Abstract
Protoporphyrin IX-based all-solid-state choline (Ch) ion-selective electrodes (ISEs) were fabricated and characterized. Poly (3,4-ethylene dioxythiophene) doped with poly (styrene sulfonate) (PEDOT/PSS) functioning as an ion-to-electron transducer was electropolymerized on the gold wire (0.5 mm diameter). The conductive polymer was covered with a Ch selective membrane containing protoporphyrin IX as an ionophore, which exhibited a lower detection limit of 0.49 μM with the potentiometric method. The Ch sensor performed a wide linear range from 1 μM to 1 mM, a fast response time of less than 5 s, and a decent selectivity of common inorganic and organic ions in the human body. Characteristics such as pH and temperature stability, life span, reproducibility and repeatability were also investigated to be satisfied. With the background of artificial cerebrospinal fluid, the recovery rate in 10 M of Ch solution was measured by the standard addition method, revealing the potential for biological application.
1. Introduction
The neurotransmitter choline (Ch) plays a crucial role in brain and body functions. As the crucial precursor of acetylcholine, it is directly involved in the synthesis of acetylcholine in the brain, with the function of modulating attentional capacities, memory, learning and normal cognition. The cholinergic system dysfunction increases disease risk and age-related cognitive degradation such as Alzheimer’s disease [1,2,3,4]. The brain Ch concentration is also a quantitative marker of ischemia and infarct expansion, which can be applied to predict tissue prognosis better [5]. In addition, the Ch in plasma is associated with organ protection, as well as cardiovascular and cerebrovascular conditions. The growing concentration of Ch in plasma may lead to a detrimental cardiometabolic risk factor, while Ch deficiency increases the risk of several cancers [6]. In addition, Ch is essential for fetal development as a critical source of nutrition. Under a higher maternal status of Ch and other nutrition, immunity to the effects of maternal immune activation is strengthened, which is increasingly recognized as a latent risk factor for various neurological disorders [7,8]. However, the physiological concentrations of Ch are close to micromole, for example, 0.5–4.0 μM in human cerebrospinal fluid. When stimulated, the Ch concentration could increase to more than 30 μM [9,10]. Therefore, a rapid and sensitive method for Ch determination in biological fluids is vital and necessary to promote fundamental neuroscience research and monitor early-stage diagnoses.
Recently, various Ch measurement strategies have been reported, including fluorescence and colorimetric methods [11,12], chemiluminescence [13,14], liquid chromatography [15], and capillary electrophoresis [16]. Although these methods have significant sensitivity and selectivity, the requirement of complicated procedures and devices, professional operations, and pre-treatments of samples restrict their application in real-time detection [17]. In comparison with those above techniques, electrochemical analysis has been rapidly developed in recent years with the advantages of fast response time, less sample consumption, portability, convenient operation, and good sensitivity [18]. As one of the most popular methods in electrochemical analysis, the amperometric sensors provide quality characteristics such as relatively wide linear range, low detection limit, high sensitivity and selectivity. However, they suffer from the significant interference of redox products due to the requirement of redox reactions to generate current characteristics [19,20]. Moreover, the lack of an electroactive group in Ch causes insensitivity to electric potential. Therefore, enzymes for catalyzation in amperometric detection are necessary [21]. As a result, the immobilization of enzymes on the substrate and maintaining enzyme activities are also non-negligible barriers for solving the problems of storage and long-term stability of the sensors [22].
