Simulation and Experimental Study of the Near Field Probe in the Form of a Folded Dipole for Measuring Glucose Concentration
Abstract
Featured Application
Abstract
1. Introduction
2. Numerical Simulations
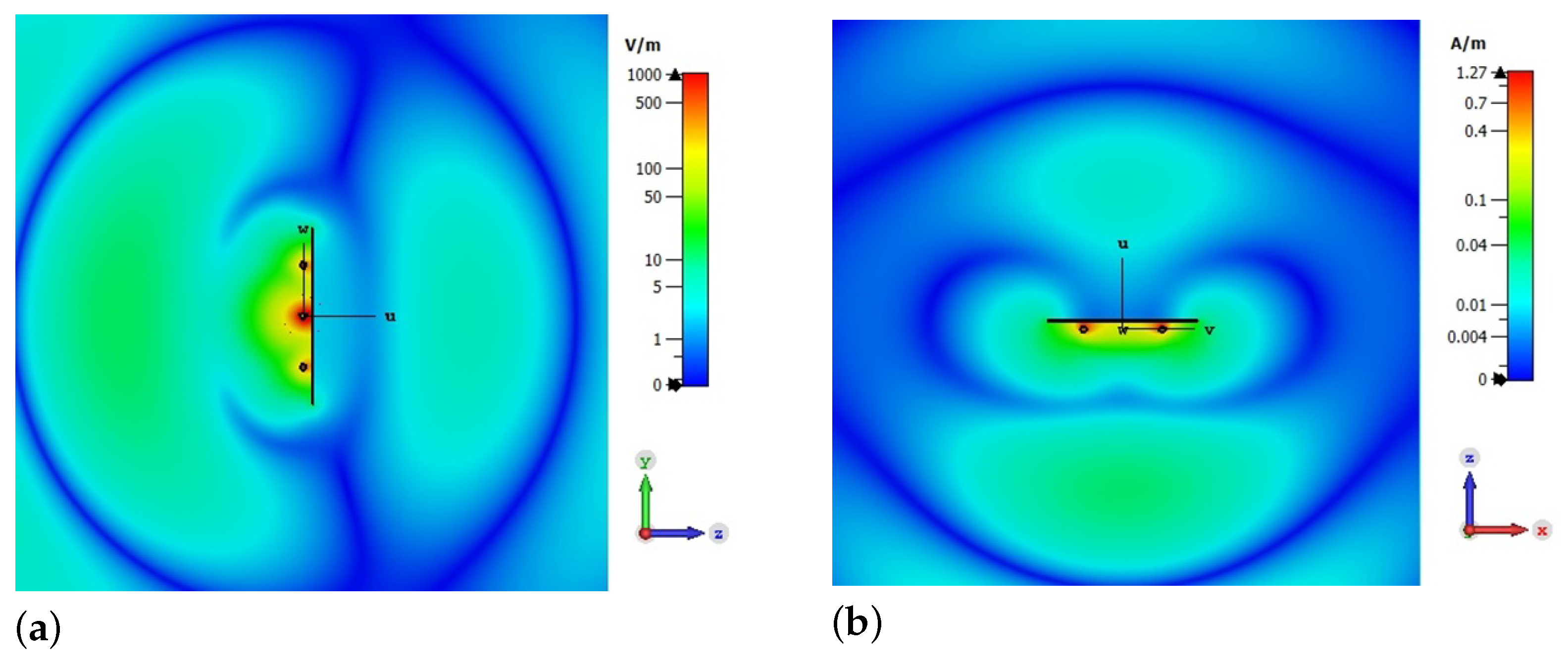
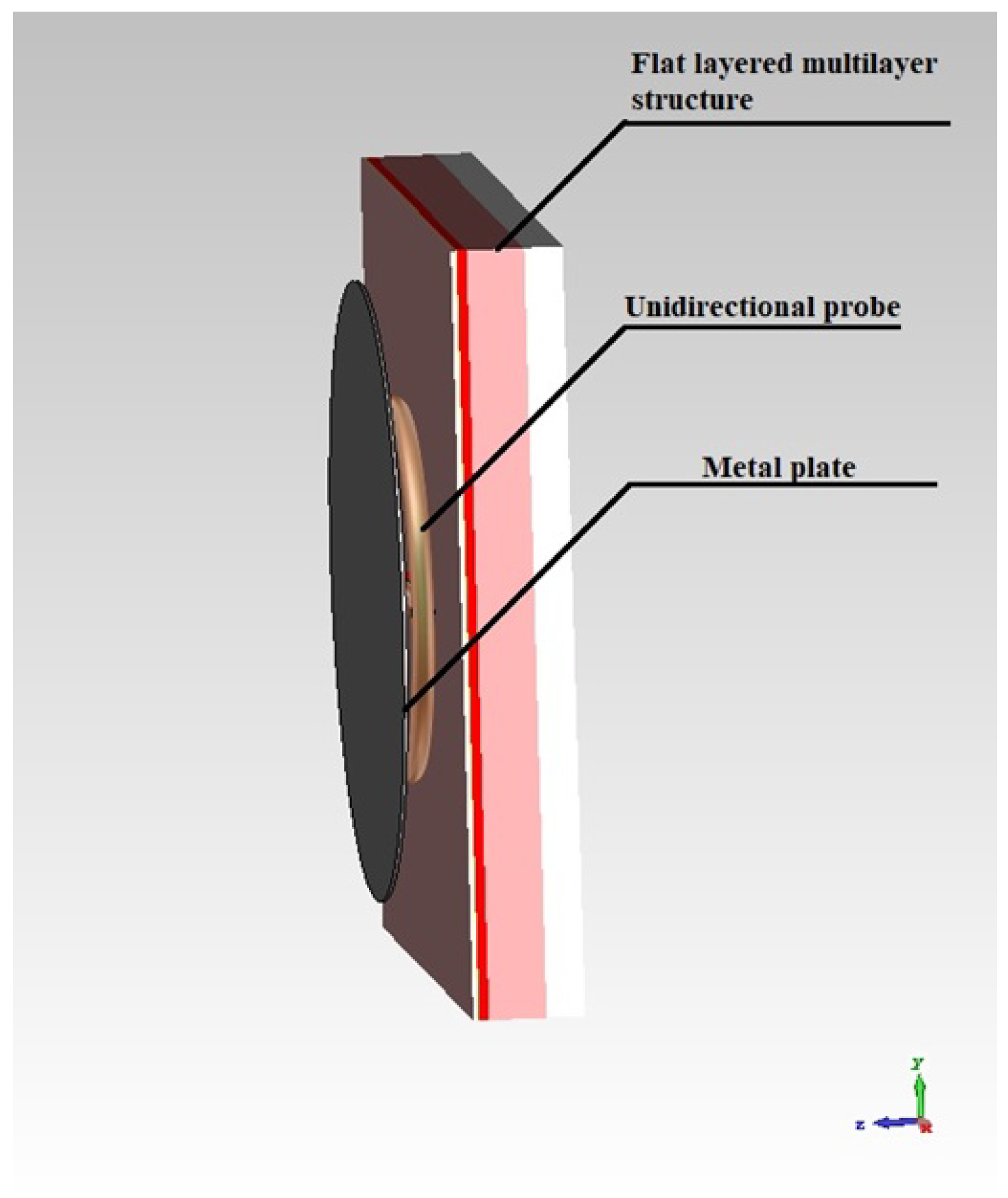
2.1. Simulation of a Unidirectional Probe
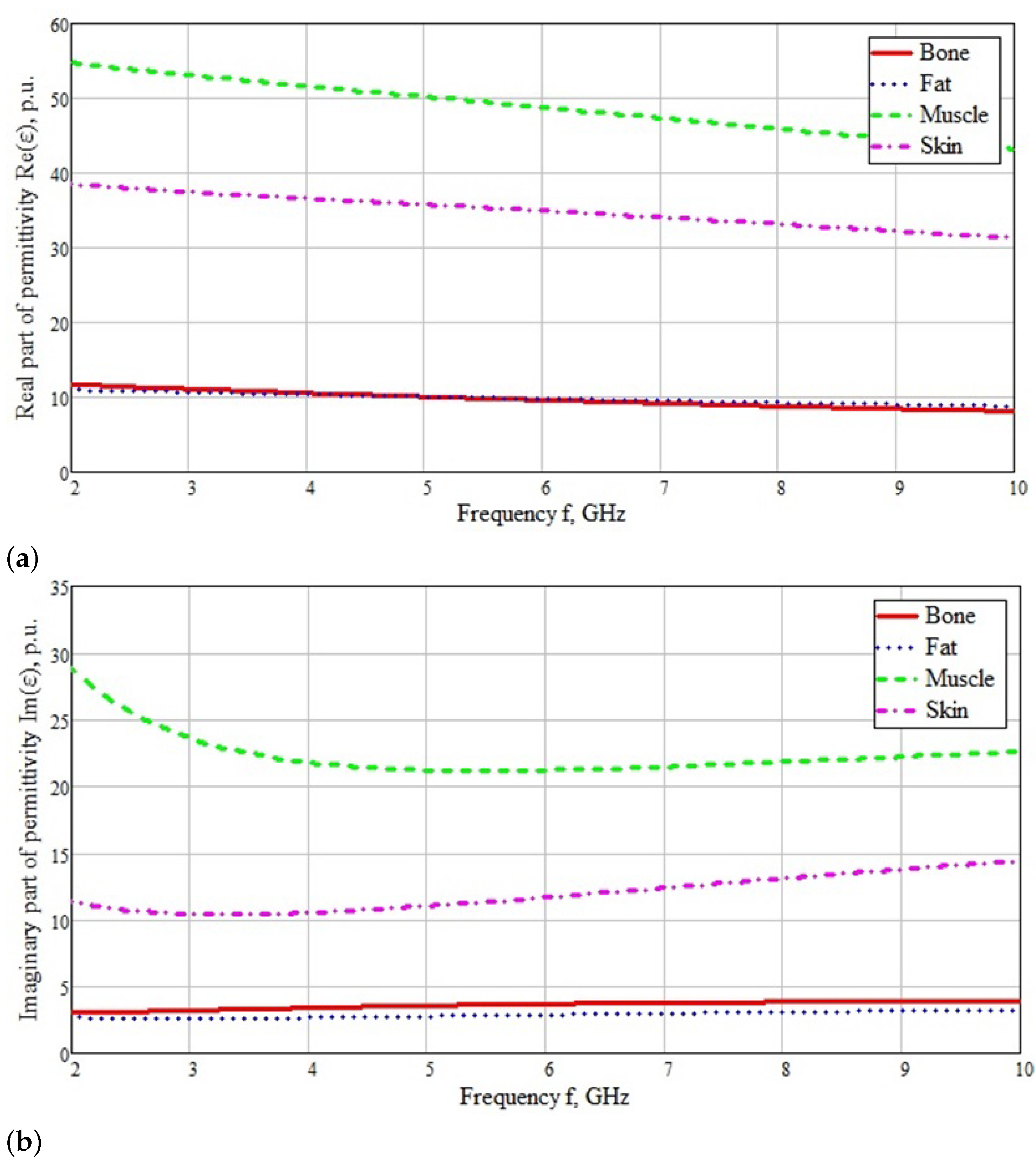
2.2. Creation of a Flat-Layered Biological Environment in the Form of a Human Forearm Phantom
3. Experiments and Results
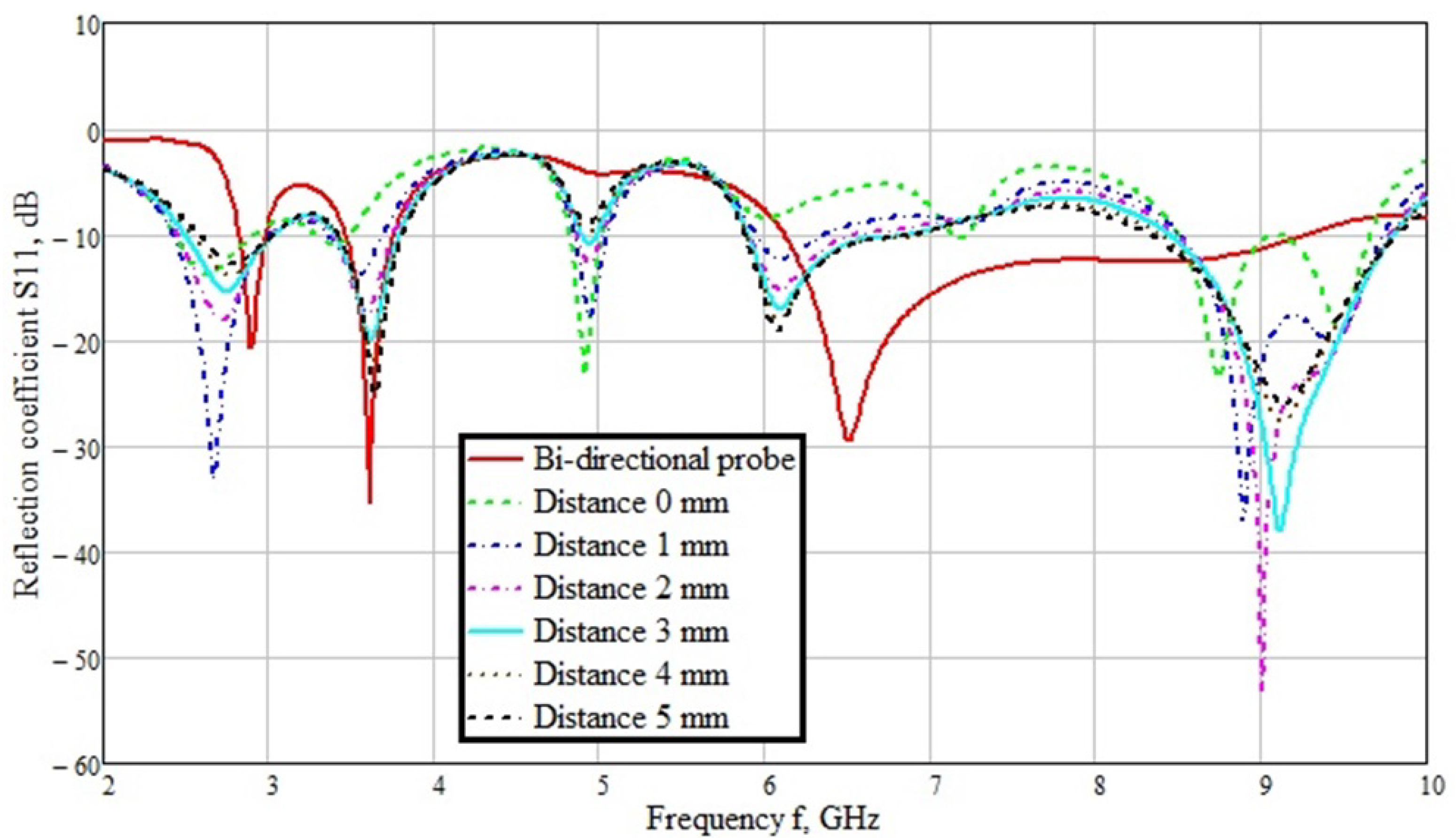
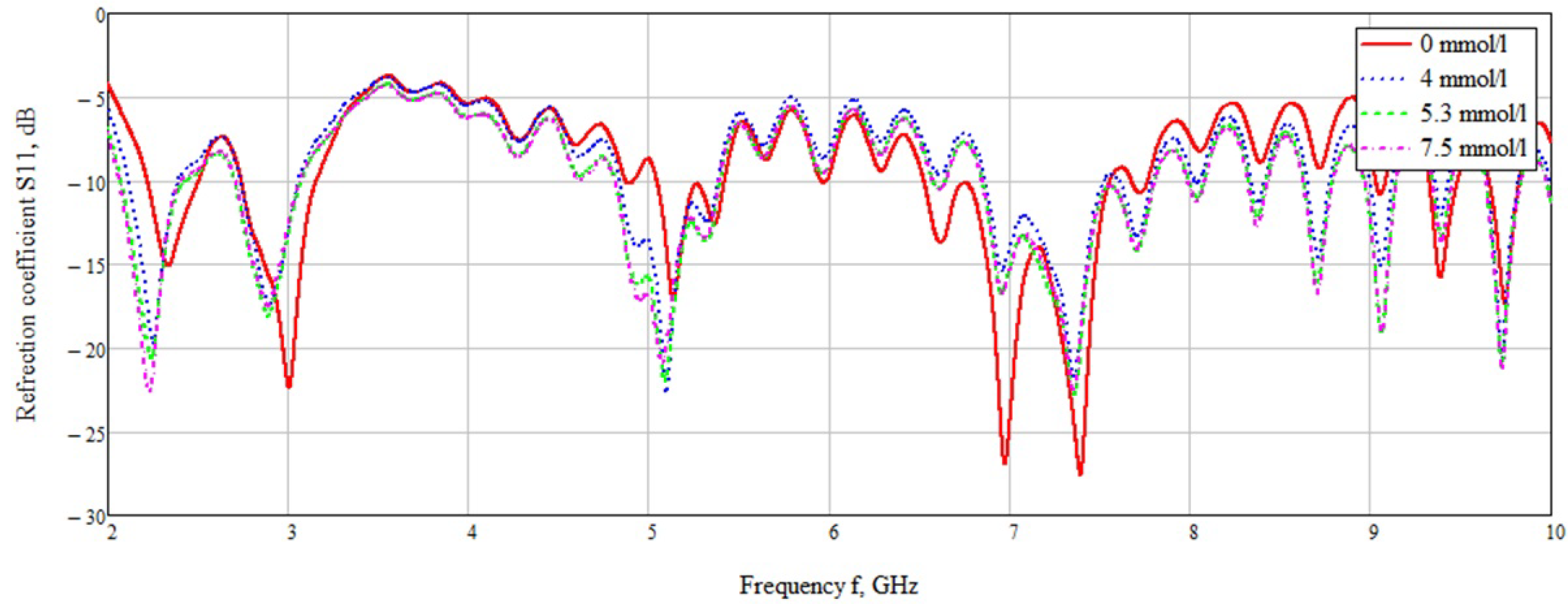
3.1. Unidirectional Probe
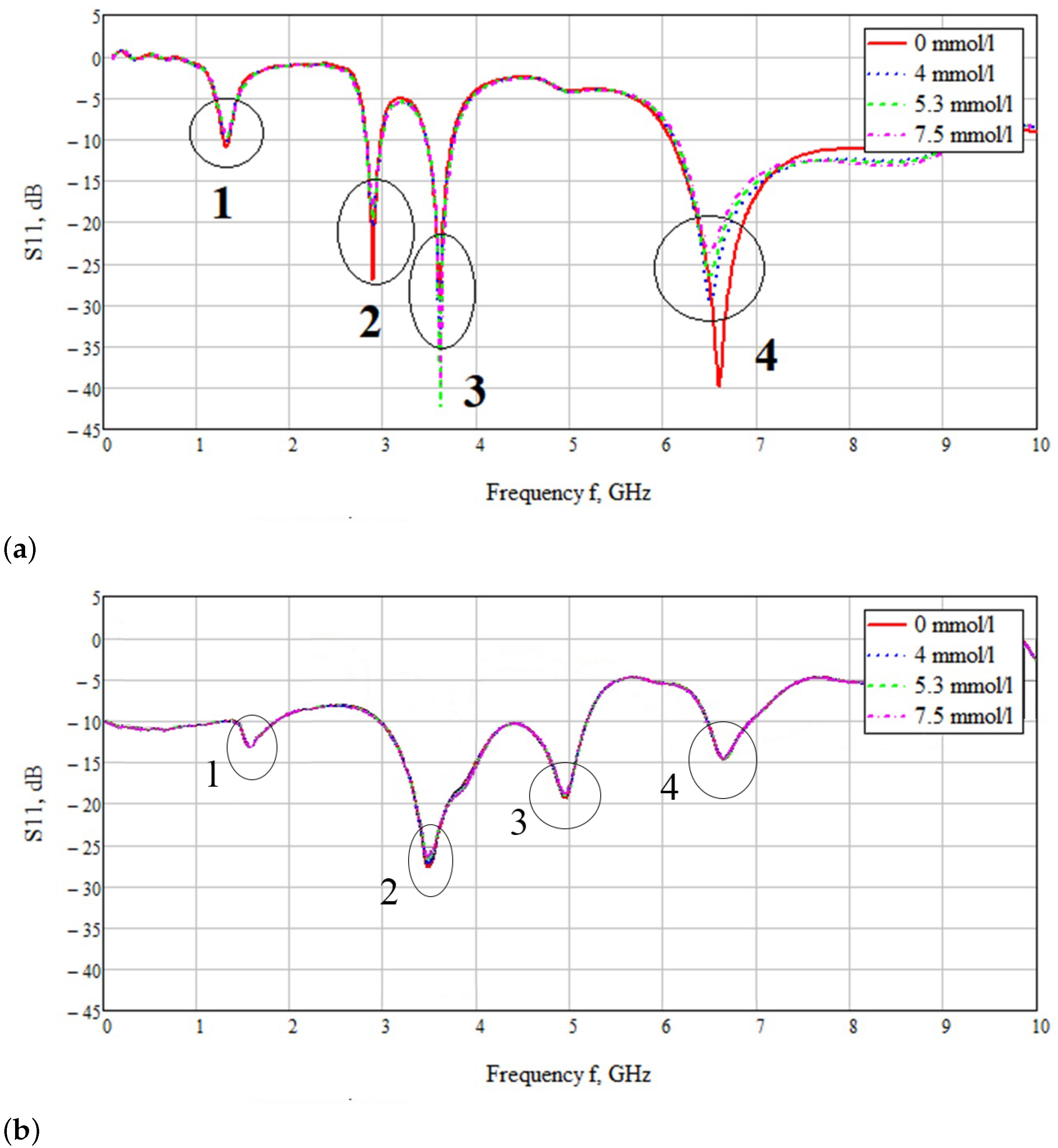
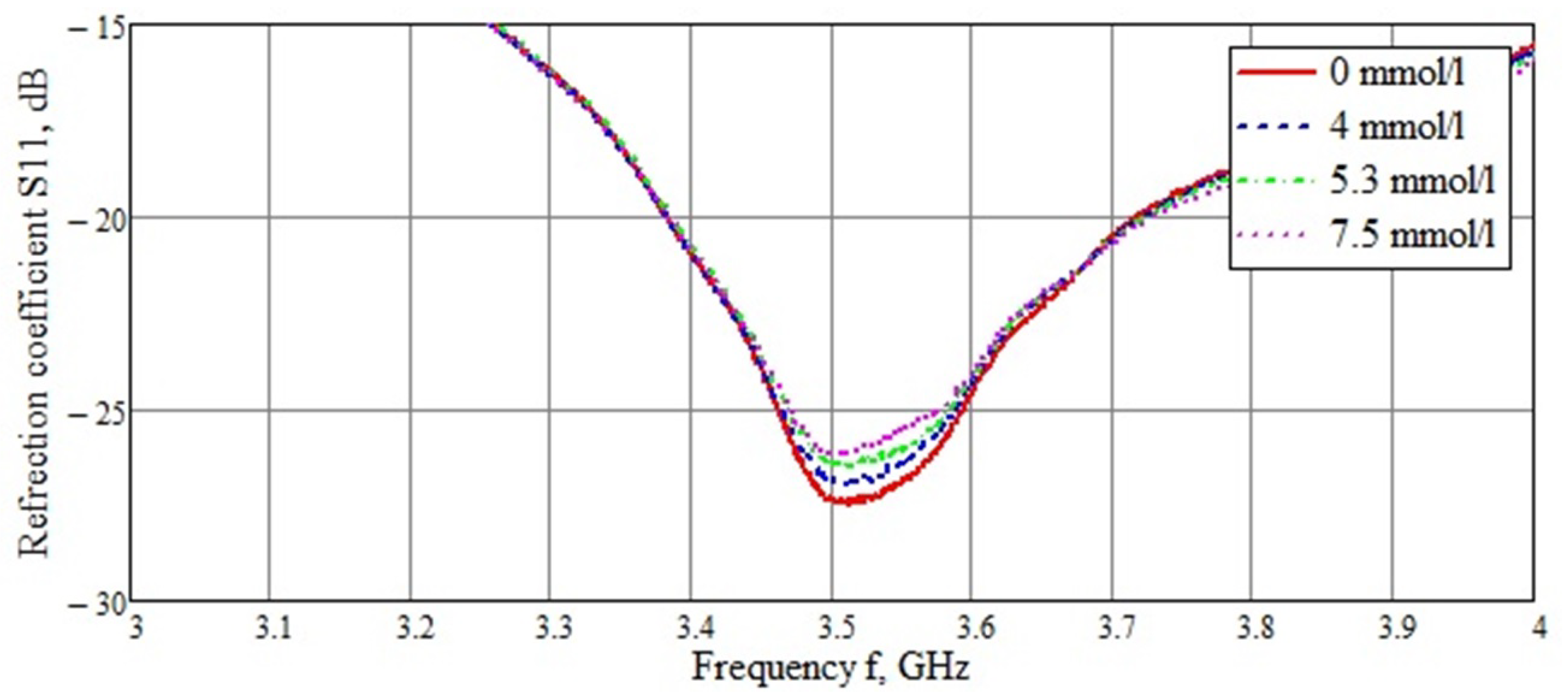
3.2. Bidirectional Probe
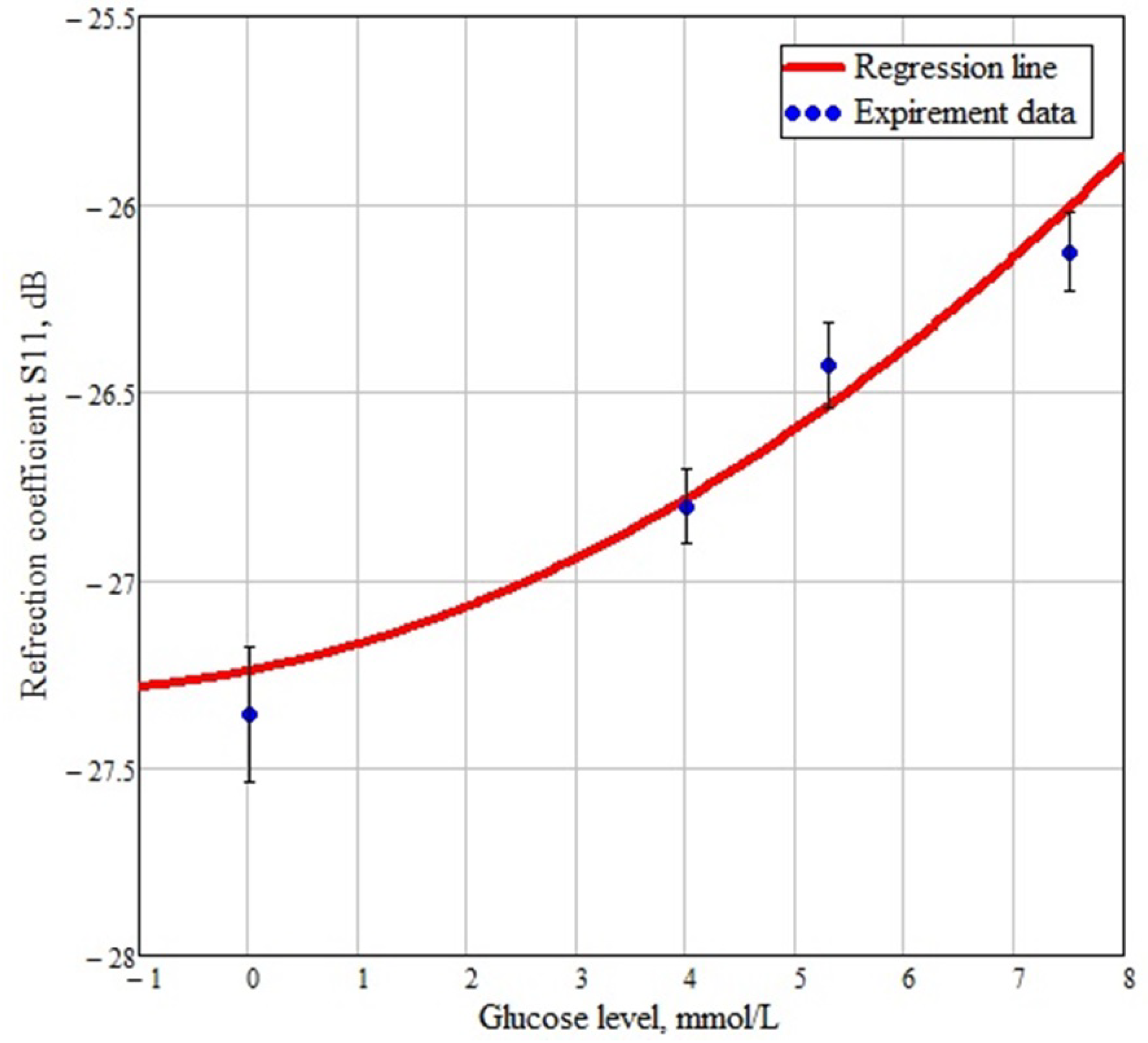
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Foster, R.; Hao, Y. Radio-frequency and microwave techniques for non-invasive measurement of blood glucose levels. Diagnostics 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, M.; Schmelzeisen-Redeker, G.; Jager, J. CGM sensor design principles for reliable and accurate glucose monitoring in the subcutaneous tissue. In Proceedings of the 7th International Conference on Advanced Technologies & Treatments