Innovative Biotechnology for Generation of Cardiac Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
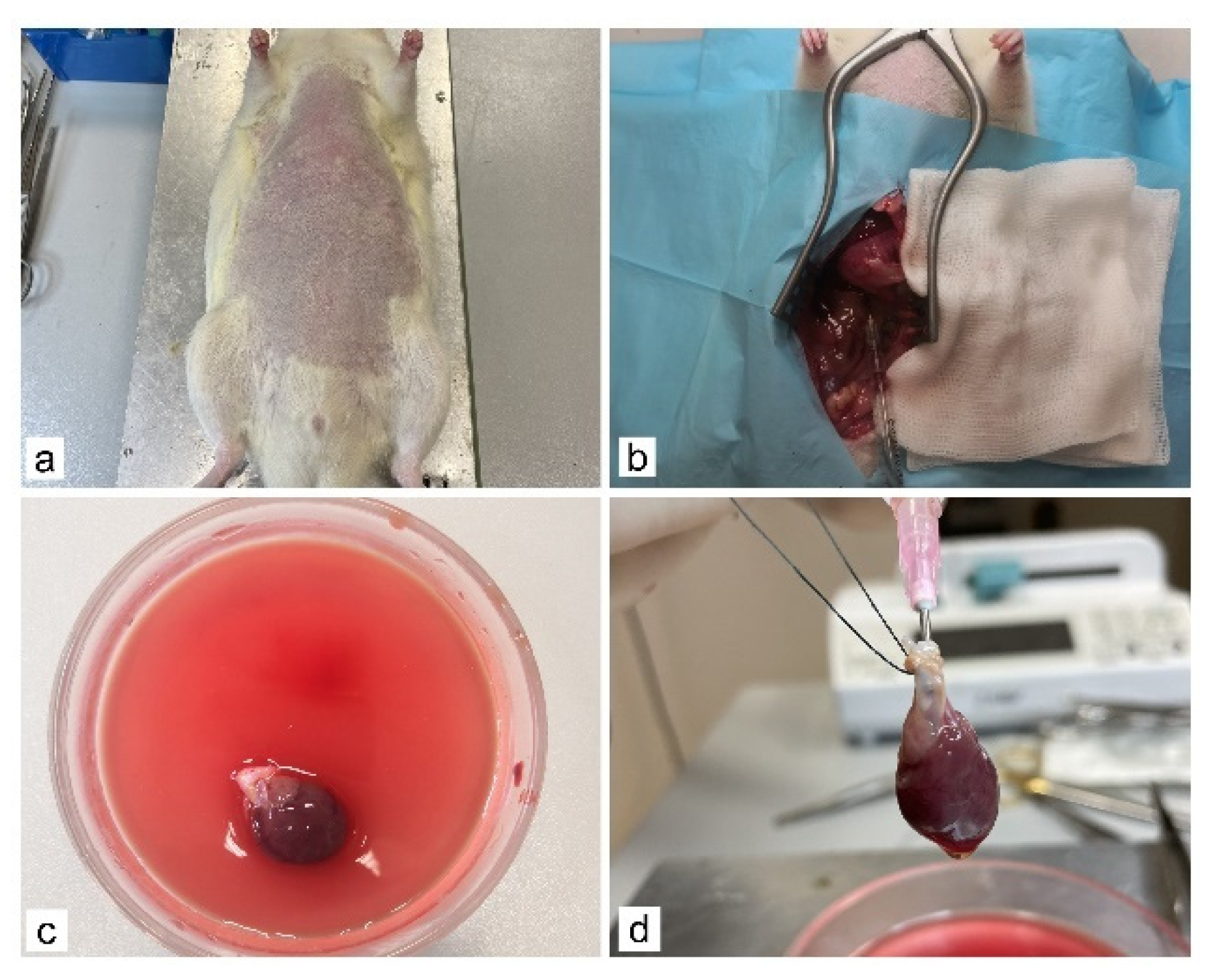
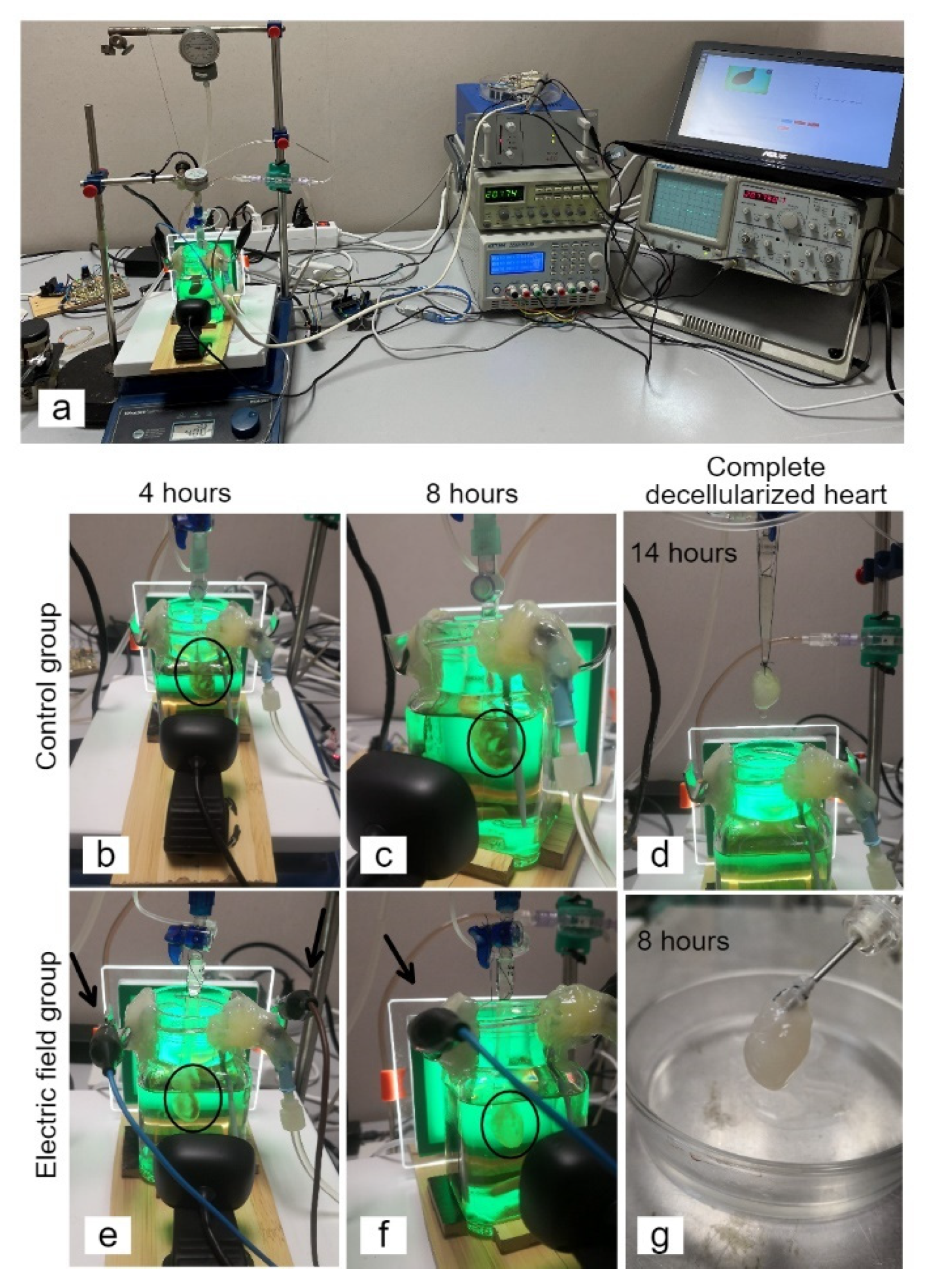
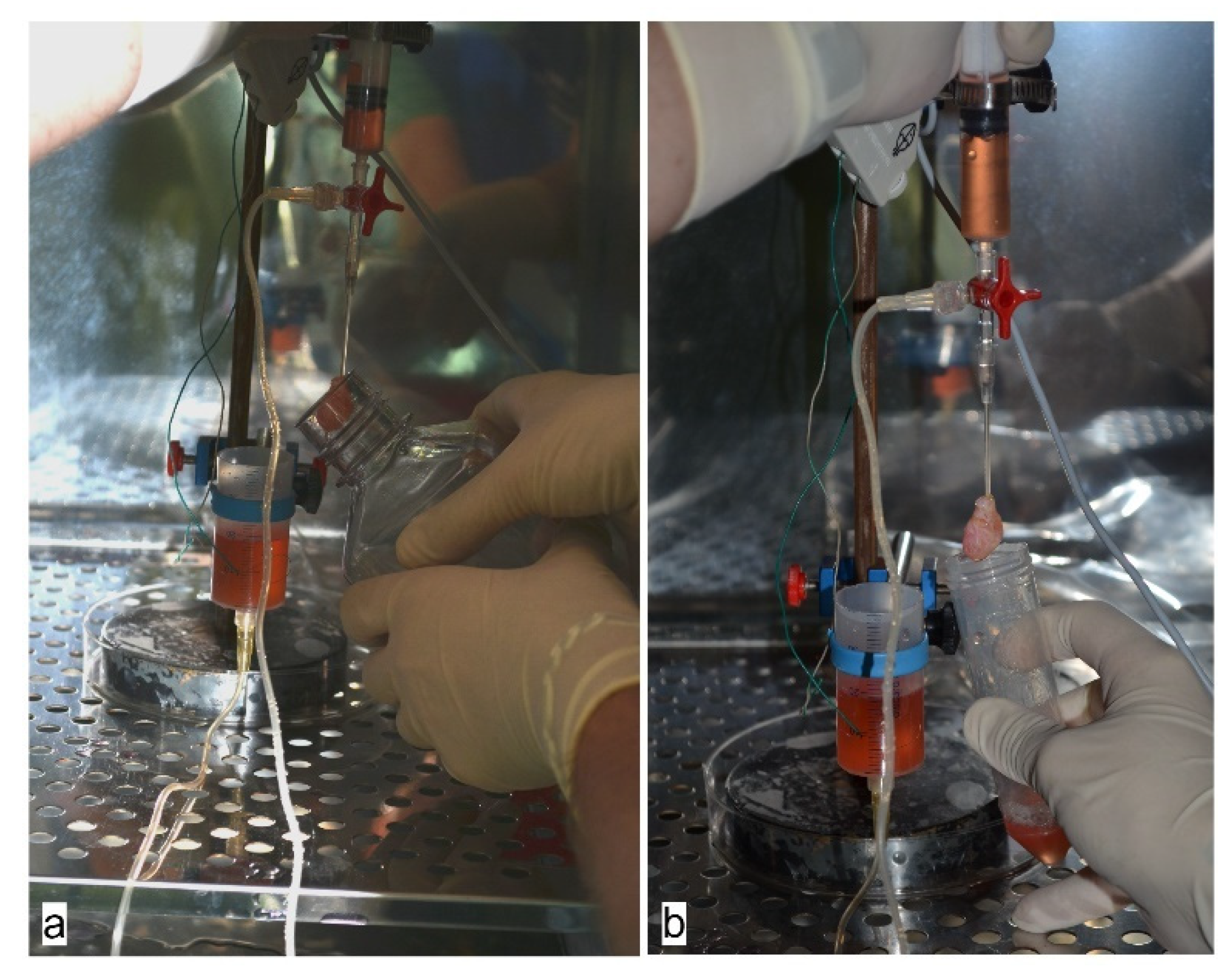
2.2. Whole-Heart Decellularization through Coronary Perfusion
2.3. Extracellular Matrix Characterization
2.4. Cell Culture
2.5. Recellularization of Whole Rat Heart
3. Results
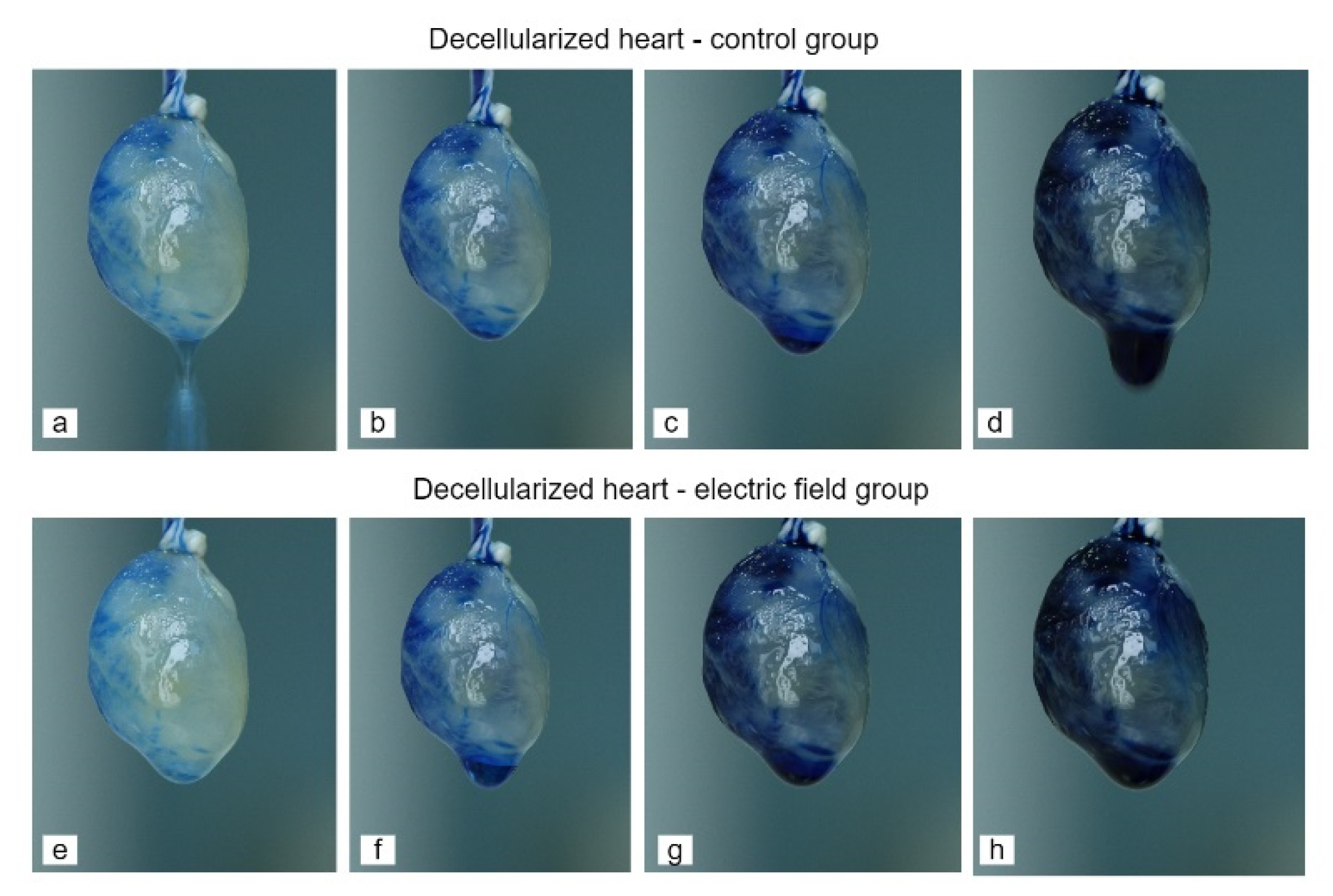
3.1. Generation of an Acellular Rat Heart using Perfusion Decellularization
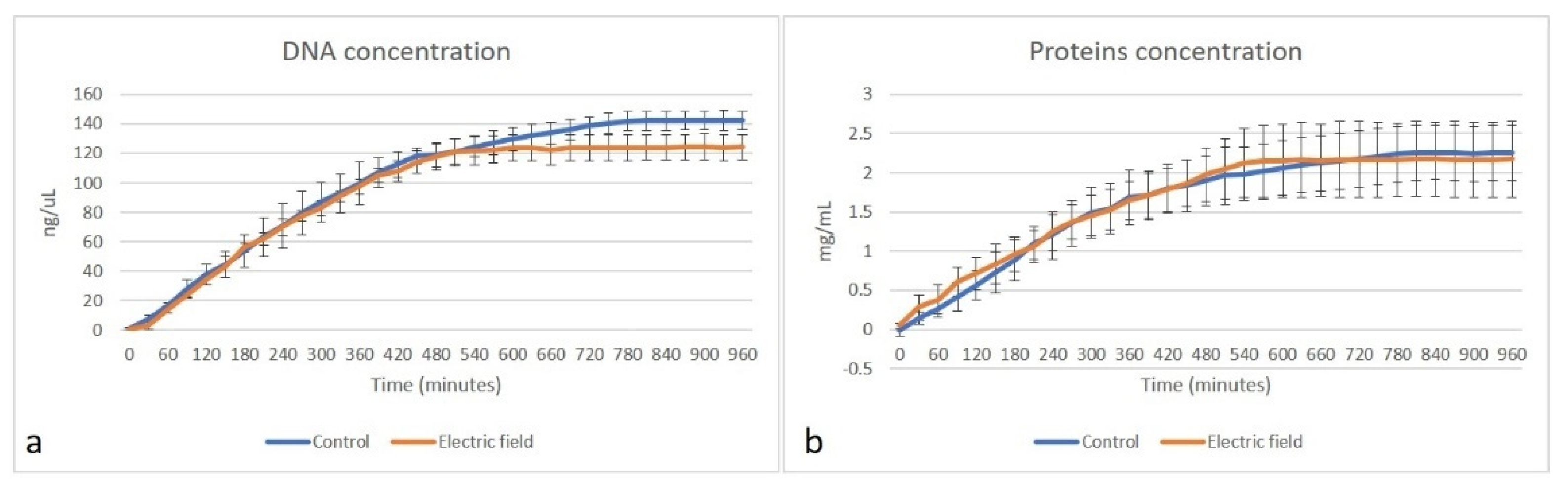
3.2. Evaluation of Matrix Content
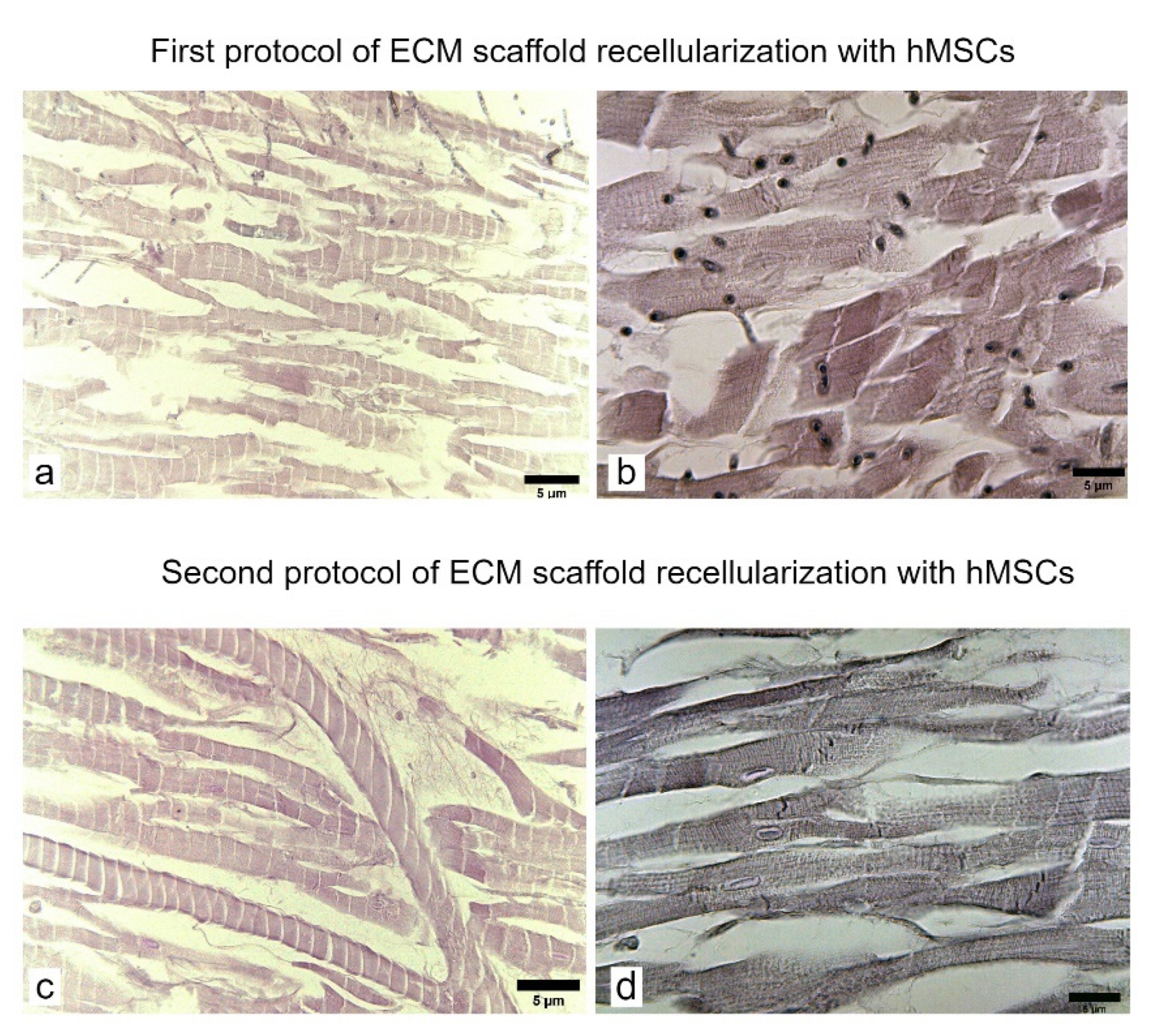
3.3. Formation of Bioartificial Whole Rat Heart
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cohn, J.N. Continue what we are doing to treat HF, but do it better. Nat. Rev. Cardiol. 2013, 11, 69–70. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analy-sis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Tonsho, M.; Michel, S.; Ahmed, Z.; Alessandrini, A.; Madsen, J.C. Heart Transplantation: Challenges Facing the Field. Cold Spring Harb. Perspect. Med. 2014, 4, a015636. [Google Scholar] [CrossRef]