With their advantages including easy storage, low power consumption, and no need for enzymes and redox potential, several potentiometric ion-selective electrodes (ISEs) for analyses in actual samples have been developed in recent years [23,24]. However, the amount of Ch ISEs is still inadequate. An ion-selective Ch electrode based on a membrane containing a valinomycin derivative for the determination of a cholinergic agent showed excellent sensitivity from 2 × 10–10 M [25], and a novel Ch ISE for concentration analyses in milk powder and infant formulas exhibited a low detection limit of 0.061 μM [26]. Nevertheless, the excellent properties relied on the internal filling solution containing a high concentration of Ch bromide or hydrophilic (water-soluble) p-sulfonated calixarene, increasing the difficulty to miniaturize and store. Another solid-contact ISE for Ch and derivatives determination based on octa amide cavitand and carbon nanotubes dealt with the problem of inner filling solution in conventional electrodes, however, the linear range of 10–10 M and selectivity over common ions such as ammonium and sodium were not yet satisfactory [27]. The ionophore plays a central role in the selective binding with the compounds. As a macrocycle aromatic compound with tetrapyrrole, protoporphyrin IX shown in Figure 1 can bind Ch via cation- bonding [28,29]. The porphyrin core and hydrogen of the O–H group in Ch can also form the hydrogen bonds [30]. Moreover, the hydrophobic property of protoporphyrin is more suitable for lipophilic polymer membranes. It minimizes the leakage from the membrane into the aqueous solutions [31], seemingly a proper ionophore for Ch.
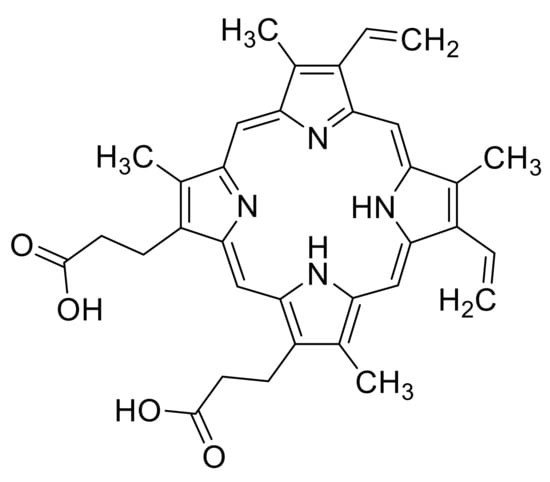
Figure 1.
Molecular structure of protoporphyrin IX.
In recent years, solid-state ISEs draw increasing attention, rather than classical liquid-contact ISEs in potentiometric methods. Between the ion-selective membrane and electron-conducting substrate, a solid conductive layer replacing the conventional internal filling solution makes the most difference. It acts as an ion-to-electron transducer to transfer the ionic conductivity in the selective polymer membrane into the electronic signal in the electron-conducting substrate [32]. Various solid conductive polymers have been tested and applied. Poly (3,4-ethylene dioxythiophene) doped with poly (styrene sulfonate) (PEDOT/PSS), one of the most well-known organic conductors, is commonly applied in electrodes due to low redox potential, high conductivity, good electrochemical stability and biocompatibility [33,34]. During electropolymerization, the hydrophobic EDOT interacts with the hydrophilic PSS to form PEDOT/PSS hybrid fibers. Electrostatic forces of attraction between -conjugated PEDOT chains and negatively charged PSS chains dominate the conductivity [35,36]. As a redox pair, PEDOT/PSS transmits the ions to electrons.
In this paper, an all-solid-state ISE for Ch determination was fabricated. By electropolymerization, the PEDOT/PSS layer performing as an ion-to-electron transducer was coated on the gold wire. Furthermore, the PVC selective membrane containing protoporphyrin IX as a specific ionophore for Ch determination was covered on the solid conductive layer. The fabricated Ch ISE showed a detection limit of 0.49 μM and a fast response time of less than 5 s. The characteristics such as stability, selectivity, reproducibility and repeatability were also investigated. Moreover, the performance in artificial cerebrospinal fluid was studied as well.
2. Materials and Methods
2.1. Reagents
Protoporphyrin IX, 2-nitrophenyl octyl ether (NPOE), high molecular weight poly(vinyl chloride) (PVC), potassium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (KTFPB), 3,4-ethylenedioxythiophene (EDOT), poly(styrene sulfonic acid sodium salt) (NaPSS), choline chloride (ChCl), histamine (HA), dopamine hydrochloride (DA), D-(+)-glucose (GC), and L-ascorbic acid (AA) were purchased from Sigma Aldrich (Shanghai, China). Gold wires (diameter 0.5 mm, 99.999% metals basis) were purchased from Alfa Aesar (Shanghai, China). Tetrahydrofuran (THF), sodium chloride, potassium chloride, calcium chloride, hydrochloric acid, sodium acetate, acetate acid and sodium hydroxide were purchased from Sinopharm Chemical Reagent (Shanghai, China). The artificial cerebrospinal fluid was obtained from Coolaber Technology Co., Ltd. (Beijing, China). All of the chemicals were of analytical grade and were used without further purification. Deionized water was used throughout.