for Diabetes, Vienna, Austria, 5–8 February 2014; pp. 5–8. [Google Scholar]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Imani, S.; Nunez-Flores, R.; Kumar, R.; Wang, C.; Mohan, A.V.; Wang, J.; Mercier, P.P. Re-usable electrochemical glucose sensors integrated into a smartphone platform. Biosens. Bioelectron. 2018, 101, 181–187. [Google Scholar] [CrossRef]
- Bazaev, N.; Masloboev, Y.P.; Selishchev, S. Optical methods for noninvasive blood glucose monitoring. Biomed. Eng. 2012, 45, 229–233. [Google Scholar] [CrossRef]
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487. [Google Scholar] [CrossRef]
- Jung, W.; Kim, J.; Jeon, M.; Chaney, E.J.; Stewart, C.N.; Boppart, S.A. Handheld optical coherence tomography scanner for primary care diagnostics. IEEE Trans. Biomed. Eng. 2010, 58, 741–744. [Google Scholar] [CrossRef]
- Xiao, X.; Li, Q. A noninvasive measurement of blood glucose concentration by UWB microwave spectrum. Ieee Antennas Wirel. Propag. Lett. 2016, 16, 1040–1043. [Google Scholar] [CrossRef]
- Jha, A.K.; Akhter, Z.; Tiwari, N.; Shafi, K.M.; Samant, H.; Akhtar, M.J.; Cifra, M. Broadband wireless sensing system for non-invasive testing of biological samples. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 251–259. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A. Coplanar waveguide transmission line loaded with electric-LC resonator for determination of glucose concentration sensing. IEEE Sens. J. 2017, 17, 1635–1640. [Google Scholar] [CrossRef]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and in vitro interference test of microwave noninvasive blood glucose monitoring sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Turgul, V.; Kale, I. Simulating the effects of skin thickness and fingerprints to highlight problems with non-invasive RF blood glucose sensing from fingertips. IEEE Sens. J. 2017, 17, 7553–7560. [Google Scholar] [CrossRef]
- Pimentel, S.; Agüero, P.D.; Uriz, A.J.; Bonadero, J.C.; Liberatori, M.; Moreira, J.C. Simulation of a non-invasive glucometer based on a microwave resonator sensor. J. Phys. Conf. Ser. IOP Publ. 2013, 477, 012020. [Google Scholar] [CrossRef]
- Adhikari, K.K.; Kim, N.Y. Ultrahigh-sensitivity mediator-free biosensor based on a microfabricated microwave resonator for the detection of micromolar glucose concentrations. IEEE Trans. Microw. Theory Tech. 2015, 64, 319–327. [Google Scholar] [CrossRef]
