Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies
Abstract
1. Overview on Gas Sensors
1.1. The Human Olfactory System
1.2. Research Background on Gas Sensors
1.3. World Market
2. Towards Optical Gas Sensing: Plasmonic Nanoparticles
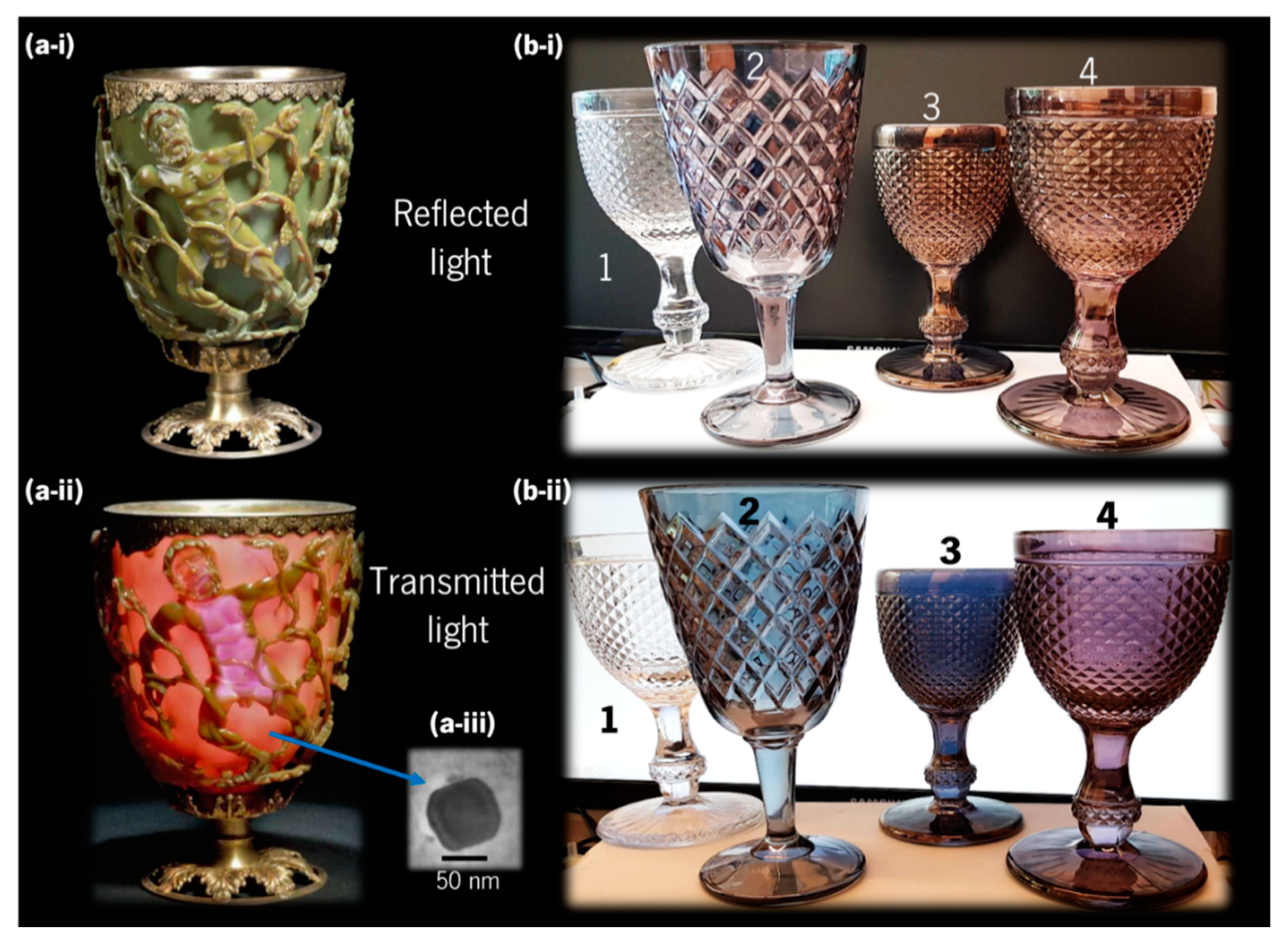
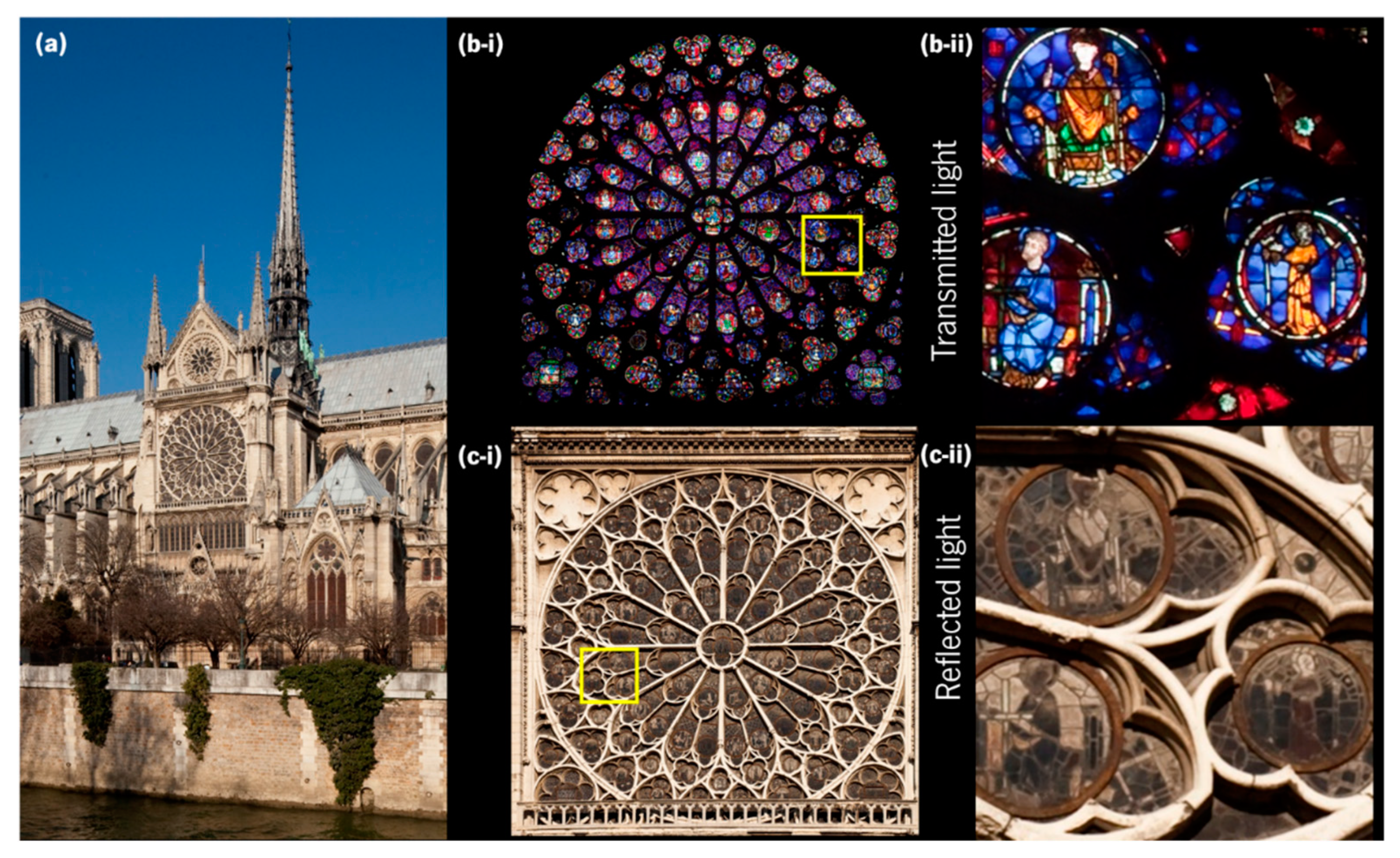
2.1. Ancient Stained Glass
2.2. Background of Surface Plasmons
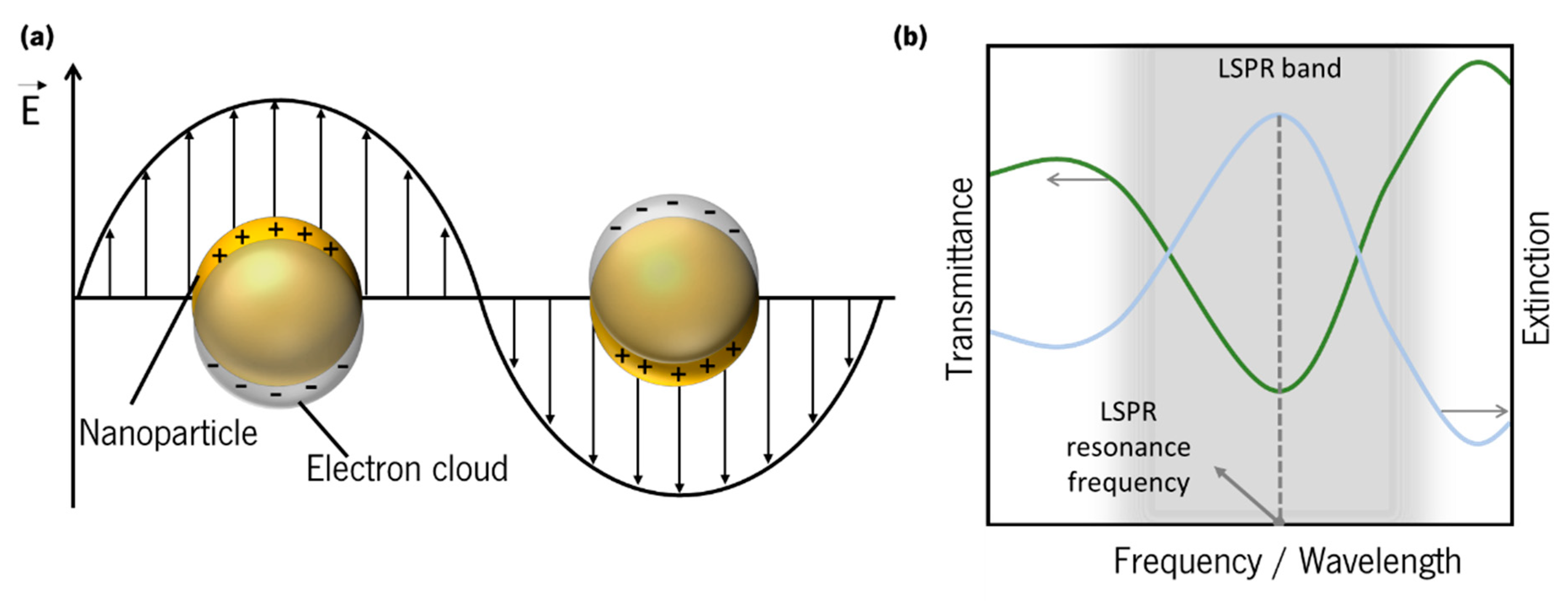
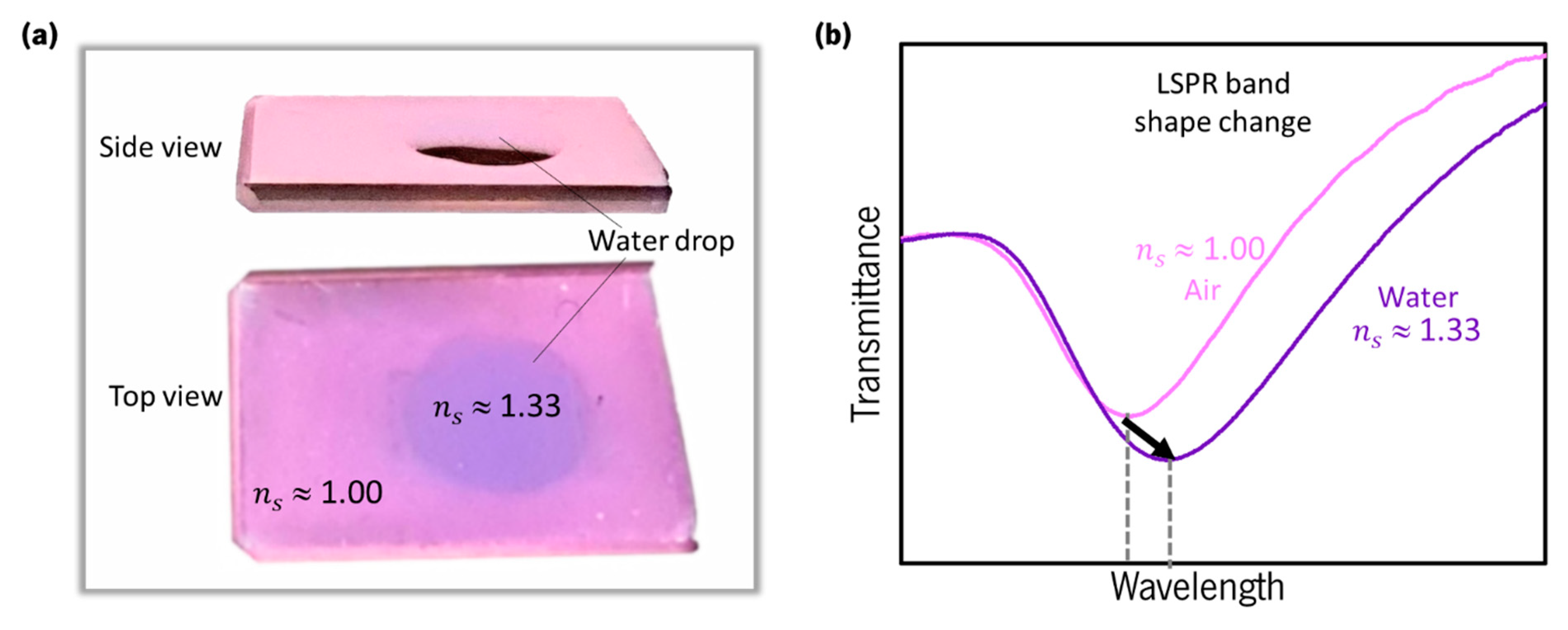
2.3. Localized Surface Plasmon Resonance (LSPR)
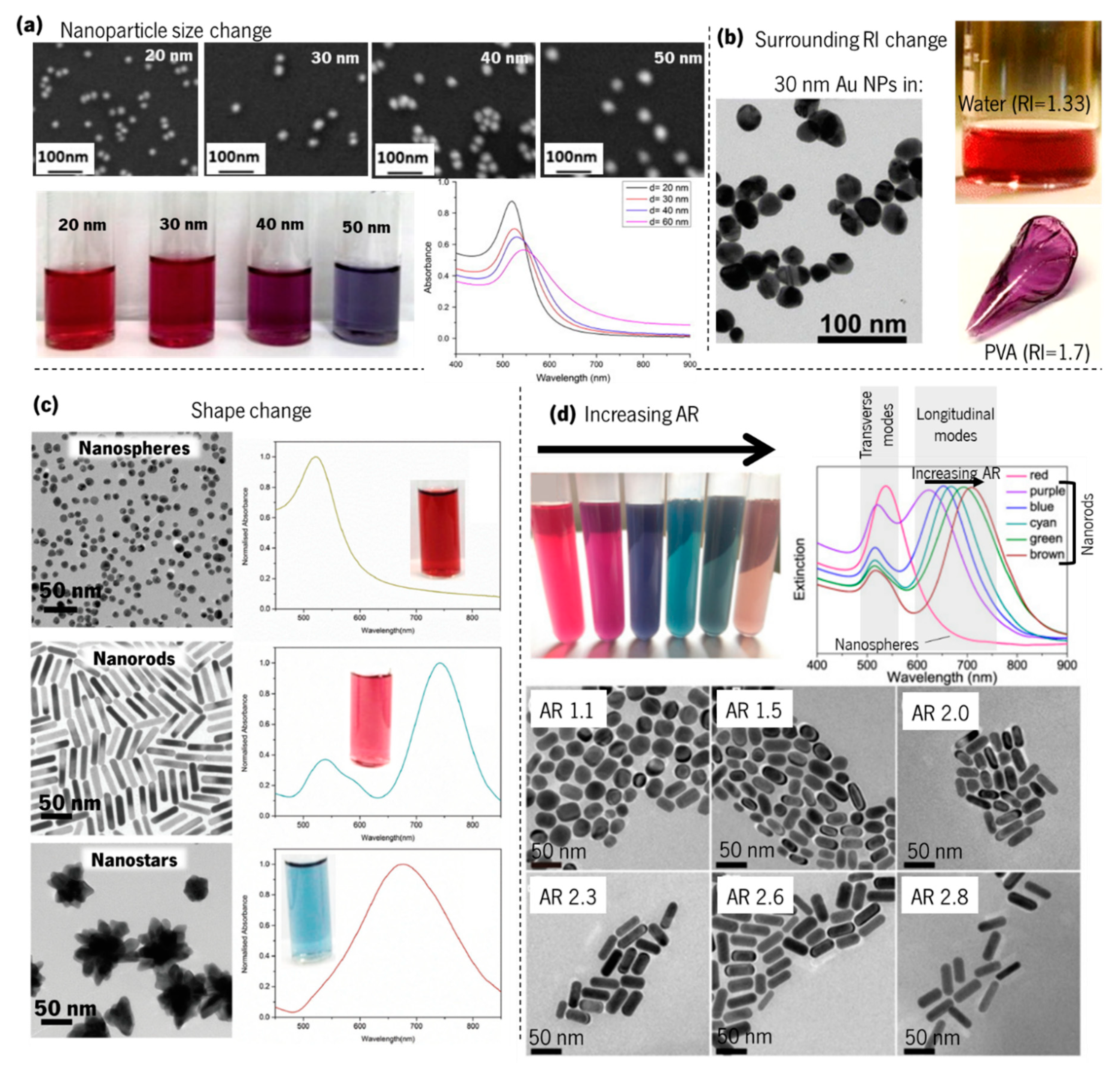
2.4. Gold Nanoparticles: LSPR Band Curvature and Applications
3. Metal Oxides as Robust Platforms to Host Plasmonic Nanoparticles
4. Preparation of Plasmonic Thin Film Sensors
4.1. Thin Film Deposition Methods
4.2. Reactive Magnetron Sputtering Deposition
4.3. Thermal Annealing to Promote Gold Nanoparticle Growth
4.4. Low-Pressure Plasma Treatment for Surface Etching
4.5. Glancing Angle Deposition (GLAD) to Tailor the Micro/Nano Structure
5. Tailoring the Sensitivity of LSPR-Based Sensors Using Simulation Models
5.1. Important Parameters Influencing the LSPR-Based Sensor Performance
5.2. Optical Properties Modelling
- (i)
- Nanoparticle shape (for instance, Mie codes and their extensions work only with high-symmetry shapes and the DDA uses a large amount of memory if the simulations use both large and very small shapes at the same time);
- (ii)
- Number of simulations to run (for instance, spectral calculations are time consuming for FDTD, FEM, and DDA);
- (iii)
- Type of output one is interested in, namely, only the local field intensity in the vicinity of a plasmonic structure, to find the most intense hot spots, or angular distribution of the scattered light intensity and polarization in the far field zone (for instance, COMSOL is ideal for the former but not so well-suited for the latter).
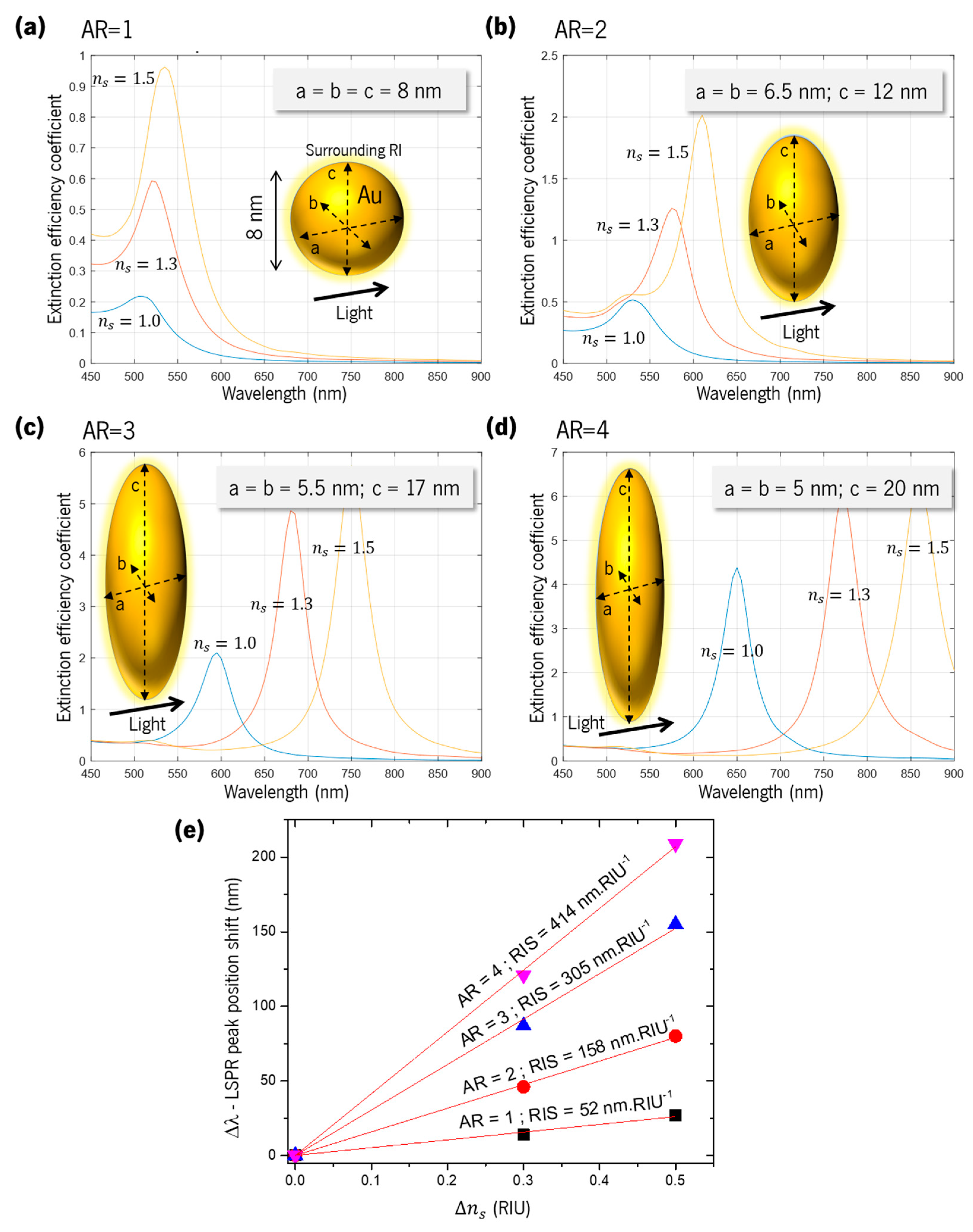
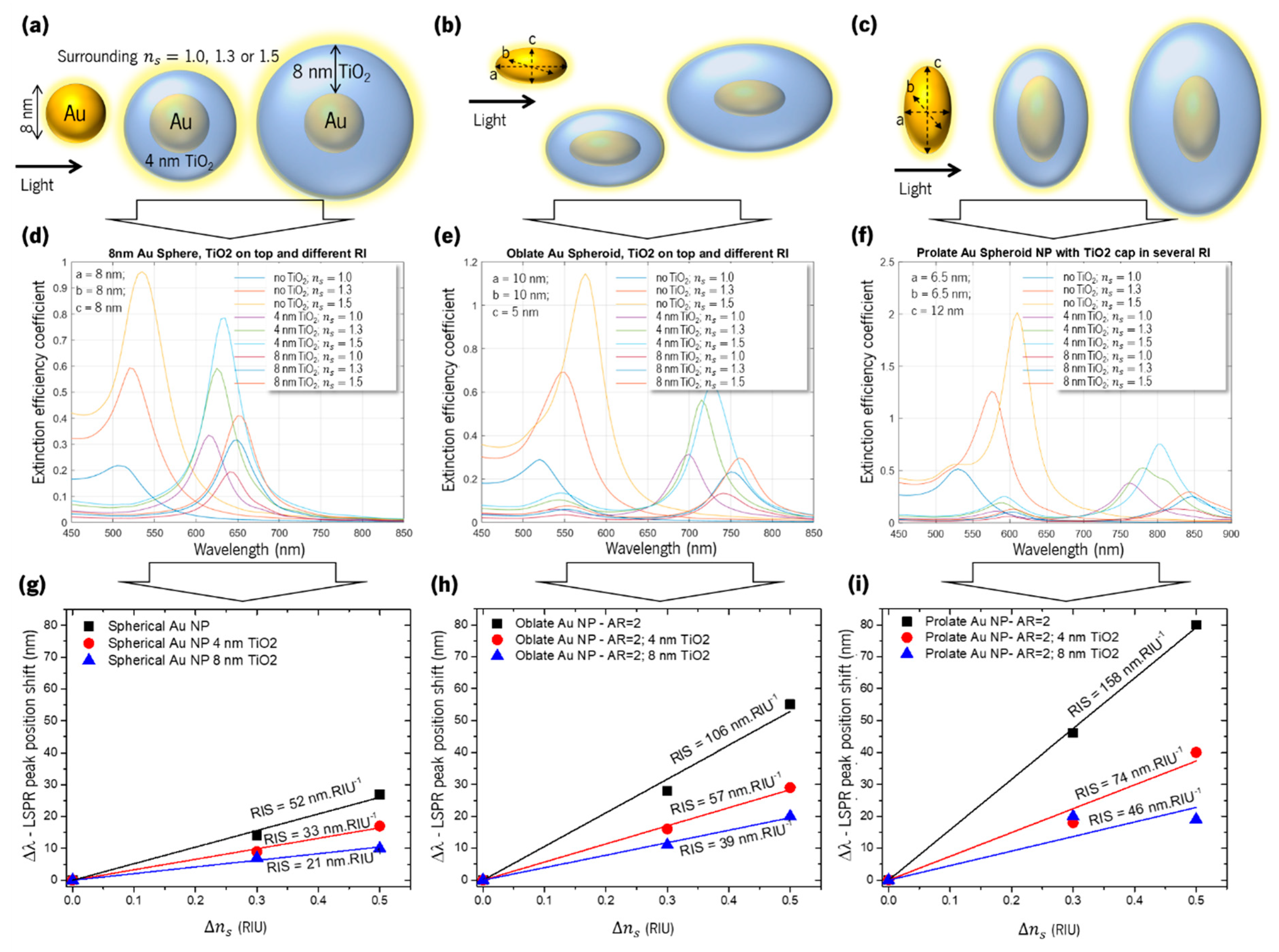
5.3. Sensitivity of a Spheroid Au Nanoparticle Embedded in TiO2
- (i)
- Unpolarized light;
- (ii)
- Four dipoles per nm;
- (iii)
- Au dielectric function from Johnson and Christy [230];
- (iv)
- TiO2 dielectric constant (ε = 6.8121, or = 2.61);
- (v)
- Surrounding external refractive index set either to 1.0, 1.3, or 1.5, as a “semi-infinite” layer;
- (vi)
- A wavelength range from 450 to 900 nm with a 5 nm step.
6. Technoeconomic Challenges
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Lafreniere, D.; Mann, N. Anosmia: Loss of Smell in the Elderly. Otolaryngol. Clin. N. Am. 2009, 42, 123–131. [Google Scholar] [CrossRef]
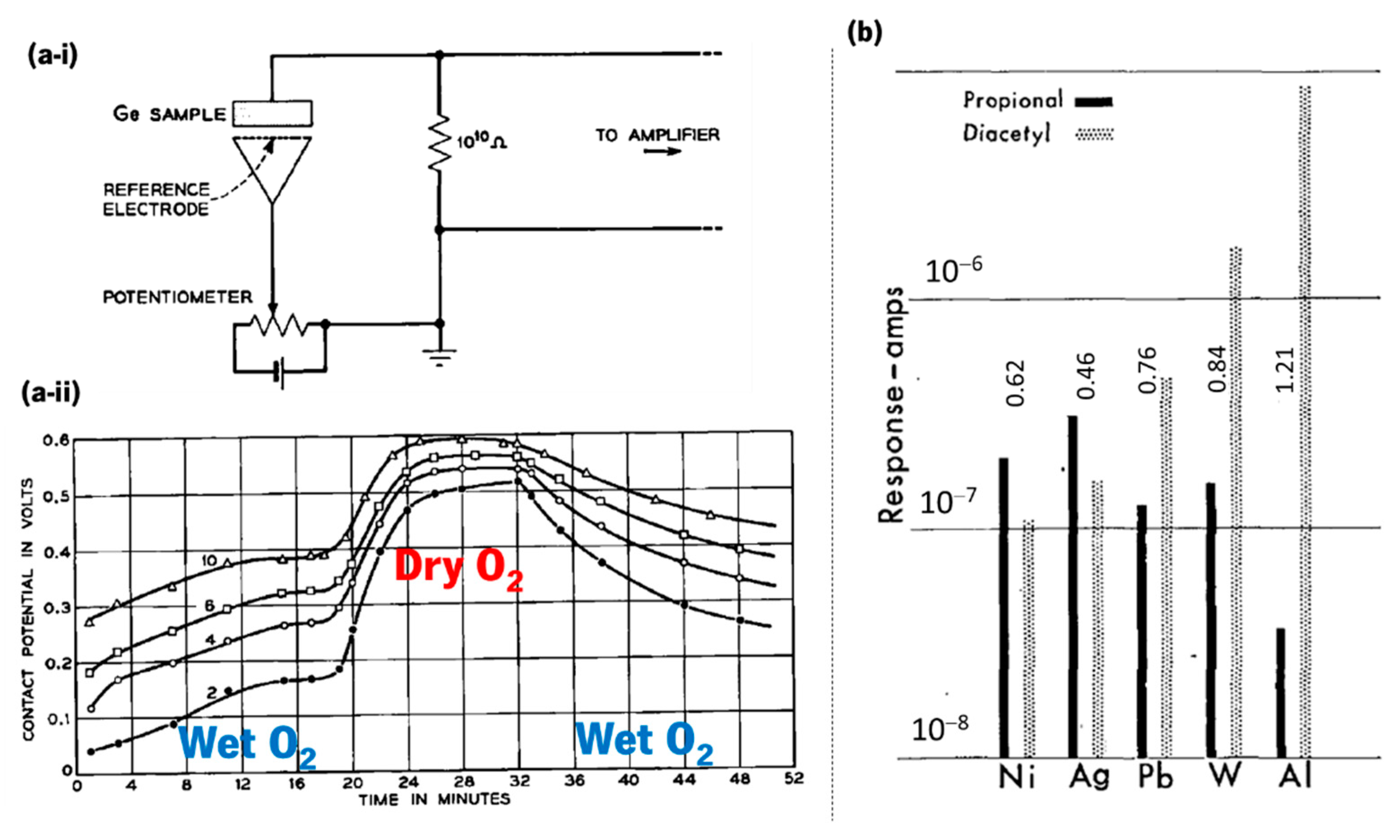
- Brattain, W.H.; Bardeen, J. Surface properties of germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Heiland, G. Zum Einfluß von adsorbiertem Sauerstoff auf die elektrische Leitfähigkeit von Zinkoxydkristallen. Z. Für Phys. 1954, 138, 459–464. [Google Scholar] [CrossRef]
- Wilkens, W.F.; Hartman, J.D. An Electronic Analog for the Olfactory Processesa. J. Food Sci. 1964, 29, 372–378. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Göpel, W. Solid-state chemical sensors: Atomistic models and research trends. Sens. Actuators 1989, 16, 167–193. [Google Scholar] [CrossRef]
- Xu, K.; Fu, C.; Gao, Z.; Wei, F.; Ying, Y.; Xu, C.; Fu, G. Nanomaterial-based gas sensors: A review. Instrum. Sci. Technol. 2018, 46, 115–145. [Google Scholar] [CrossRef]
- Schierbaum, K.D.; Vaihinger, S.; Göpel, W.; van den Vlekkert, H.H.; Kloeck, B.; de Rooij, N.F. Prototype structure for systematic investigations of thin-film gas sensors. Sens. Actuators B Chem. 1990, 1, 171–175. [Google Scholar] [CrossRef]