2.2. Preparation of Ch ISEs
One gold wire was sealed in a capillary tube with resin, leaving both ends open. After polishing with alumina slurries, one side of the gold wire was successively immersed in ethanol solution (50.0 vol%), sulfuric acid (8.0 wt.%) and deionized water for ultrasonic cleaning lasting 10 min. Then, the gold wire was rinsed with deionized water and dried with nitrogen.
The PEDOT/PSS layer was developed in an aqueous solution containing 0.01 M EDOT and 0.05 M NaPSS. Before electropolymerized, the mixture of monomer EDOT and NaPSS was stirred for 12 h until homogeneous and transparent electrolyte was received. Nitrogen was inlet for 10 min to eliminate the air in the electrolyte. After preparation, 6 mm length of the gold wire was immersed in the electrolyte and formed a three-electrode system with an Ag/AgCl electrode as a reference electrode and a Pt electrode as a counter electrode. With the method of chronopotentiometry, a constant current of 18.84 μA with a density of 0.2 mA/cm was provided for 1800 s. After this step, the PEDOT/PSS layer was rinsed with deionized water and dried in a closet for 24 h.
A protoporphyrin IX-based membrane cocktail solution was prepared by dissolving 32.3 mg of PVC as a membrane matrix, 62.2 μL of NPOE as a plasticizer, 2.5 mg of protoporphyrin IX as an ionophore and 0.5 mg of KTFPB as an ion exchanger in 370 μL of THF as a solvent. The solution was vibrated until homogeneous and then stored in 4 °C for four hours before used. The gold wire modified with PEDOT/PSS was dipped in the polymer membrane solution for 3 s and then taken out to dry. After repeating for five times, the electrode was covered with the selective membrane and then dried overnight. Before use, the electrodes were conditioned in 10 M of ChCl solution for one hour.
2.3. Electrochemical Instrumentation and Measurements
The potentiometric measurements were operated with a ChI660 Electrochemical Work Station (Shanghai Chenhua Instrument Corporation, Shanghai, China) and an Ag/AgCl reference electrode (Tianjin Aida Hengsheng Technology, Tianjin, China). A pH electrode (Mettler Toledo, Shanghai, China) was used to prepare ChCl solutions with different pH values. A shaking water bath (Shanghai Lichen Instrument Corporation, Shanghai, China) was applied in the experiment of temperature stability.
2.4. Evaluation of Potentiometric Performance
The acetate buffers with different pH were prepared as the background. We added 0.6981 g of ChCl to 50 mL of acetate buffer to obtain 0.1 M of ChCl stock solution. By diluting ChCl stock solution in acetate buffers, other solutions with different concentrations were acquired. Freshly prepared solutions were used during each experiment.
The CHI660 Electrochemical Work Station was used to measure the potentiometric response with a two-electrode system, including a reference and a working electrode, as shown in Figure 2A. The structure and photograph of Ch ISE are shown in Figure 2B,C, respectively. All potentiometric measurements were carried out at a pH of 7.4 and 25 °C except for the pH and temperature experiments, respectively. During the test of the temperature effect, a shaking water bath was used. The fixed interference method (FIM) was used to calculate the selective coefficients [37]. The recovery rate of Ch sensors was investigated and calculated, referring to the standard addition method, by diluting ChCl stock solution with a high concentration in the background of artificial cerebrospinal fluid [38].
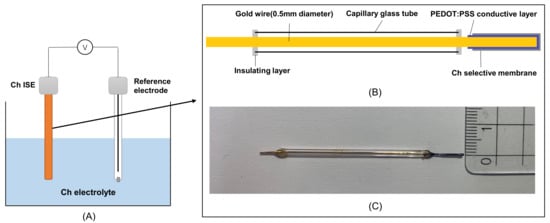
Figure 2.