- Rienks, M.; Papageorgiou, A.-P.; Frangogiannis, N.; Heymans, S. Myocardial Extracellular Matrix: An Ever-Changing and Diverse Entity. Circ. Res. 2014, 114, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tedder, M.E.; Perez, C.E.; Wang, G.; Curry, A.L.D.J.; To, F.; Elder, S.H.; Williams, L.N.; Simionescu, D.T.; Liao, J. Structural and biomechanical characterizations of porcine myocardial extracellular matrix. J. Mater. Sci. Mater. Med. 2012, 23, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.J.; Coulombe, K.L.K. Physiologically inspired cardiac scaffolds for tailored in vivo function and heart regeneration. Biomed. Mater. 2015, 10, 034003. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Moser, P.T.; Ott, H.C. Recellularization of organs: What is the future for solid organ transplantation? Curr. Opin. Organ Transplant. 2014, 19, 603–609. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Zia, S.; Mozafari, M.; Natasha, G.; Tan, A.; Cui, Z.; Seifalian, A. Hearts beating through decellularized scaffolds: Whole-organ engineering for cardiac regeneration and transplantation. Crit. Rev. Biotechnol. 2015, 36, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, P.L.; Santos, M.E.F.; Costanza, S.; Climent, A.M.; Moscoso, I.; Gonzalez-Nicolas, M.A.; Sanz-Ruiz, R.; Rodríguez, H.; Kren, S.M.; Garrido, G.; et al. Acellular human heart matrix: A critical step toward whole heart grafts. Biomaterials 2015, 61, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Weymann, A.; Loganathan, S.; Takahashi, H.; Schies, C.; Claus, B.; Hirschberg, K.; Soós, P.; Korkmaz, S.; Schmack, B.; Karck, M.; et al. Development and Evaluation of a Perfusion Decellularization Porcine Heart Model—Generation of 3-Dimensional Myocardial Neoscaffolds: Generation of 3-Dimensional Myocardial Neoscaffolds. Circ. J. 2011, 75, 852–860. [Google Scholar] [CrossRef]
- Weymann, A.; Patil, N.P.; Sabashnikov, A.; Jungebluth, P.; Korkmaz, S.; Li, S.; Veres, G.; Soos, P.; Ishtok, R.; Chaimow, N.; et al. Bioartificial Heart: A Human-Sized Porcine Model—The Way Ahead. PLoS ONE 2014, 9, e111591. [Google Scholar] [CrossRef]
