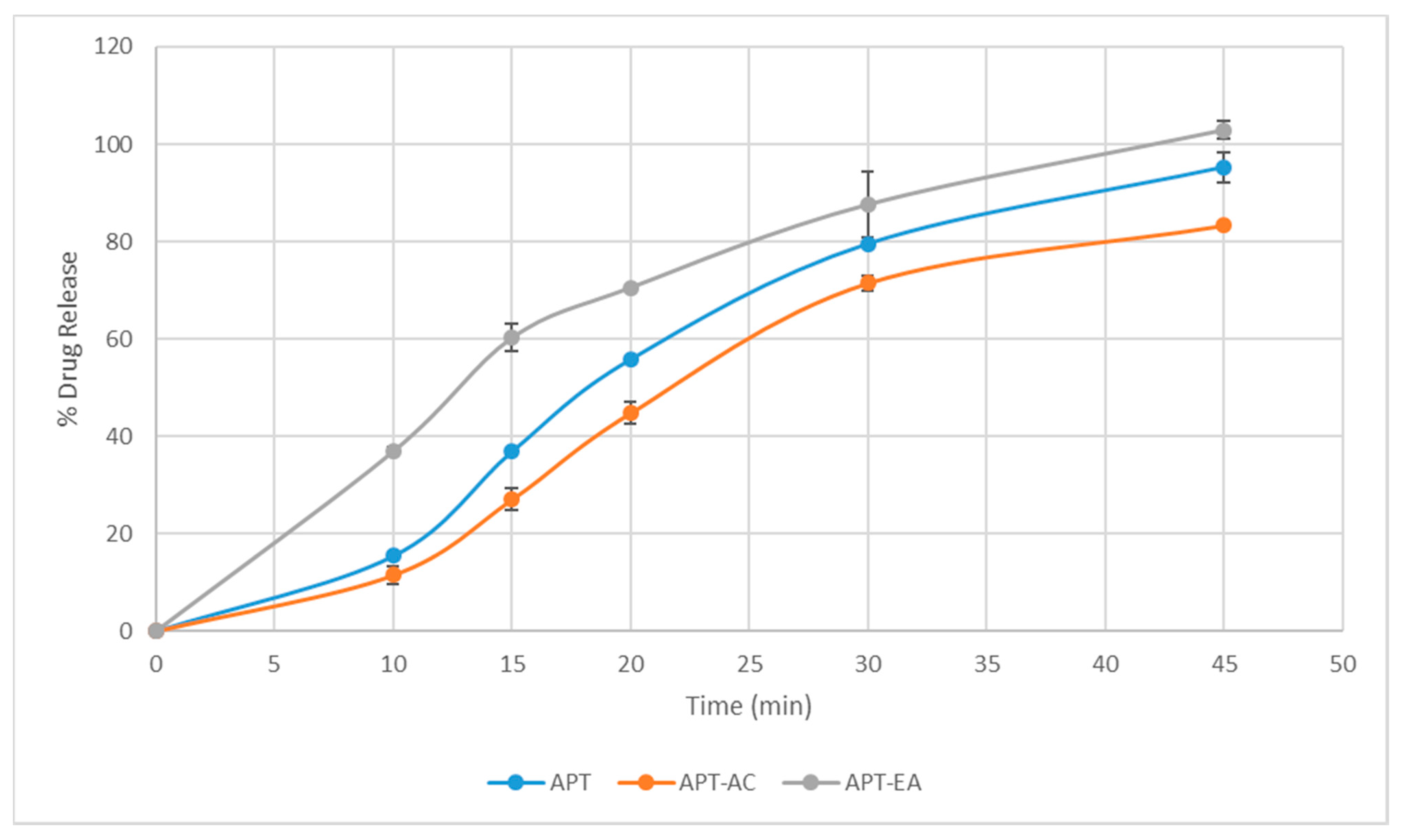
3.12. Dissolution Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef]
- Lee, E.H. A practical guide to pharmaceutical polymorph screening & selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ghassami, E.; Ahmadipour, S. Crystal Engineering for Enhanced Solubility and Bioavailability of Poorly Soluble Drugs. Curr. Pharm. Des. 2018, 24, 2473–2496. [Google Scholar] [CrossRef]
- Li, Y.; Yao, F.; Shang, C.; Ren, F.; Zhang, J.; Li, Y.; Yang, G.; Lin, R.; Wang, W. Preparation, characterization and in vivo evaluation of pharmacological activity of different crystal forms of ibuprofen. Pak. J. Pharm. Sci. 2019, 32, 2139–2147. [Google Scholar]
- Rasenack, N.; Müller, B.W. Crystal habit and tableting behavior. Int. J. Pharm. 2002, 244, 45–57. [Google Scholar] [CrossRef]
- Burt, H.M.; Mitchell, A.G. Effect of habit modification on dissolution rate. Int. J. Pharm. 1980, 5, 239–251. [Google Scholar] [CrossRef]
- Aziz, M.S.; Gupta, C.; Tyagi, L.K. Development of Novel Crystal Forms of Metaxalone for Solubility Enhancement. Indian J. Pharm. Sci. 2020, 82, 974–980. [Google Scholar] [CrossRef]
- Joiris, E. Joiris_1998_PhRes.pdf. Pharm. Res. 1998, 15, No. 7.