(A) Potentiometric detection system in Ch solutions; and (B) structure of the Ch all-solid-state ISE; (C) photograph of the Ch ISE with a centimeter ruler as a reference.
3. Results and Discussion
3.1. Dynamic Responses and Calibration Curve
The potentiometric response of the Ch sensor was evaluated by diluting 0.1 M of ChCl solution with a continuous stir at 400 rpm in the background of acetate buffers at pH 7.4. The Ch ISE was calibrated from 10 M to 3 × 10 M, and a typical stepwise trace is shown in Figure 3A. With the addition of ChCl stock solution each time, the sensor reaches a stable state in 5 s. The results demonstrated that the Ch ISE would be the perspective to be used in real-time Ch monitoring.
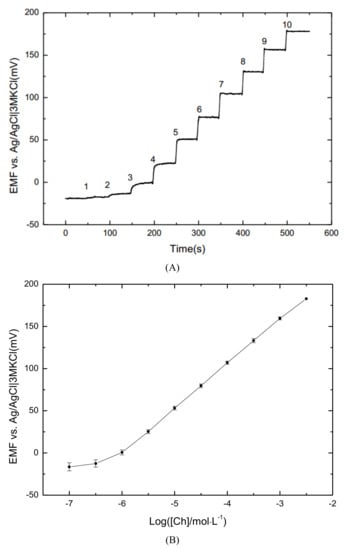
Figure 3.
(A) Dynamic responses with the addition of ChCl stock solutions every 50 s (1: 10 M; 2: 3 × 10 M; 3: 10 M; 4: 3 × 10 M; 5: 10 M; 6: 3 × 10 M; 7: 10 M; 8: 3 × 10 M; 9: 10 M; 10: 3 × 10 M); and (B) calibration curve of ChCl solutions with a concentration in the range from 10 M to 3 × 10 M.
The calibration curve was plotted in Figure 3B. The sensitivity of the sensor was in the linear range of 10–3 × 10 M is 52.71 ± 0.95 mV/decade. According to the IUPAC recommendation, the detection limit is 0.49 μM, where the linear response and nonresponsive segment in the calibration curve intersect [37]. Compared with the ionophore of octa amide cavitand, the cation– bonding between Ch and tetrapyrrole of protoporphyrin IX, as well as hydrogen bonds formed between the O–H group in Ch and the porphyrin core enhanced the interaction, which improves the performance of Ch sensor [28,30].
3.2. Selective Coefficients to Common Cations
The selectivity of the Ch sensor was tested and the common inorganic and organic ions, including K, Na, Ca, GC, DA, AA, and HA, were taken into account as possible interferences in biological fluid. The selective coefficients were determined by FIM with constant concentrations of these interferences [37]. According to Table 1, the negative coefficients demonstrating that the Ch sensor is more sensitive to Ch rather than the interferences. Although the selective coefficients to DA and HA are relative higher than other ions, the selectivity to Ch is close to being 100 times higher. Moreover, the physiological concentrations of these ions, such as K less than 5 mM, Na less than 150 mM, Ca less than 3 mM, GC less than 7.1 mM, AA less than 0.1 mM and a lower concentration of DA and HA in plasma and cerebrospinal fluid are far less than that set in the experiments [39,40], demonstrating that the interference of these ions could be neglected.

Table 1.
Selective coefficients to K, Na, Ca, GC, DA, AA, HA.
3.3. pH and Temperature Stability
The stability of the Ch ISE at various pH levels was investigated. The pH range of 7.0–8.0 was selected in coordination with the human body’s physiological condition. The influence of the pH was studied in 10–10 M of ChCl solutions at different pH. Figure 4A shows the basically stable response, and the results indicated that the Ch sensor was not sensitive to pH in the range of 7.0–8.0. The pKa of Ch is 13.9, so the Ch cation is protonated in the environment of pH less than 13.9 [41].