- Pinto, R.M.R.; Brito, P.; Chu, V.; Conde, J.P. Thin-Film Silicon MEMS for Dynamic Mass Sensing in Vacuum and Air: Phase Noise, Allan Deviation, Mass Sensitivity and Limits of Detection. J. Microelectromech. Syst. 2019, 28, 390–400. [Google Scholar] [CrossRef]
- Behzadi pour, G.; Fekri aval, L. Highly sensitive work function hydrogen gas sensor based on PdNPs/SiO2/Si structure at room temperature. Results Phys. 2017, 7, 1993–1999. [Google Scholar] [CrossRef]
- Soares, L.; Csáki, A.; Jatschka, J.; Fritzsche, W.; Flores, O.; Franco, R.; Pereira, E. Localized surface plasmon resonance (LSPR) biosensing using gold nanotriangles: Detection of DNA hybridization events at room temperature. Analyst 2014, 139, 4964–4973. [Google Scholar] [CrossRef]
- Bingham, J.M.; Anker, J.N.; Kreno, L.E.; Van Duyne, R.P. Gas Sensing with High-Resolution Localized Surface Plasmon Resonance Spectroscopy. J. Am. Chem. Soc. 2010, 132, 17358–17359. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Lin, H.-T.; Lai, M.-S.; Shieh, T.-Y.; Peng, C.-C.; Shih, M.-H.; Tung, Y.-C. Flexible Localized Surface Plasmon Resonance Sensor with Metal–Insulator–Metal Nanodisks on PDMS Substrate. Sci. Rep. 2018, 8, 11812. [Google Scholar] [CrossRef]
- Farooq, S.; Neves, W.W.; Pandoli, O.; Del Rosso, T.; de Lima, L.M.; Dutra, R.F.; Araujo, R.E. de Engineering a plasmonic sensing platform for Candida albicans antigen identification. J. Nanophotonics 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Koutsioubas, A.G.; Spiliopoulos, N.; Anastassopoulos, D.L.; Vradis, A.A.; Priftis, G.D. On the implementation of nano-structured materials in surface plasmon resonance sensors. Mater. Sci. Eng. B 2009, 165, 270–273. [Google Scholar] [CrossRef]
- Yang, Z.; Sassa, F.; Hayashi, K. A robot equipped with a high-speed LSPR gas sensor module for collecting spatial odor information from on-ground invisible odor sources. ACS Sens. 2018, 3, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Heo, N.S.; Bajpai, V.K.; Jang, S.-C.; Ok, G.; Cho, Y.; Huh, Y.S. Development of a Cuvette-Based LSPR Sensor Chip Using a Plasmonically Active Transparent Strip. Front. Bioeng. Biotechnol. 2019, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Castillero, P.; Roales, J.; Lopes-Costa, T.; Sánchez-Valencia, J.; Barranco, A.; González-Elipe, A.; Pedrosa, J. Optical Gas Sensing of Ammonia and Amines Based on Protonated Porphyrin/TiO2 Composite Thin Films. Sensors 2016, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, P.; Costa-Barbosa, A.; Costa, D.; Rodrigues, M.S.; Carvalho, P.; Borges, J.; Vaz, F.; Sampaio, P. Antifungal activity of ZnO thin films prepared by glancing angle deposition. Thin Solid Film. 2019, 687, 137461. [Google Scholar] [CrossRef]
- Proença, M.; Rodrigues, M.S.; Borges, J.; Vaz, F. Gas Sensing with Nanoplasmonic Thin Films Composed of Nanoparticles (Au, Ag) Dispersed in a CuO Matrix. Coatings 2019, 9, 337. [Google Scholar] [CrossRef]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Ahmad, R.B.; Kamaruddin, R. Toward Optical Sensors: Review and Applications. J. Phys. Conf. Ser. 2013, 423, 12064. [Google Scholar] [CrossRef]
- Ng, S.M. 12—Carbon Dots as Optical Nanoprobes for Biosensors. In Micro and Nano Technologies; Gopinath, S.C.B., Lakshmipriya, T.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–300. ISBN 978-0-12-813900-4. [Google Scholar]
- Chemical Sensors Market Forecast, Trend Analysis & Competition Tracking—Global Market Insights 2018 to 2028; Fact.MR: Dublin, Ireland, 2020.