- Garekani, H.A.; Sadeghi, F.; Badiee, A.; Mostafa, S.A.; Rajabi-Siahboomi, A.R.; Rajabi-Siahboomi, A.R. Crystal habit modifications of ibuprofen and their physicomechanical characteristics. Drug Dev. Ind. Pharm. 2001, 27, 803–809. [Google Scholar] [CrossRef]
- Thakur, A.; Thipparaboina, R.; Kumar, D.; Sai Gouthami, K.; Shastri, N.R. Crystal engineered albendazole with improved dissolution and material attributes. CrystEngComm 2016, 18, 1489–1494. [Google Scholar] [CrossRef]
- Aisling, L.; Åke, R. Crystal Growth of Single Salicylamide Crystals. Cryst. Growth Des. 2019, 19, 7230–7239. [Google Scholar] [CrossRef]
- Tiwary, A.K. Modification of crystal habit and its role in dosage form performance. Drug Dev. Ind. Pharm. 2001, 27, 699–709. [Google Scholar] [CrossRef]
- Buckton, G. Surface Characterization: Understanding Sources of Variability in the Production and Use of Pharmaceuticals. J. Pharm. Pharmacol. 1995, 47, 265–275. [Google Scholar] [CrossRef]
- Hammouda, Y.E.; El-Khordagui, L.K.; Darwish, I.A.; El-Kamel, A.H. Manipulation of powder characteristics by interactions at the solid-liquid interface: 1-sulphadiazine. Eur. J. Pharm. Sci. 1999, 8, 283–290. [Google Scholar] [CrossRef]
- Khan, G.M.; Jiabi, Z. Preparation, characterization, and evaluation of physicochemical properties of different crystalline forms of ibuprofen. Drug Dev. Ind. Pharm. 1998, 24, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, A.K.; Panpalia, G.M. Influence of crystal habit on trimethoprim suspension formulation. Pharm. Res. 1999, 16, 261–265. [Google Scholar] [CrossRef]
- Dohme, M.S. US PIL Emend-Aprepitant Capsules; 2012; Volume 79.
- Braun, D.E.; Gelbrich, T.; Kahlenberg, V.; Laus, G.; Wieser, J.; Griesser, U.J. Packing polymorphism of a conformationally flexible molecule (aprepitant). New J. Chem. 2008, 32, 1677–1685. [Google Scholar] [CrossRef]
- Crocker Louis, M.J. Polymorphic Form of a Tachykinin Receptor Antagonist. U.S. Patent No. 6096742, 1 August 2000. [Google Scholar]
- Yamashiro, M.; Yuasa, Y.; Kawakita, K. An experimental study on the relationships between compressibility, fluidity, and cohesion of powder solids at small tapping numbers. Powder Technol. 1983, 34, 225–231. [Google Scholar] [CrossRef]
- USP Chapter 616 Bulk Density and Tapped Density of Powders. US Pharmacop. Conv. 2014, 6, 2014–2016.