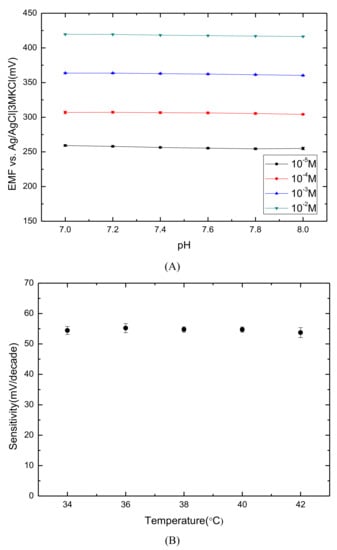
Figure 4.
(A) Potentiometric response (10–10 M of ChCl solutions) within a pH range of 7.0–8.0 in the background of acetate buffers; and (B) the effect of temperature on the sensitivities of the Ch ISE within the range of 34–42 °C.
The effect of temperature was also tested in a range that is close to the human body temperature. The potentiometric response was examined in 10–10 M of ChCl solutions. As shown in Figure 4B, the sensitivities of Ch sensors within the range of 34–42 °C are 54.47 ± 0.73 mV/decade. The result illustrated that the temperature range located in the human body made no difference in the performance of Ch ISEs.
3.4. Reproducibility and Repeatability
To investigate the reproducibility, four Ch sensors were randomly selected to detect the ChCl solutions with concentrations of 3 μM, 10 μM, and 100 μM. The means of the concentrations (Mean), standard deviations (S. D.) and relative standard deviations (R. S. D.) were calculated. As shown in Table 2, the detected concentrations of four sensors were both close to the actual concentrations, with the R. S. D. less than 10%. The results demonstrated that the reproducibility of the Ch sensors was acceptable.

Table 2.
Reproducibility of four Ch sensors.
The repeatability was evaluated with five Ch electrodes. By testing the response of five sensors in 10–10 M of ChCl solutions, the sensitivities were calculated according to the calibration curves. As shown in Figure 5, the sensitivities of 51.63 ± 0.88 mV/decade reveal that the repeatability is satisfied.
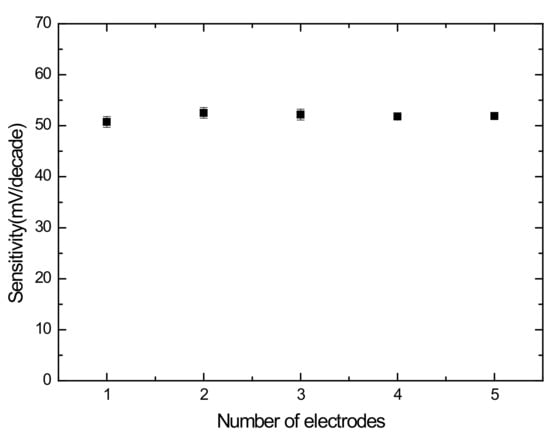
Figure 5.
Repeatability of five Ch sensors.
3.5. Life Span
The long-term stability of Ch sensors was examined by the potentiometric response in 35 days. The experiments were carried out every five days in freshly prepared ChCl solutions, with a concentration range of 10–10 M. After each experiment, the sensor was rinsed with deionized water and stored in a dark closet. The sensitivities of 54.40 ± 0.79 mV/decade in Figure 6 illustrates that Ch sensor could perform stably for at least one month.
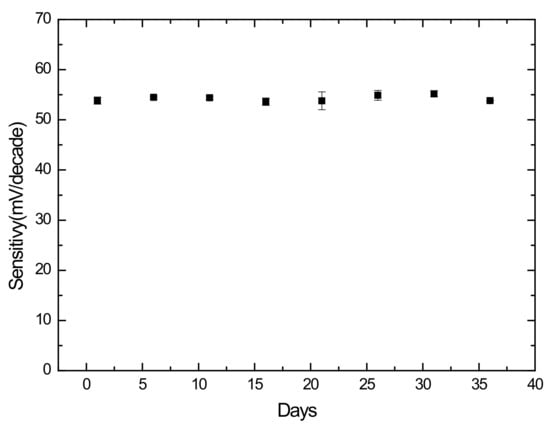
Figure 6.
Sensitivities of the Ch sensor during 35 days.