- Chemical Sensors Market: Global Demand Analysis & Opportunity Outlook 2024; Research Nester: New York, NY, USA, 2020.
- Gas Sensors Market by Gas Type (Oxygen, Carbon Monoxide, Carbon Dioxide, Ammonia, Chlorine, Hydrogen Sulfide, Nitrogen Oxide, Volatile Organic Compounds, Hydrocarbons), Technology, Application, Geography—Global Forecast 2024; MarketsandMarkets: Northbrook, IL, USA, 2021.
- Olson, K.R. Carbon monoxide poisoning: Mechanisms, presentation, and controversies in management. J. Emerg. Med. 1984, 1, 233–243. [Google Scholar] [CrossRef]
- Rose, J.J.; Wang, L.; Xu, Q.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am. J. Respir. Crit. Care Med. 2016, 195, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.L. Chapter 38—Hypoxemia; Zaoutis, L.B., Ed.; Mosby: Philadelphia, PA, USA, 2007; pp. 181–189. ISBN 978-0-323-03004-5. [Google Scholar]
- Eckhoff, R.K. Explosion Hazards in the Process Industries, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128032732. [Google Scholar]
- Stockman, M.I. Nanoplasmonics: The physics behind the applications. Phys. Today 2011, 64, 39–44. [Google Scholar] [CrossRef]
- Barber, D.J.; Freestone, I.C. An investigation of the origin of the colour of the lycurgus cup by analytical transmission electron microscopy. Archaeometry 1990, 32, 33–45. [Google Scholar] [CrossRef]
- Dekker, F.; Kool, L.; Bunschoten, A.; Velders, A.H.; Saggiomo, V. Syntheses of gold and silver dichroic nanoparticles; looking at the Lycurgus cup colors. Chem. Teach. Int. 2019. [Google Scholar] [CrossRef]
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus Cup—A Roman nanotechnology. Gold Bull. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Kool, L.; Bunschoten, A.; Velders, A.H.; Saggiomo, V. Gold nanoparticles embedded in a polymer as a 3D-printable dichroic nanocomposite material. Beilstein J. Nanotechnol. 2019, 10, 442–447. [Google Scholar] [CrossRef]
- Borges, J.; Rodrigues, M.S.; Kubart, T.; Kumar, S.; Leifer, K.; Evaristo, M.; Cavaleiro, A.; Apreutesei, M.; Pereira, R.M.S.; Vasilevskiy, M.I.; et al. Thin films composed of gold nanoparticles dispersed in a dielectric matrix: The influence of the host matrix on the optical and mechanical responses. Thin Solid Film. 2015, 596, 8–17. [Google Scholar] [CrossRef]
- Gonçalves, M.R. Plasmonic nanoparticles: Fabrication, simulation and experiments. J. Phys. D Appl. Phys. 2014, 47, 213001. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley Online Books; Wiley: New York, NY, USA, 1998; ISBN 9780471293408. [Google Scholar]
- Landau, L.D.; Pitaevskii, L.P.; Lifshitz, E.M. Electrodynamics of Continuous Media, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1984; ISBN 9780080570600. [Google Scholar]
- Pettine, J.; Choo, P.; Medeghini, F.; Odom, T.W.; Nesbitt, D.J. Plasmonic nanostar photocathodes for optically-controlled directional currents. Nat. Commun. 2020, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Pitarke, J.M.; Silkin, V.M.; Chulkov, E.V.; Echenique, P.M. Theory of surface plasmons and surface-plasmon polaritons. Rep. Prog. Phys. 2007, 70, 1–87. [Google Scholar] [CrossRef]
- Bludov, Y.U.V.; Ferreira, A.; Peres, N.M.R.; Vasilevskiy, M.I. A primer on surface plasmon-polaritons in graphene. Int. J. Mod. Phys. B 2013, 27, 1341001. [Google Scholar] [CrossRef]
- McQuillan, A.J. The discovery of surface-enhanced Raman scattering. Notes Rec. R. Soc. 2009, 63, 105–109. [Google Scholar] [CrossRef]