- Guariti, G.; Hofmann, M.; Weigel, R.; Fischer, G.; Kissinger, D. Determination of sugar concentration in aqueous solutions using ultra-wideband microwave impedance spectroscopy. In Proceedings of the 2013 IEEE MTT-S International Microwave Symposium Digest (MTT), Seattle, WA, USA, 2–7 June 2013; pp. 1–4. [Google Scholar]
- Lin, Y.R.; Hung, C.C.; Chiu, H.Y.; Chang, P.H.; Li, B.R.; Cheng, S.J.; Yang, J.W.; Lin, S.F.; Chen, G.Y. Noninvasive glucose monitoring with a contact lens and smartphone. Sensors 2018, 18, 3208. [Google Scholar] [CrossRef]
- Gorst, A.; Zavyalova, K.; Yakubov, V.; Mironchev, A.; Zapasnoy, A. Theoretical Simulation of the Near-Field Probe for Non-Invasive Measurements on Planar Layers with Biological Characteristics. Bioengineering 2020, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271. [Google Scholar] [CrossRef] [PubMed]
- Freer, B.; Venkataraman, J. Feasibility study for non-invasive blood glucose monitoring. In Proceedings of the 2010 IEEE Antennas and Propagation Society International Symposium, Toronto, ON, Canadan, 11–17 July 2010; pp. 1–4. [Google Scholar]