3.6. Performance in Artificial Cerebrospinal Fluid
Considering the complicated interferences in samples, the recovery rate of the Ch sensor was detected and calculated in artificial cerebrospinal fluid. The 10 M of the ChCl standard solution with the background of artificial cerebrospinal fluid was prepared. According to the standard addition method, certain amounts of 10 M ChCl solution were added to the samples. After repeating for three times, the predicted concentration was tested to be 1.02 × 10 M. The recovery rate of 102.02% in 10 M actual ChCl solution indicated that the existence of common cations such as K, Na, Ca, Mg, GC, AA would hardly affect the performance of the Ch sensor. Referring to the Ch concentration of more than 30 μM in cerebrospinal fluid when stimulated [42,43], the results in coordination with the selective coefficients provided the possible usage for Ch determination in clinical conditions. Moreover, the relatively higher concentration of more than 1.5 mM in breast lesions and 1 mg/100 g in Ch-rich food suggested that the Ch sensors were also suitable for monitoring tumor signals and nutrition [44,45,46].
4. Conclusions
All-solid-state Ch ISEs were presented with an excellent detection limit of 0.49 μM and high selectivity toward common cations. The PEDOT/PSS was electropolymerized on the gold wire as a solid conductive layer, to provide ion-to-electron transfer. The PVC selective membrane covered on the conductive layer contained protoporphyrin IX as an ionophore, and showed specific interaction with Ch. The experimental results demonstrated the stability to pH and temperature within the physiological range. The reproducibility, repeatability and life span were also investigated to be satisfactory. Moreover, the recovery rate close to 100% in artificial cerebrospinal fluid revealed the possibility to determine Ch in biological fluid and some Ch-rich nutrition. With further miniaturization, the low detection limit provided the potential application of Ch sensors in the clinical field.
Author Contributions
Conceptualization, G.L. and Y.W.; methodology, S.M.; validation, S.M.; formal analysis, S.M.; investigation, S.M., H.Z., and Z.W.; resources, Y.W.; data curation, S.M.; writing—original draft preparation, S.M.; writing—review and editing, G.L., Y.W. and Z.L.; visualization, S.M.; supervision, G.L., Y.W. and Z.L.; project administration, Y.W.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (Grant No. 61773342) and the Autonomous Research Project of the State Key Laboratory of Industrial Control Technology, China (Grant No. ICT2021A13).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef]
- Shea, T.B. Choline and phosphatidylcholine may maintain cognitive performance by multiple mechanisms. Am. J. Clin. Nutr. 2019, 110, 1268–1269. [Google Scholar] [CrossRef]
- Soreq, H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 2015, 38, 448–458. [Google Scholar] [CrossRef]
- Karaszewski, B.; Thomas, R.; Chappell, F.; Armitage, P.; Carpenter, T.; Lymer, G.; Dennis, M.; Marshall, I.; Wardlaw, J. Brain choline concentration: Early quantitative marker of ischemia and infarct expansion? Neurology 2010, 75, 850–856. [Google Scholar] [CrossRef]
- Fu, B.C.; Hullar, M.A.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Le Marchand, L.; et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the growing science on its benefits for moms and babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef]