- Park, S.M.; Yang, S.; Rye, S.-M.; Choi, S.W. Effect of pulsatile flow perfusion on decellularization. Biomed. Eng. Online 2018, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Parikh, R.B.; Sampaio, L.C. Bioengineering Hearts: Simple yet Complex. Curr. Stem Cell Rep. 2017, 3, 35–44. [Google Scholar] [CrossRef]
- Vats, A.; Tolley, N.S.; Bishop, A.E.; Polak, J.M. Embryonic stem cells and tissue engineering: Delivering stem cells to the clinic. J. R. Soc. Med. 2005, 98, 346–350. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human Mesenchymal Stem Cells Differentiate to a Cardiomyocyte Phenotype in the Adult Murine Heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef]
- Karantalis, V.; Hare, J.M. Use of Mesenchymal Stem Cells for Therapy of Cardiac Disease. Circ. Res. 2015, 116, 1413–1430. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.-Y.; Cho, H.-J.; Kang, H.-J.; Kim, T.-S.; Kim, M.-H.; Chung, J.-H.; Bae, J.-W.; Oh, B.-H.; Park, Y.-B.; Kim, H.-S. Pre-Treatment of Mesenchymal Stem Cells With a Combination of Growth Factors Enhances Gap Junction Formation, Cytoprotective Effect on Cardiomyocytes, and Therapeutic Efficacy for Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.-H.; Zhang, T.-T.; Li, Y.; Yan, H.-J.; Qi, H.; Li, F.-R. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell. Mol. Immunol. 2014, 12, 444–455. [Google Scholar] [CrossRef]
- Bonciog, D.D.; Matiu-Iovan, L.; Barbulescu, G.-I.; Burian, C.A.; Goje, I.-D.; Buica, T.P.; Paunescu, V.; Ordodi, V.L. Modified Langendorff Device for Rat Heart Decellularization. Physiology 2019, 2, 17–20. [Google Scholar]
- Bonciog, D.D.; Lascu, M.R.; Matiu-Iovan, L.; Ionel, R.; Burian, C.A.; Ordodi, V.L. Modified Langendorff vibrating fluid column device for improved rat heart decellularization experiments. In Proceedings of the 2020 International Symposium on Electronics and Telecommunications (ISETC), Timisoara, Romania, 5–6 November 2020. [Google Scholar]