- Di Meo, S.; Pasotti, L.; Iliopoulos, I.; Pasian, M.; Ettorre, M.; Zhadobov, M.; Matrone, G. Tissue-mimicking materials for breast phantoms up to 50 GHz. Phys. Med. Biol. 2019, 64, 055006. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Madsen, E.L.; Frank, G.R.; Hagness, S.C. Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications. Phys. Med. Biol. 2005, 50, 4245. [Google Scholar] [CrossRef]
- Di Meo, S.; Matrone, G.; Pasian, M. Experimental Validation on Tissue-Mimicking Phantoms of Millimeter-Wave Imaging for Breast Cancer Detection. Appl. Sci. 2021, 11, 432. [Google Scholar] [CrossRef]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Gorst, A.; Zavyalova, K.; Shipilov, S.; Yakubov, V.; Mironchev, A. Microwave Method for Measuring Electrical Properties of the Materials. Appl. Sci. 2020, 10, 8936. [Google Scholar] [CrossRef]
- Huang, S.Y.; Yoshida, Y.; Inda, A.J.G.; Xavier, C.X.; Mu, W.C.; Meng, Y.S.; Yu, W. Microstrip line-based glucose sensor for noninvasive continuous monitoring using the main field for sensing and multivariable crosschecking. IEEE Sens. J. 2018, 19, 535–547. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Babajanyan, A.; Lee, K.; Friedman, B. Noncontact characterization of glucose by a waveguide microwave probe. Curr. Appl. Phys. 2009, 9, 856–860. [Google Scholar] [CrossRef]