- Ilkka, J.; Paronen, P. Prediction of the compression behaviour of powder mixtures by the Heckel equation. Int. J. Pharm. 1993, 94, 181–187. [Google Scholar] [CrossRef]
- Prakash, S.S.; Niranjan Patra, C.; Santanu, C.; Kumar, P.H.; Jagannath Patro, V.; Vimala Devi, M. Studies on flowability, compressibility and in-vitro release of Terminalia chebula fruit powder tablets. Iran. J. Pharm. Res. 2011, 10, 393–401. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Dissolution Test 3. United States Pharmacopeia; USP: Rockville, MD, USA, 2020; pp. 1–3. [Google Scholar]
- Garti, N.; Tibika, F. Habit modifications of nitrofurantoin crystallized from formic acid mixtures. Drug Dev. Ind. Pharm. 1980, 6, 379–398. [Google Scholar] [CrossRef]
- Turner, T.D.; Hatcher, L.E.; Wilson, C.C.; Roberts, K.J. Habit Modification of the Active Pharmaceutical Ingredient Lovastatin Through a Predictive Solvent Selection Approach. J. Pharm. Sci. 2019, 108, 1779–1787. [Google Scholar] [CrossRef]
- Harris, K.D.M. Powder diffraction crystallography of molecular solids. Top. Curr. Chem. 2012, 315, 133–178. [Google Scholar] [CrossRef] [PubMed]
- Tremayne, M. The impact of powder diffraction on the structural characterization of organic crystalline materials. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2691–2707. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.Y.Y.; Thielmann, F.; Williams, D.R. The effects of milling on the surface properties of form I paracetamol crystals. Pharm. Res. 2006, 23, 1918–1927. [Google Scholar] [CrossRef]
- Heng, J.Y.Y.; Williams, D.R. Wettability of paracetamol polymorphic forms I and II. Langmuir 2006, 22, 6905–6909. [Google Scholar] [CrossRef]
- Heng, J.Y.Y.; Bismarck, A.; Williams, D.R. Anisotropic surface chemistry of crystalline pharmaceutical solids. AAPS PharmSciTech 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. Particle Engineering in Pharmaceutical Solids Processing: Surface Energy Considerations. Curr. Pharm. Des. 2015, 21, 2677–2694. [Google Scholar] [CrossRef]


| Solvent | Solubility (mg/mL) |
|---|---|
| Methanol | 25.64 |
| Acetone | 23.25 |
| Ethyl Acetate | 14.71 |
| DCM | 4.07 |
| Chloroform | 55.55 |
| tert-Butyl methyl ether | Milky suspension |
| Ethanol | 10.64 |
| Acetonitrile | 45.45 |
| DMF | 35.71 |
| DMSO | 60.2 |
| Crystal Habit | d10 (µm) | d50 (µm) | d90 (µm) | Span | Surface Area m2/g | Aspect Ratio |
|---|---|---|---|---|---|---|
| APT-AC | 3.932 | 27.041 | 110.634 | 3.946 | 0.7 | 1.13 |
| APT-EA | 2.589 | 16.204 | 44.241 | 2.571 | 0.993 | 2.27 |
| Powder | Compactibility (a) | Cohesiveness (1/b) | Coefficient of Determination (r2) |
|---|---|---|---|
| APT | 0.014 ± 0.00002 | 12.81 ± 0.73 | 0.9749 |
| APT-AC | 0.037 ± 0.00012 | 12.01 ± 0.40 | 0.9902 |
| APT-EA | 0.039 ± 0.00041 | 14.82 ± 0.088 | 0.9652 |
| f Value | 9718 | 14.66 | NA |
| f critical | 5.14 | 9.55 | NA |
| Powder | Slope (K) | Intercept (A) | Yield Pressure (Py) | Coefficient of Determination r2 |
|---|---|---|---|---|
| APT | 0.0005 ± 0.00006 | 0.037 ± 0.007 | 1888.89 ± 192.45 | 0.8674 |
| APT-AC | 0.0007 ± 0.00006 | 0.030 ± 0.0006 | 1369.05 ± 103.10 | 0.9919 |
| APT-EA | 0.0006 ± 0.00006 | 0.039 ± 0.0015 | 1587.30 ± 137.46 | 0.8922 |
| f-value | 9 | 69.77 | 9.21 | NA |
| f-critical | 5.14 | 5.14 | 5.14 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datir, S.R.; Kumar, D.; Kumar, P.; Jain, S.; Bansal, A.K.; Nallamothu, B.; Thakore, S.D.; Bele, M.H. Study of Different Crystal Habits of Aprepitant: Dissolution and Material Attributes. Appl. Sci. 2021, 11, 5604. https://doi.org/10.3390/app11125604
Datir SR, Kumar D, Kumar P, Jain S, Bansal AK, Nallamothu B, Thakore SD, Bele MH. Study of Different Crystal Habits of Aprepitant: Dissolution and Material Attributes. Applied Sciences. 2021; 11(12):5604. https://doi.org/10.3390/app11125604
Chicago/Turabian StyleDatir, Satyajit R., Dinesh Kumar, Pradeep Kumar, Sanyog Jain, Arvind Kumar Bansal, Bhargavi Nallamothu, Samarth D. Thakore, and Mrudula H. Bele. 2021. "Study of Different Crystal Habits of Aprepitant: Dissolution and Material Attributes" Applied Sciences 11, no. 12: 5604. https://doi.org/10.3390/app11125604
APA StyleDatir, S. R., Kumar, D., Kumar, P., Jain, S., Bansal, A. K., Nallamothu, B., Thakore, S. D., & Bele, M. H. (2021). Study of Different Crystal Habits of Aprepitant: Dissolution and Material Attributes. Applied Sciences, 11(12), 5604. https://doi.org/10.3390/app11125604