- Meyer, U. Neurodevelopmental resilience and susceptibility to maternal immune activation. Trends Neurosci. 2019, 42, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.; Markham, C.; Jenden, D. Acetylcholine and choline in cerebrospinal fluid of patients with Parkinson’s disease and Huntington’s chorea. J. Neurol. Neurosurg. Psychiatry 1976, 39, 367–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Awwad, H.M.; Kirsch, S.H.; Geisel, J.; Obeid, R. Measurement of concentrations of whole blood levels of choline, betaine, and dimethylglycine and their relations to plasma levels. J. Chromatogr. B 2014, 957, 41–45. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Q.; He, K.; Liu, M.; Zhang, Y.; Yao, S. A cyclic signal amplification strategy to fluorescence and colorimetric dual-readout assay for the detection of H2O2-related analytes and application to colorimetric logic gate. Sens. Actuators B Chem. 2018, 260, 908–917. [Google Scholar] [CrossRef]
- Hua, B.; Shao, L.; Zhang, Z.; Sun, J.; Yang, J. Pillar[6]arene/acridine orange host–guest complexes as colorimetric and fluorescence sensors for choline compounds and further application in monitoring enzymatic reactions. Sens. Actuators B Chem. 2018, 255, 1430–1435. [Google Scholar] [CrossRef]
- Wu, X.; Chai, Y.; Yuan, R.; Liang, W.; Yuan, D. A novel electrochemiluminescence choline biosensor based on biofunctional AMs-ChO biocomposite. Sens. Actuators B Chem. 2014, 204, 429–436. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Guo, L.; Wang, J.; Zhang, K.; He, J.; Cui, H. High-resolution temporally resolved chemiluminescence based on double-layered 3D microfluidic paper-based device for multiplexed analysis. Biosens. Bioelectron. 2019, 141, 111472. [Google Scholar] [CrossRef] [PubMed]
- Hefni, M.; McEntyre, C.; Lever, M.; Slow, S. A simple HPLC method with fluorescence detection for choline quantification in foods. Food Anal. Methods 2015, 8, 2401–2408. [Google Scholar] [CrossRef]
- Inoue, T.; Kirchhoff, J.R.; Hudson, R.A. Enhanced measurement stability and selectivity for choline and acetylcholine by capillary electrophoresis with electrochemical detection at a covalently linked enzyme-modified electrode. Anal. Chem. 2002, 74, 5321–5326. [Google Scholar] [CrossRef]
- Rahimi, P.; Joseph, Y. Enzyme-based biosensors for choline analysis: A review. TrAC Trends Anal. Chem. 2019, 110, 367–374. [Google Scholar] [CrossRef]
- Yan, K.; Nandhakumar, P.; Bhatia, A.; Lee, N.S.; Yoon, Y.H.; Yang, H. Electrochemical immunoassay based on choline oxidase-peroxidase enzymatic cascade. Biosens. Bioelectron. 2021, 171, 112727. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Baker, K.L.; Bolger, F.B.; Lowry, J.P. Development of a microelectrochemical biosensor for the real-time detection of choline. Sens. Actuators B Chem. 2017, 243, 412–420. [Google Scholar] [CrossRef]
- Shadlaghani, A.; Farzaneh, M.; Kinser, D.; Reid, R.C. Direct electrochemical detection of glutamate, acetylcholine, choline, and adenosine using non-enzymatic electrodes. Sensors 2019, 19, 447. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Wang, Y.; Yu, L.; Wang, J.; Peng, H.; Zhu, J. Improved enzyme immobilization for enhanced bioelectrocatalytic activity of choline sensor and acetylcholine sensor. Sens. Actuators B Chem. 2014, 193, 904–910. [Google Scholar] [CrossRef]
- He, C.; Wang, Z.; Wang, Y.; Hu, R.; Li, G. Nonenzymatic all-solid-state coated wire electrode for acetylcholine determination in vitro. Biosens. Bioelectron. 2016, 85, 679–683. [Google Scholar] [CrossRef]
- Durka, M.; Durka, K.; Adamczyk-Wozniak, A.; Wroblewski, W. Dopamine/2-Phenylethylamine sensitivity of ion-selective electrodes based on bifunctional-symmetrical boron receptors. Sensors 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Nikol’skaya, E.; Kormilitsyn, B.; Kugusheva, L. Determination of trace cholinergic compounds and the evaluation of their relative receptor activity with the use of a receptor biosensor and an ion-selective choline electrode. J. Anal. Chem. 2003, 58, 277–281. [Google Scholar] [CrossRef]
- Abd El-Rahman, M.K.; Mazzone, G.; Mahmoud, A.M.; Sicilia, E.; Shoeib, T. Novel choline selective electrochemical membrane sensor with application in milk powders and infant formulas. Talanta 2021, 221, 121409. [Google Scholar] [CrossRef] [PubMed]