- Hu, S.; Nagae, S.; Hirose, A. Millimeter-wave adaptive glucose concentration estimation with complex-valued neural networks. IEEE Trans. Biomed. Eng. 2018, 66, 2065–2071. [Google Scholar] [CrossRef]
- Omer, A.E.; Gigoyan, S.; Shaker, G.; Safavi-Naeini, S. WGM-based sensing of characterized glucose-aqueous solutions at mm-waves. IEEE Access 2020, 8, 38809–38825. [Google Scholar] [CrossRef]


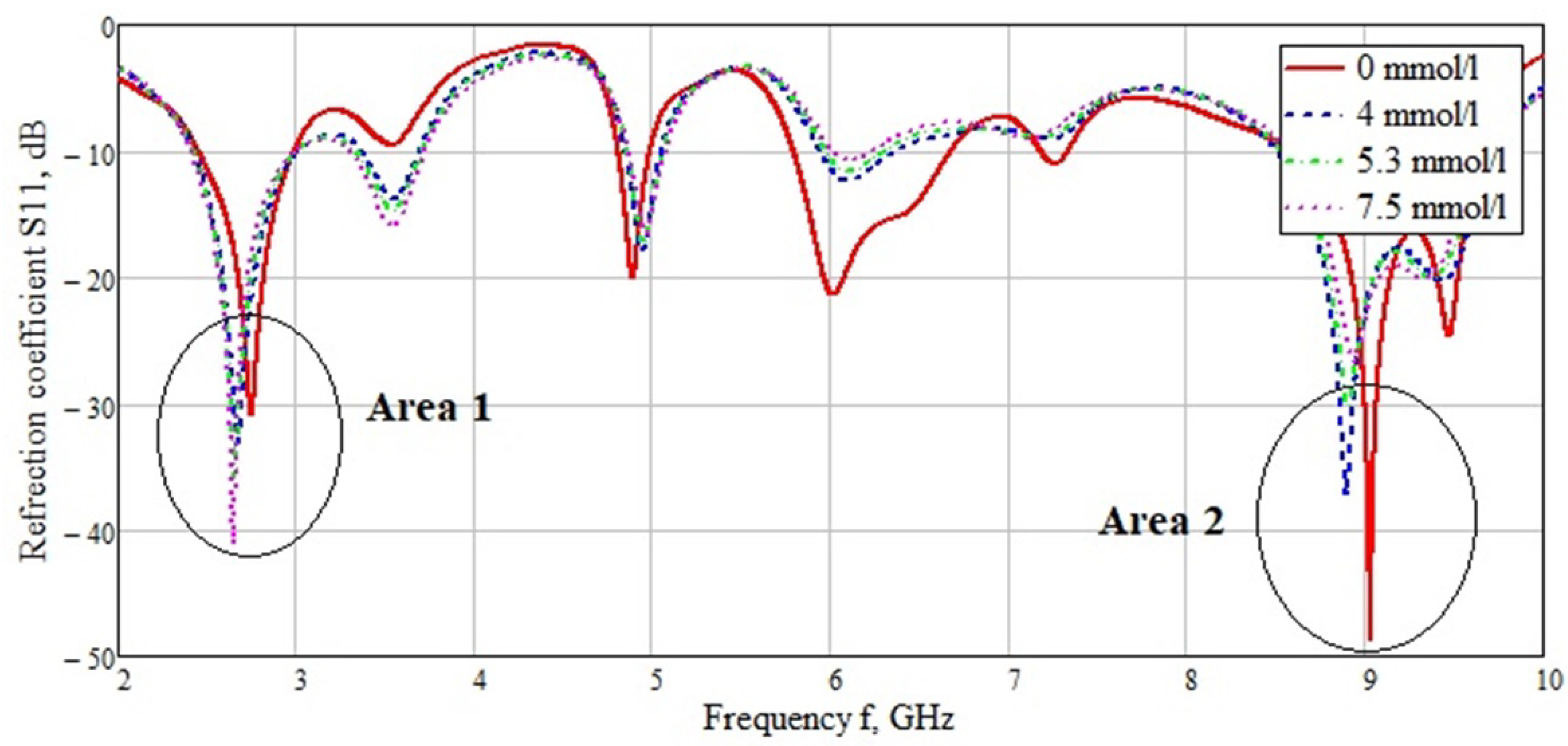
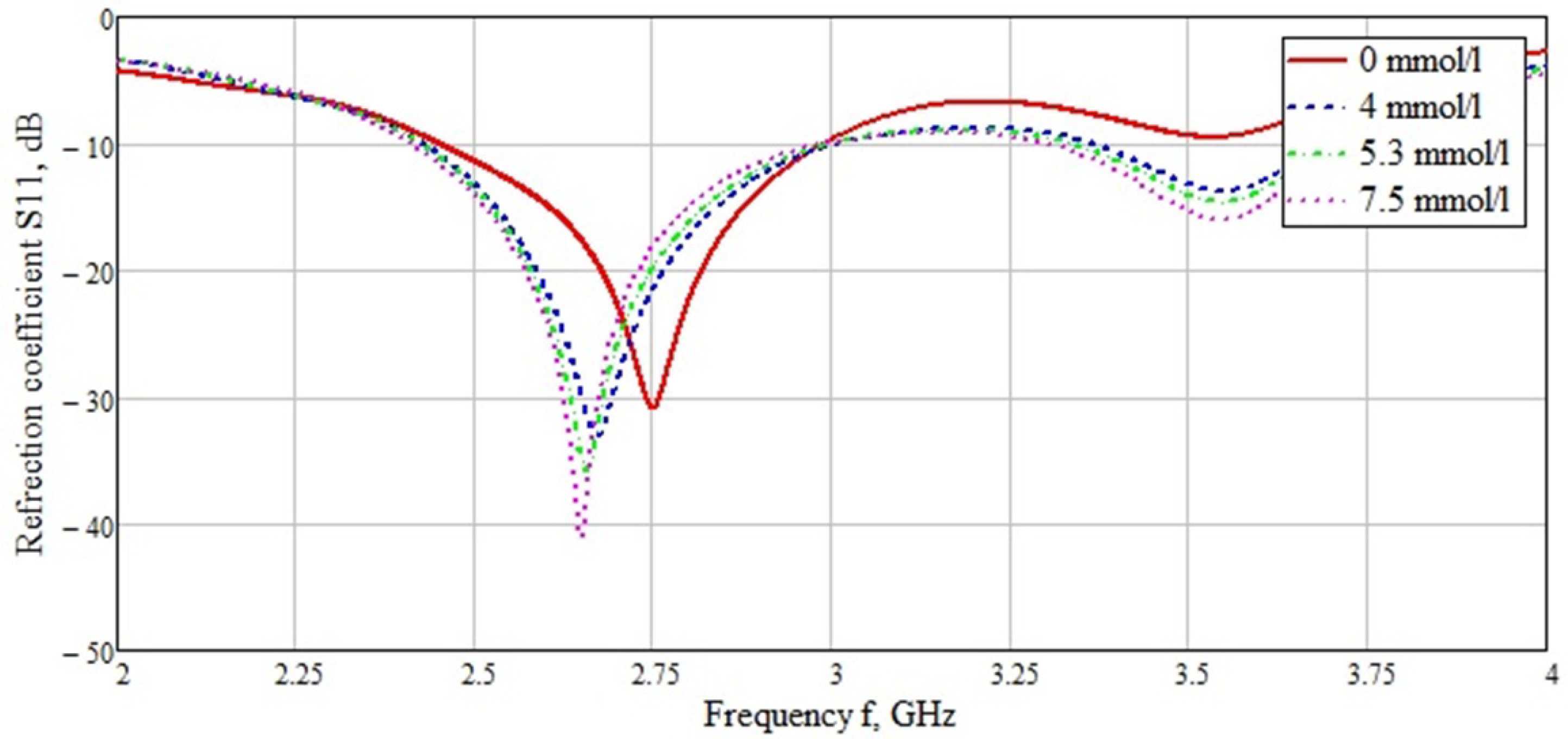

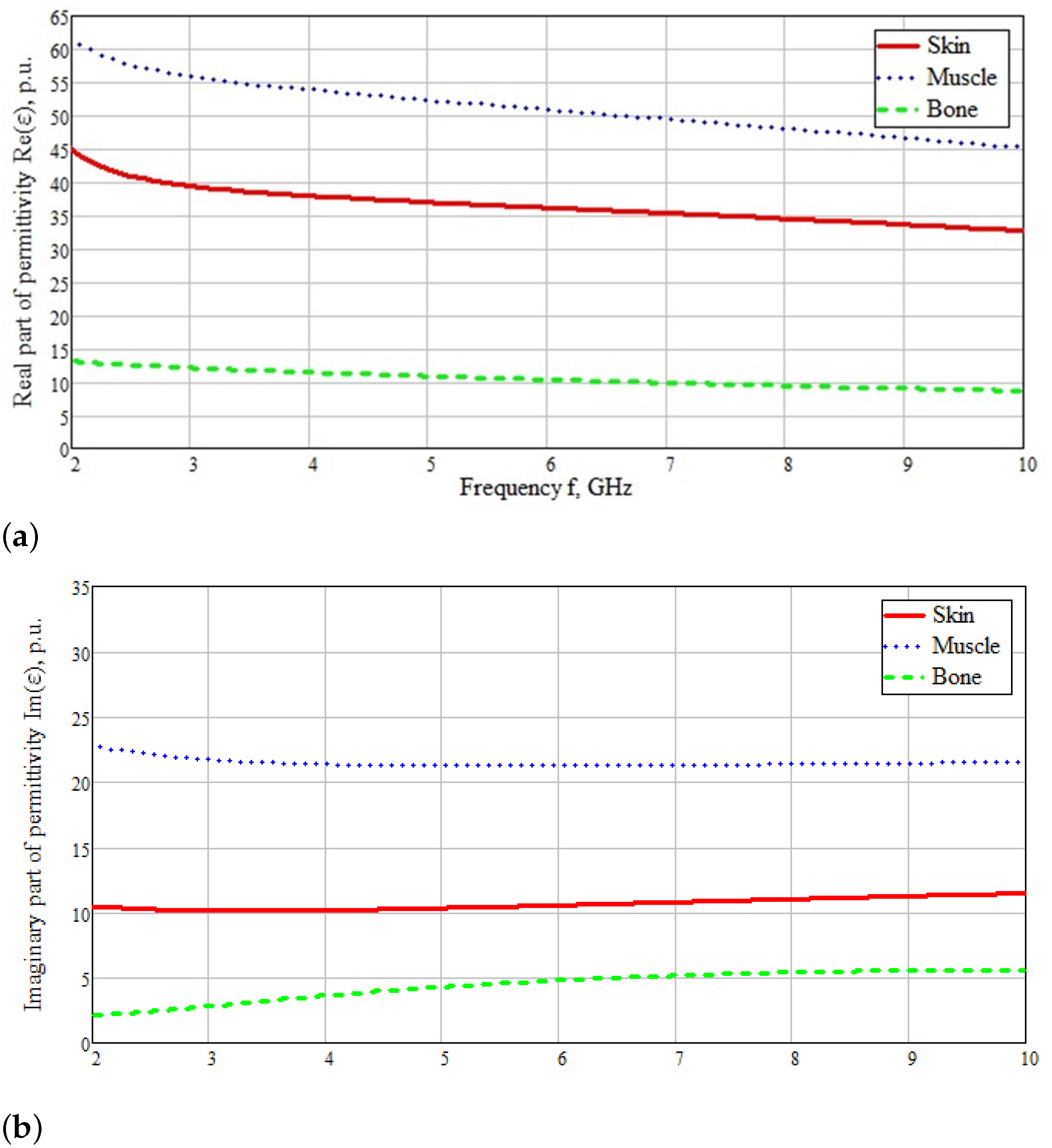
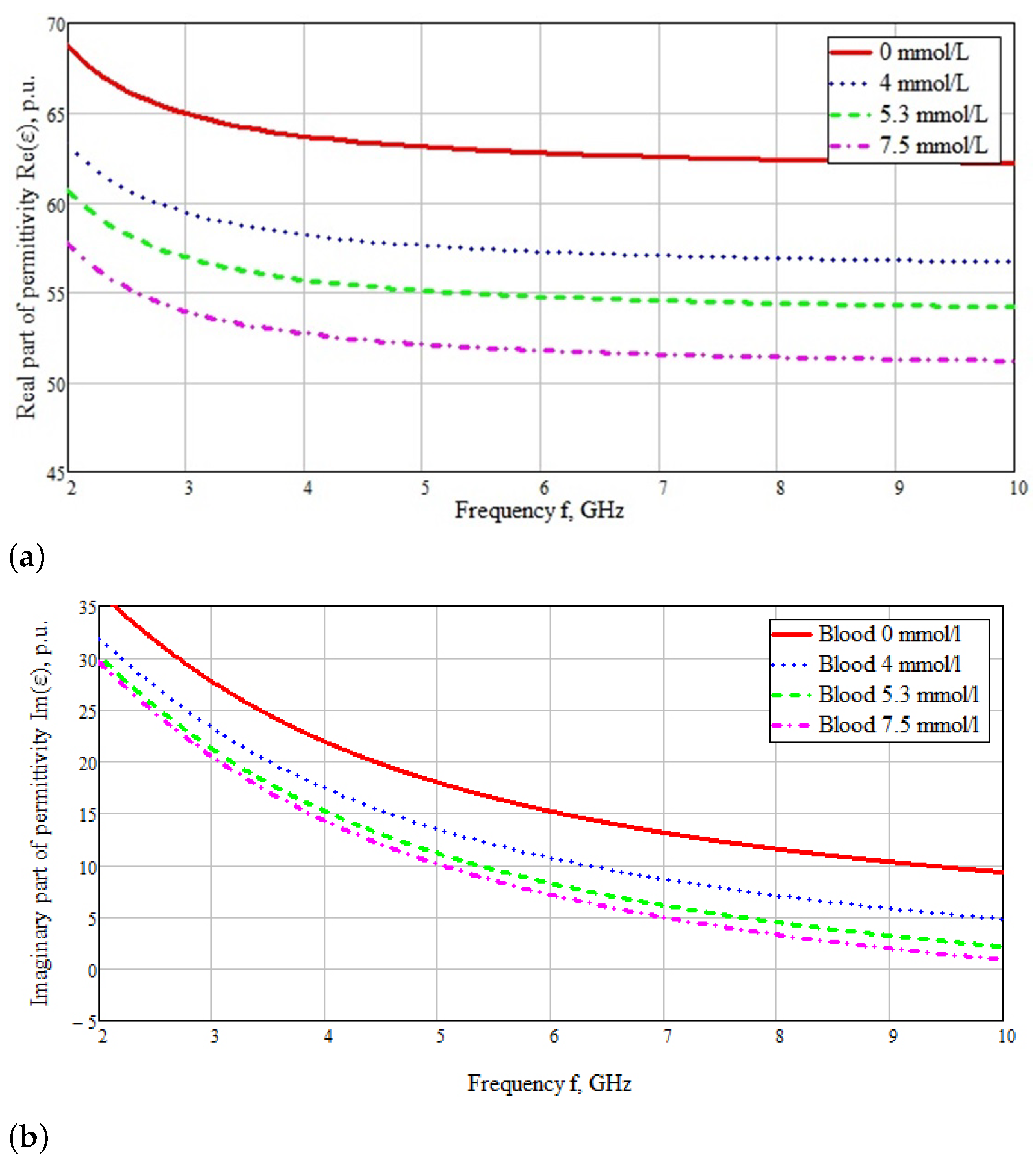



| Skin | Fat | Muscle | Bone | |
|---|---|---|---|---|
| 4 | 2.5 | 4 | 2.5 | |
| 0.0002 | 0.035 | 0.2 | 0.02 | |
| 0 | 0.2 | 0.1 | 0.2 | |
| 0.2 | 0.1 | 0.1 | 0.2 | |
| — | 0.05 | 0.1 | 0.2 | |
| — | 0.1 | 0 | 0 | |
| 32 | 9 | 50 | 10 | |
| 1100 | 35 | 7 × 10 | 180 | |
| — | 33 × 10 | 12 × 10 | 5 × 10 | |
| — | 1 × 10 | 2.5 × 10 | 1 × 10 | |
| 7.234 × 10 | 7.958 × 10 | 7.234 × 10 | 13.263 × 10 | |
| 0.324 × 10 | 0.159 × 10 | 3.537 × 10 | 0.795 × 10 | |
| — | 15.915 × 10 | 31.831 × 10 | 15.915 × 10 | |
| — | 1.595 × 10 | 0.274 × 10 | 1.591 × 10 |
| Material | Polyuret. HP40 % | Two-Comp. Polyuret.// % | Graphite, % | Acetone, mL/100 g |