- Pereira, R.M.S.; Borges, J.; Peres, F.C.R.; Pereira, P.A.S.; Smirnov, G.V.; Vaz, F.; Cavaleiro, A.; Vasilevskiy, M.I. Effect of clustering on the surface plasmon band in thin films of metallic nanoparticles. J. Nanophotonics 2015, 9, 093796. [Google Scholar] [CrossRef]
- Toudert, J.; Simonot, L.; Camelio, S.; Babonneau, D. Advanced optical effective medium modeling for a single layer of polydisperse ellipsoidal nanoparticles embedded in a homogeneous dielectric medium: Surface plasmon resonances. Phys. Rev. B 2012, 86, 45415. [Google Scholar] [CrossRef]
- Scholl, J.A.; Koh, A.L.; Dionne, J.A. Quantum plasmon resonances of individual metallic nanoparticles. Nature 2012, 483, 421–427. [Google Scholar] [CrossRef]
- Stockman, M.I.; Kneipp, K.; Bozhevolnyi, S.I.; Saha, S.; Dutta, A.; Ndukaife, J.; Kinsey, N.; Reddy, H.; Guler, U.; Shalaev, V.M.; et al. Roadmap on plasmonics. J. Opt. 2018, 20, 043001. [Google Scholar] [CrossRef]
- Giannini, V.; Fernández-Domínguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic Nanoantennas: Fundamentals and Their Use in Controlling the Radiative Properties of Nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007; ISBN 978-0-387-37825-1. [Google Scholar]
- Kahl, P.; Podbiel, D.; Schneider, C.; Makris, A.; Sindermann, S.; Witt, C.; Kilbane, D.; Hoegen, M.H.; Aeschlimann, M.; zu Heringdorf, F.M. Direct Observation of Surface Plasmon Polariton Propagation and Interference by Time-Resolved Imaging in Normal-Incidence Two Photon Photoemission Microscopy. Plasmonics 2018, 13, 239–246. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Kedem, O.; Vaskevich, A.; Rubinstein, I. Critical Issues in Localized Plasmon Sensing. J. Phys. Chem. C 2014, 118, 8227–8244. [Google Scholar] [CrossRef]
- Li, D.-B.; Sun, X.-J.; Jia, Y.-P.; Stockman, M.I.; Paudel, H.P.; Song, H.; Jiang, H.; Li, Z.-M. Direct observation of localized surface plasmon field enhancement by Kelvin probe force microscopy. Light Sci. Appl. 2017, 6, e17038. [Google Scholar] [CrossRef] [PubMed]
- Svedendahl, M.; Chen, S.; Käll, M. An Introduction to Plasmonic Refractive Index Sensing BT—Nanoplasmonic Sensors; Dmitriev, A., Ed.; Springer: New York, NY, USA, 2012; pp. 1–26. ISBN 978-1-4614-3933-2. [Google Scholar]
- Vasilevskiy, M.I. Effective Dielectric Response of Composites Containing Uniaxial Inclusions. Phys. Status Solidi 2000, 219, 197–204. [Google Scholar] [CrossRef]
- Baida, H.; Billaud, P.; Marhaba, S.; Christofilos, D.; Cottancin, E.; Crut, A.; Lermé, J.; Maioli, P.; Pellarin, M.; Broyer, M.; et al. Quantitative Determination of the Size Dependence of Surface Plasmon Resonance Damping in Single Ag@SiO2 Nanoparticles. Nano Lett. 2009, 9, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.; Yang, J.K.W.; Bozhevolnyi, S.I.; Link, S.; Nordlander, P.; Halas, N.J.; Mortensen, N.A. Plasmonic colour generation. Nat. Rev. Mater. 2016, 2, 16088. [Google Scholar] [CrossRef]
- Adnan, N.N.M.; Cheng, Y.Y.; Ong, N.M.N.; Kamaruddin, T.T.; Rozlan, E.; Schmidt, T.W.; Duong, H.T.T.; Boyer, C. Effect of gold nanoparticle shapes for phototherapy and drug delivery. Polym. Chem. 2016, 7, 2888–2903. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Lin, S.; Liu, S.; Liu, Y. Tunable fluorescence lifetime of Eu-PMMA films with plasmonic nanostructures for multiplexing. Opt. Express 2016, 24, 8228–8236. [Google Scholar] [CrossRef]
- Song, M.; Wang, D.; Peana, S.; Choudhury, S.; Nyga, P.; Kudyshev, Z.A.; Yu, H.; Boltasseva, A.; Shalaev, V.M.; Kildishev, A. V Colors with plasmonic nanostructures: A full-spectrum review. Appl. Phys. Rev. 2019, 6, 41308. [Google Scholar] [CrossRef]