- Xu, W.; Zhang, X.; Qian, H.; Zhu, W.; Sun, X.; Hu, J.; Zhou, H.; Chen, Y. Mesenchymal Stern Cells from Adult Human Bone Marrow Differentiate into a Cardiomyocyte Phenotype In Vitro. Exp. Biol. Med. 2004, 229, 623–631. [Google Scholar] [CrossRef]
- Antonitsis, P.; Ioannidou-Papagiannaki, E.; Kaidoglou, A.; Papakonstantinou, C. In vitro cardiomyogenic differentiation of adult human bone marrow mesenchymal stem cells. The role of 5-azacytidine. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Iop, L.; Sasso, E.D.; Menabò, R.; Di Lisa, F.; Gerosa, G. The Rapidly Evolving Concept of Whole Heart Engineering. Stem Cells Int. 2017, 2017, 8920940. [Google Scholar] [CrossRef]
- Lunkenheimer, P.P.; Redmann, K.; Westermann, P.; Rothaus, K.; Cryer, C.W.; Niederer, P.; Anderson, R.H. The myocardium and its fibrous matrix working in concert as a spatially netted mesh: A critical review of the purported tertiary structure of the ventricular mass. Eur. J. Cardio-Thoracic Surg. 2006, 29, S41–S49. [Google Scholar] [CrossRef]