|---|---|---|---|---|
| Skin | 30 | 30 | 32.3 | 7.7 |
| Blood 0 mmol/L | 30 | 30 | 33.8 | 6.2 |
| Blood 4 mmol/L | 30 | 30 | 30.2 | 9.8 |
| Blood 5.3 mmol/L | 30 | 30 | 29 | 11 |
| Blood 7.5 mmol/L | 30 | 30 | 27.1 | 12.9 |
| Muscle | 33.7 | 33.7 | 25.8 | 6.8 |
| Bone | 40 | 40 | 15.6 | 4.4 |
| Concentration Level | Amplitude of the Reflected Signal at a Frequency of 2.235 GHz (dB) | The Minimum Amplitude of the Reflected Signal (dB) | Frequency at Minimum Amplitude (GHz) |
|---|---|---|---|
| Blood 0 mmol/L | |||
| Blood 4 mmol/L | |||
| Blood 5.3 mmol/L | |||
| Blood 7.5 mmol/L |
| Concentration Level | Frequency | Reflected Signal Amplitude (dB) |
|---|---|---|
| Blood 0 mmol/L | 3.5 GHz | |
| Blood 4 mmol/L | 3.5 GHz | |
| Blood 5.3 mmol/L | 3.5 GHz | |
| Blood 7.5 mmol/L | 3.5 GHz |
| Reference | Concentration (mg/mL) | Frequency (GHz) | Sensitivity Parameter | S (dB per mg/mL) |
|---|---|---|---|---|
| [26] | 0.78–50 | 1.4–1.9 | S11 | 0.18 |
| [27] | 0–300 | 2.0–2.5 | S11 | 0.003 |
| [28] | 0–3 | 60–80 | S12 | 0.23 |
| [29] | 0.7–1.2 | 50–70 | S12 | 0.8–1 |
| [11] | 40–200 | 2.5–6 | S12 | 0.01 |
| Unidirectional probe | 0–1.81 | 2.1–2.5 | S11 | 0.94–1.1 |