- Notarianni, M.; Vernon, K.; Chou, A.; Aljada, M.; Liu, J.; Motta, N. Plasmonic effect of gold nanoparticles in organic solar cells. Sol. Energy 2014, 106, 23–37. [Google Scholar] [CrossRef]
- Pluchery, O.; Remita, H.; Schaming, D. Demonstrative experiments about gold nanoparticles and nanofilms: An introduction to nanoscience. Gold Bull. 2013, 46, 319–327. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
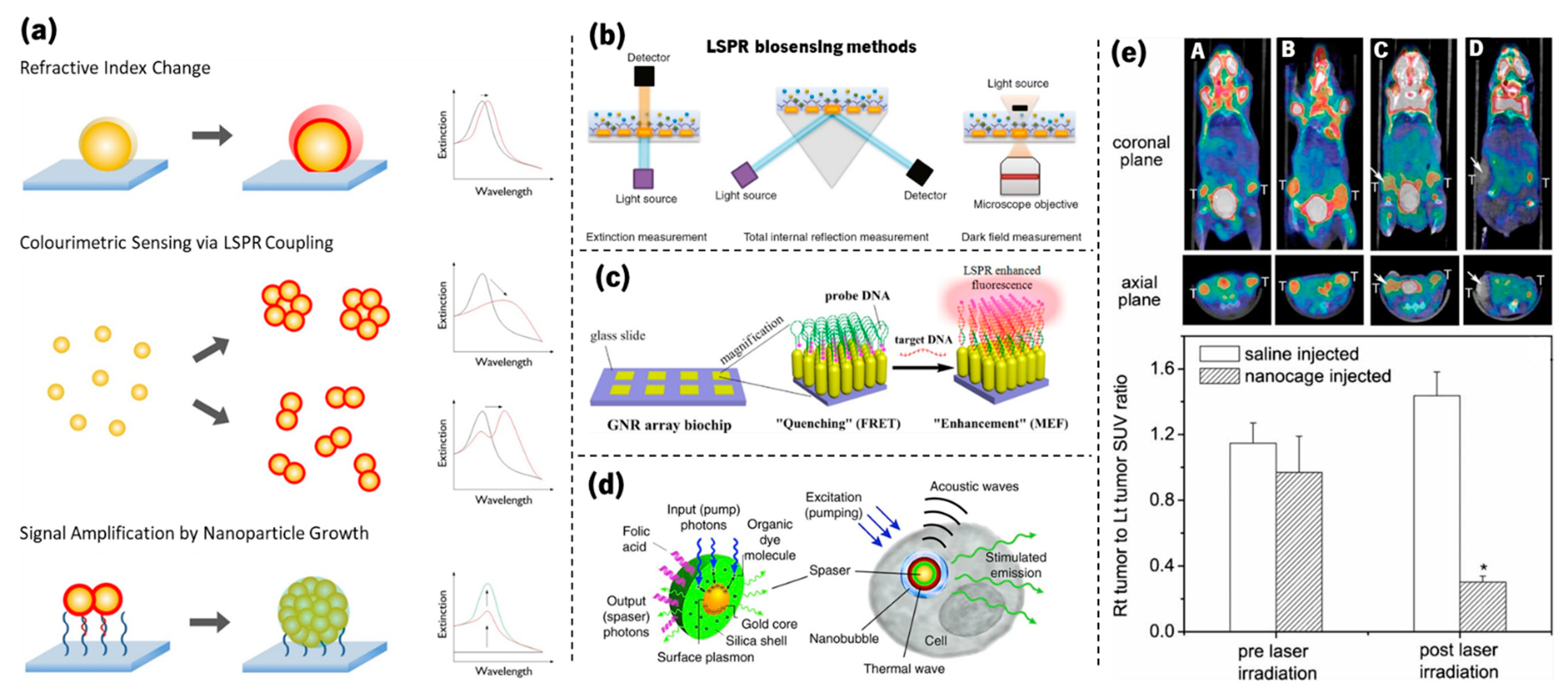
- Guo, L.; Jackman, J.A.; Yang, H.H.; Chen, P.; Cho, N.J.; Kim, D.H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Kazuma, E.; Tatsuma, T. Localized surface plasmon resonance sensors based on wavelength-tunable spectral dips. Nanoscale 2014, 6, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Sannomiya, T.; Vörös, J. Single plasmonic nanoparticles for biosensing. Trends Biotechnol. 2011, 29, 343–351. [Google Scholar] [CrossRef]
- Piliarik, M.; Šípová, H.; Kvasnička, P.; Galler, N.; Krenn, J.R.; Homola, J. High-resolution biosensor based on localized surface plasmons. Opt. Express 2012, 20, 672. [Google Scholar] [CrossRef]
- Soler, M.; Huertas, C.S.; Lechuga, L.M. Label-free plasmonic biosensors for point-of-care diagnostics: A review. Expert Rev. Mol. Diagn. 2019, 19, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Cappi, G.; Spiga, F.M.; Moncada, Y.; Ferretti, A.; Beyeler, M.; Bianchessi, M.; Decosterd, L.; Buclin, T.; Guiducci, C. Label-Free Detection of Tobramycin in Serum by Transmission-Localized Surface Plasmon Resonance. Anal. Chem. 2015, 87, 5278–5285. [Google Scholar] [CrossRef]
- Lopez, G.A.; Estevez, M.C.; Soler, M.; Lechuga, L.M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonics 2017, 6, 123–136. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Pereira, R.M.S.; Vasilevskiy, M.I.; Borges, J.; Vaz, F. NANOPTICS: In-depth analysis of NANomaterials for OPTICal localized surface plasmon resonance Sensing. SoftwareX 2020, 12, 100522. [Google Scholar] [CrossRef]
- Mei, Z.; Tang, L. Surface-Plasmon-Coupled Fluorescence Enhancement Based on Ordered Gold Nanorod Array Biochip for Ultrasensitive DNA Analysis. Anal. Chem. 2017, 89, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Li, C.-Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.K.; Radhakrishnan, T.P. Tuning the SERS Response with Ag-Au Nanoparticle-Embedded Polymer Thin Film Substrates. Acs Appl. Mater. Interfaces 2015, 7, 12767–12773. [Google Scholar] [CrossRef]
- Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Liz-Marzán, L.M.; García de Abajo, F.J. Optimization of Nanoparticle-Based SERS Substrates through Large-Scale Realistic Simulations. ACS Photonics 2017, 4, 329–337. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Jeong, K.-H. Optofluidic SERS chip with plasmonic nanoprobes self-aligned along microfluidic channels. Lab Chip 2014, 14, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, M.; Liu, W.; Liu, Y.; Li, Z.; Wang, X.; Yang, F. Absorption enhancement in thin film solar cells with bilayer silver nanoparticle arrays. J. Phys. Commun. 2018, 2, 055032. [Google Scholar] [CrossRef]
- Liu, W.-L.; Lin, F.-C.; Yang, Y.-C.; Huang, C.-H.; Gwo, S.; Huang, M.H.; Huang, J.-S. The influence of shell thickness of Au@TiO2 core–shell nanoparticles on the plasmonic enhancement effect in dye-sensitized solar cells. Nanoscale 2013, 5, 7953. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Che, X.; Qin, G. Large Absorption Enhancement in Ultrathin Solar Cells Patterned by Metallic Nanocavity Arrays. Sci. Rep. 2016, 6, 34219. [Google Scholar] [CrossRef]