- Ampurdanés, J.; Crespo, G.A.; Maroto, A.; Sarmentero, M.A.; Ballester, P.; Rius, F.X. Determination of choline and derivatives with a solid-contact ion-selective electrode based on octaamide cavitand and carbon nanotubes. Biosens. Bioelectron. 2009, 25, 344–349. [Google Scholar] [CrossRef]
- Blaser, G.; Sanderson, J.M.; Wilson, M.R. Free-energy relationships for the interactions of tryptophan with phosphocholines. Org. Biomol. Chem. 2009, 7, 5119–5128. [Google Scholar] [CrossRef]
- Liu, X.; Ji, H.; Tang, J.; Tao, F.; Zhang, X.; Yao, Z.; Song, H.; Li, C.; Wang, F. Electrochemical activation and renewal of pyrrole nitrogen sites in porphyrin-based conjugated polymer for simultaneous determination of uric acid and adrenaline. J. Electroanal. Chem. 2021, 884, 115055. [Google Scholar] [CrossRef]
- Kielmann, M.; Senge, M.O. Molecular Engineering of Free-Base Porphyrins as Ligands—The N-H...X Binding Motif in Tetrapyrroles. Angew. Chem. Int. Ed. 2019, 58, 418–441. [Google Scholar] [CrossRef]
- Braegelman, A.S.; Webber, M.J. Integrating stimuli-responsive properties in host-guest supramolecular drug delivery systems. Theranostics 2019, 9, 3017. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable conductive polymers and composites based on PEDOT and PEDOT: PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef]
- Bodart, C.; Rossetti, N.; Hagler, J.; Chevreau, P.; Chhin, D.; Soavi, F.; Schougaard, S.B.; Amzica, F.; Cicoira, F. Electropolymerized poly (3,4-ethylenedioxythiophene)(PEDOT) coatings for implantable deep-brain-stimulating microelectrodes. ACS Appl. Mater. Interfaces 2019, 11, 17226–17233. [Google Scholar] [CrossRef]
- Green, R.A.; Hassarati, R.T.; Bouchinet, L.; Lee, C.S.; Cheong, G.L.; Jin, F.Y.; Dodds, C.W.; Suaning, G.J.; Poole-Warren, L.A.; Lovell, N.H. Substrate dependent stability of conducting polymer coatings on medical electrodes. Biomaterials 2012, 33, 5875–5886. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure Pedot: Pss hydrogels. Nat. Commun. 2019, 10, 1–10. [Google Scholar]
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef]
- Alberty, R.A. Recommendations for nomenclature and tables in biochemical thermodynamics (IUPAC recommendations 1994). Pure Appl. Chem. 1994, 66, 1641–1666. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Santos, J.R.; Rangel, A.O. Standard addition flow method for potentiometric measurements at low concentration levels: Application to the determination of fluoride in food samples. Talanta 2015, 133, 1–6. [Google Scholar] [CrossRef]
- Chapp, A.D.; Schum, S.; Behnke, J.E.; Hahka, T.; Huber, M.J.; Jiang, E.; Larson, R.A.; Shan, Z.; Chen, Q.H. Measurement of cations, anions, and acetate in serum, urine, cerebrospinal fluid, and tissue by ion chromatography. Physiol. Rep. 2018, 6, e13666. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 1–32. [Google Scholar] [CrossRef]
- Berton, P.; Tian, H.; Rogers, R.D. Phase Behavior of Aqueous Biphasic Systems with Choline Alkanoate Ionic Liquids and Phosphate Solutions: The Influence of pH. Molecules 2021, 26, 1702. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K. Acetylcholine and Choline Amperometric Enzyme Sensors Characterized In Vitro and In Vivo. Anal. Chem. 2004, 76, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Brashear, R.; Mattingly, P. Choline Concentration in Normal Blood Donor and Cardiac Troponin-Positive Plasma Samples. Clin. Chem. 2006, 52, 2123–2124. [Google Scholar] [CrossRef][Green Version]
- Graaf, M. In vivo magnetic resonance spectroscopy: Basic methodology and clinical applications. Eur. Biophys. J. 2009, 39, 527–540. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- Dorrius, M.D.; Pijnappel, R.M.; Jansen-van der Weide, M.C.; Jansen, L.; Kappert, P.; Oudkerk, M.; Sijens, P.E. Determination of choline concentration in breast lesions: Quantitative multivoxel proton MR spectroscopy as a promising noninvasive assessment tool to exclude benign lesions. Radiology 2011, 259, 695–703. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).