- Costa, K.D.; Lee, E.J.; Holmes, J. Creating Alignment and Anisotropy in Engineered Heart Tissue: Role of Boundary Conditions in a Model Three-Dimensional Culture System. Tissue Eng. 2003, 9, 567–577. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Zimmermann, W.H. Engineering Myocardial Tissue. Circ. Res. 2005, 97, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, H.; Gong, W.; Li, S.; Li, H.; Wang, Z.; Zhao, Q. Impact of decellularization on porcine myocardium as scaffold for tissue engineered heart tissue. J. Mater. Sci. Mater. Electron. 2016, 27, 70. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Gilbert, T. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef]
- Remlinger, N.T.; Wearden, P.D.; Gilbert, T.W. Procedure for Decellularization of Porcine Heart by Retrograde Coronary Perfusion. J. Vis. Exp. 2012, e50059. [Google Scholar] [CrossRef]
- Robertson, M.J.; Dries-Devlin, J.L.; Kren, S.M.; Burchfield, J.S.; Taylor, D.A. Optimizing Recellularization of Whole Decellularized Heart Extracellular Matrix. PLoS ONE 2014, 9, e90406. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Buica, T.P.; Gavriliuc, O.I.; Baderca, F.; Hoinoiu, T.; Paunescu, V. Innovative Biotechnology for Generation of Cardiac Tissue. Appl. Sci. 2021, 11, 5603. https://doi.org/10.3390/app11125603
Barbulescu GI, Bojin FM, Ordodi VL, Goje ID, Buica TP, Gavriliuc OI, Baderca F, Hoinoiu T, Paunescu V. Innovative Biotechnology for Generation of Cardiac Tissue. Applied Sciences. 2021; 11(12):5603. https://doi.org/10.3390/app11125603
Chicago/Turabian StyleBarbulescu, Greta Ionela, Florina Maria Bojin, Valentin Laurentiu Ordodi, Iacob Daniel Goje, Taddeus Paul Buica, Oana Isabella Gavriliuc, Flavia Baderca, Teodora Hoinoiu, and Virgil Paunescu. 2021. "Innovative Biotechnology for Generation of Cardiac Tissue" Applied Sciences 11, no. 12: 5603. https://doi.org/10.3390/app11125603
APA StyleBarbulescu, G. I., Bojin, F. M., Ordodi, V. L., Goje, I. D., Buica, T. P., Gavriliuc, O. I., Baderca, F., Hoinoiu, T., & Paunescu, V. (2021). Innovative Biotechnology for Generation of Cardiac Tissue. Applied Sciences, 11(12), 5603. https://doi.org/10.3390/app11125603