| Bidirectional probe | 0–1.81 | 3.4–3.6 | S11 | 0.16–0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorst, A.; Zavyalova, K.; Mironchev, A.; Zapasnoy, A.; Klokov, A. Simulation and Experimental Study of the Near Field Probe in the Form of a Folded Dipole for Measuring Glucose Concentration. Appl. Sci. 2021, 11, 5415. https://doi.org/10.3390/app11125415
Gorst A, Zavyalova K, Mironchev A, Zapasnoy A, Klokov A. Simulation and Experimental Study of the Near Field Probe in the Form of a Folded Dipole for Measuring Glucose Concentration. Applied Sciences. 2021; 11(12):5415. https://doi.org/10.3390/app11125415
Chicago/Turabian StyleGorst, Aleksandr, Kseniya Zavyalova, Aleksandr Mironchev, Andrey Zapasnoy, and Andrey Klokov. 2021. "Simulation and Experimental Study of the Near Field Probe in the Form of a Folded Dipole for Measuring Glucose Concentration" Applied Sciences 11, no. 12: 5415. https://doi.org/10.3390/app11125415
APA StyleGorst, A., Zavyalova, K., Mironchev, A., Zapasnoy, A., & Klokov, A. (2021). Simulation and Experimental Study of the Near Field Probe in the Form of a Folded Dipole for Measuring Glucose Concentration. Applied Sciences, 11(12), 5415. https://doi.org/10.3390/app11125415