- Kim, I.; Seok Jeong, D.; Seong Lee, T.; Seong Lee, W.; Lee, K.-S. Plasmonic absorption enhancement in organic solar cells by nano disks in a buffer layer. J. Appl. Phys. 2012, 111, 103121. [Google Scholar] [CrossRef]
- Peng, Y.; Xiong, B.; Peng, L.; Li, H.; He, Y.; Yeung, E.S. Recent Advances in Optical Imaging with Anisotropic Plasmonic Nanoparticles. Anal. Chem. 2015, 87, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Lee, B.-R.; Lee, H.; Jo, S.D.; Kim, H.; Won, Y.-Y.; Lee, J. Near-Infrared Plasmonic Assemblies of Gold Nanoparticles with Multimodal Function for Targeted Cancer Theragnosis. Sci. Rep. 2017, 7, 17327. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Weingold, R.; Nedosekin, D.A.; Sarimollaoglu, M.; Nolan, J.; Harrington, W.; Kuchyanov, A.S.; Parkhomenko, R.G.; Watanabe, F.; Nima, Z.; et al. Spaser as a biological probe. Nat. Commun. 2017, 8, 15528. [Google Scholar] [CrossRef]
- STOCKMAN, M.I. Spaser, Plasmonic Amplification, and Loss Compensation. Act. Plasmon. Tuneable Plasmonic Metamater. 2013, 1–39. [Google Scholar] [CrossRef]
- Mendes, R.; Pedrosa, P.; Lima, J.C.; Fernandes, A.R.; Baptista, P.V. Photothermal enhancement of chemotherapy in breast cancer by visible irradiation of Gold Nanoparticles. Sci. Rep. 2017, 7, 10872. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold Nanocages as Photothermal Transducers for Cancer Treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Cetin, S.S.; Corekci, S.; Cakmak, M.; Ozcelik, S. Structural investigation and electronic band transitions of nanostructured TiO2 thin films. Cryst. Res. Technol. 2011, 46, 1207–1214. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Prasai, B.; Cai, B.; Underwood, M.K.; Lewis, J.P.; Drabold, D.A. Properties of amorphous and crystalline titanium dioxide from first principles. J. Mater. Sci. 2012, 47, 7515–7521. [Google Scholar] [CrossRef]
- Rojviroon, T.; Sirivithayapakorn, S. Properties of TiO2 thin films prepared using sol–gel process. Surf. Eng. 2013, 29, 77–80. [Google Scholar] [CrossRef]
- Kang, S.H.; Kang, M.S.; Kim, H.S.; Kim, J.Y.; Chung, Y.H.; Smyri, W.H.; Sung, Y.E. Columnar rutile TiO2 based dye-sensitized solar cells by radio-frequency magnetron sputtering. J. Power Sources 2008, 184, 331–335. [Google Scholar] [CrossRef]
- Song, D.H.; Uhm, S.H.; Lee, S.B.; Han, J.G.; Kim, K.N. Antimicrobial silver-containing titanium oxide nanocomposite coatings by a reactive magnetron sputtering. Thin Solid Film. 2011, 519, 7079–7085. [Google Scholar] [CrossRef]
- Shibata, T.; Irie, H.; Hashimoto, K. Photoinduced hardness change on TiO2 single crystal surfaces. Chem. Commun. 2009, 3735–3737. [Google Scholar] [CrossRef]
- Torrell, M.; Cunha, L.; Kabir, M.R.; Cavaleiro, A.; Vasilevskiy, M.I.; Vaz, F. Nanoscale color control of TiO2 films with embedded Au nanoparticles. Mater. Lett. 2010, 64, 2624–2626. [Google Scholar] [CrossRef]
- Eufinger, K.; Janssen, E.N.; Poelman, H.; Poelman, D.; De Gryse, R.; Marin, G.B. The effect of argon pressure on the structural and photocatalytic characteristics of TiO2 thin films deposited by d.c. magnetron sputtering. Thin Solid Film. 2006, 515, 425–429. [Google Scholar] [CrossRef]
- Gonzalez-García, L.; Parra-Barranco, J.; Sanchez-Valencia, J.R.; Ferrer, J.; Garcia-Gutierrez, M.-C.; Barranco, A.; Gonzalez-Elipe, A.R. Tuning Dichroic Plasmon Resonance Modes of Gold Nanoparticles in Optical Thin Films. Adv. Funct. Mater. 2013, 23, 1655–1663. [Google Scholar] [CrossRef]
- Lee, H.; Park, Y.K.; Kim, S.J.; Park, J.H.; Ki, S.J.; Jung, S.C. TiO2 photocatalyst film using circulating fluidised bed–chemical vapour deposition. Surf. Eng. 2015, 31, 134–139. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhao, H.; Li, D.; Zheng, M.; Du, P.; Zhang, G.; Qu, D.; Sun, Z.; Fan, H. Orientated anatase TiO2 nanocrystal array thin films for self-cleaning coating. Chem. Commun. 2013, 49, 8958–8960. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.J. Effect of substrates on the photocatalytic activity of nanometer TiO2 thin films. Mater. Res. Bull. 2000, 35, 1293–1301. [Google Scholar] [CrossRef]
- Liu, B.S.; Zhao, X.J.; Zhao, Q.N.; Li, C.L.; He, X. The effect of O-2 partial pressure on the structure and photocatalytic property of TiO2 films prepared by sputtering. Mater. Chem. Phys. 2005, 90, 207–212. [Google Scholar] [CrossRef]
- Raza, M.A.; Habib, A.; Kanwal, Z.; Hussain, S.S.; Iqbal, M.J.; Saleem, M.; Riaz, S.; Naseem, S. Optical CO2 Gas Sensing Based on TiO2 Thin Films of Diverse Thickness Decorated with Silver Nanoparticles. Adv. Mater. Sci. Eng. 2018, 2018, 2780203. [Google Scholar] [CrossRef]
- Krško, O.; Plecenik, T.; Roch, T.; Grančič, B.; Satrapinskyy, L.; Truchlý, M.; Ďurina, P.; Gregor, M.; Kúš, P.; Plecenik, A. Flexible highly sensitive hydrogen gas sensor based on a TiO2 thin film on polyimide foil. Sens. Actuators B Chem. 2017, 240, 1058–1065. [Google Scholar] [CrossRef]
- Ali Haidry, A.; Sun, L.; Saruhan, B.; Plecenik, A.; Plecenik, T.; Shen, H.; Yao, Z. Cost-effective fabrication of polycrystalline TiO2 with tunable n/p response for selective hydrogen monitoring. Sens. Actuators B Chem. 2018, 274, 10–21. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Radecka, M. TiO2-Based Nanomaterials for Gas Sensing—Influence of Anatase and Rutile Contributions. Nanoscale Res. Lett. 2017, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Comert, B.; Akin, N.; Donmez, M.; Saglam, S.; Ozcelik, S. Titanium Dioxide Thin Films as Methane Gas Sensors. IEEE Sens. J. 2016, 16, 8890–8896. [Google Scholar] [CrossRef]
- Plecenik, T.; Moško, M.; Haidry, A.A.; Ďurina, P.; Truchlý, M.; Grančič, B.; Gregor, M.; Roch, T.; Satrapinskyy, L.; Mošková, A.; et al. Fast highly-sensitive room-temperature semiconductor gas sensor based on the nanoscale Pt–TiO2–Pt sandwich. Sens. Actuators B Chem. 2015, 207, 351–361. [Google Scholar] [CrossRef]
- Presmanes, L.; Thimont, Y.; el Younsi, I.; Chapelle, A.; Blanc, F.; Talhi, C.; Bonningue, C.; Barnabé, A.; Menini, P.; Tailhades, P. Integration of P-CuO Thin Sputtered Layers onto Microsensor Platforms for Gas Sensing. Sensors 2017, 17, 1409. [Google Scholar] [CrossRef] [PubMed]
- Edelmannová, M.; Lin, K.-Y.; Wu, J.C.S.; Troppová, I.; Čapek, L.; Kočí, K. Photocatalytic hydrogenation and reduction of CO2 over CuO/ TiO2 photocatalysts. Appl. Surf. Sci. 2018, 454, 313–318. [Google Scholar] [CrossRef]
- Mugwang’a, F.K.; Karimi, P.K.; Njoroge, W.K.; Omayio, O.; Waita, S.M. Optical characterization of Copper Oxide thin films prepared by reactive dc magnetron sputtering for solar cell applications. Int. J. Thin Film. Sci. Technol. 2013, 2, 12–24. [Google Scholar]
- Lim, Y.-F.; Chua, C.S.; Lee, C.J.J.; Chi, D. Sol-gel deposited Cu2O and CuO thin films for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef]
- Hossain, S.T.; Almesned, Y.; Zhang, K.; Zell, E.T.; Bernard, D.T.; Balaz, S.; Wang, R. Support structure effect on CO oxidation: A comparative study on SiO2 nanospheres and CeO2 nanorods supported CuOx catalysts. Appl. Surf. Sci. 2018, 428, 598–608. [Google Scholar] [CrossRef]
- Shen, J.; Rao, C.; Fu, Z.; Feng, X.; Liu, J.; Fan, X.; Peng, H.; Xu, X.; Tan, C.; Wang, X. The influence on the structural and redox property of CuO by using different precursors and precipitants for catalytic soot combustion. Appl. Surf. Sci. 2018, 453, 204–213. [Google Scholar] [CrossRef]
- Wanjala, K.S.; Njoroge, W.K.; Makori, N.E.; Ngaruiya, J.M. Optical and Electrical Characterization of CuO Thin Films as Absorber Material for Solar Cell Applications. Am. J. Condens. Matter Phys. 2016, 6, 1–6. [Google Scholar]
- Wongratanaphisan, D.; Kaewyai, K.; Choopun, S.; Gardchareon, A.; Ruankham, P.; Phadungdhitidhada, S. CuO-Cu2O nanocomposite layer for light-harvesting enhancement in ZnO dye-sensitized solar cells. Appl. Surf. Sci. 2019, 474, 85–90. [Google Scholar] [CrossRef]
- Daoudi, O.; Qachaou, Y.; Raidou, A.; Nouneh, K.; Lharch, M.; Fahoume, M. Study of the physical properties of CuO thin films grown by modified SILAR method for solar cells applications. Superlattices Microstruct. 2019, 127, 93–99. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, M.; Xia, X.; Shao, G. Role of materials chemistry on the electrical/electronic properties of CuO thin films. Acta Mater. 2015, 85, 122–131. [Google Scholar] [CrossRef]
- Sultana, J.; Paul, S.; Karmakar, A.; Yi, R.; Dalapati, G.K.; Chattopadhyay, S. Chemical bath deposited (CBD) CuO thin films on n-silicon substrate for electronic and optical applications: Impact of growth time. Appl. Surf. Sci. 2017, 418, 380–387. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Selective Detection of CO at Room Temperature with CuO Nanoplatelets Sensor for Indoor Air Quality Monitoring Manifested by Crystallinity. Appl. Surf. Sci. 2019, 466, 545–553. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, C.; Li, L.; Du, C.; Li, X.; Kang, X.-F.; Chen, W. CO gas sensors based on p-type CuO nanotubes and CuO nanocubes: Morphology and surface structure effects on the sensing performance. Talanta 2018, 188, 41–49. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Ahmed, R.E.; Al-Rashid, M.A.; Alarrouqi, R.A.; Saleh, B.; Abdulrehman, T.; Haik, Y.; Al-Sulaiti, L.A. Selective gas sensors using graphene and CuO nanorods. Sens. Actuators A Phys. 2018, 283, 107–112. [Google Scholar] [CrossRef]
- Sangwaranatee, N.; Chananonnawathorn, C.; Horprathum, M. Deposition of CuO thin film prepared by DC reactive magnetron sputtering. Mater. Today Proc. 2018, 5, 13896–13899. [Google Scholar] [CrossRef]
- Bai, X.; Chen, W.; Song, Y.; Zhang, J.; Ge, R.; Wei, W.; Jiao, Z.; Sun, Y. Nickel-copper oxide nanowires for highly sensitive sensing of glucose. Appl. Surf. Sci. 2017, 420, 927–934. [Google Scholar] [CrossRef]
- Rydosz, A.; Szkudlarek, A. Gas-sensing performance of M-doped CuO-based thin films working at different temperatures upon exposure to propane. Sensors 2015, 15, 20069–20085. [Google Scholar] [CrossRef]
- Batra, N.; Tomar, M.; Gupta, V. ZnO–CuO composite matrix based reagentless biosensor for detection of total cholesterol. Biosens. Bioelectron. 2015, 67, 263–271. [Google Scholar] [CrossRef]
- Pulker, H.K. Film Deposition Methods BT—Optical Interference Coatings; Kaiser, N., Pulker, H.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 131–153. ISBN 978-3-540-36386-6. [Google Scholar]
- Ashik, U.P.M.; Kudo, S.; Hayashi, J. Chapter 2—An Overview of Metal Oxide Nanostructures. In Micro and Nano Technologies; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S.B.T.-S., Eds.; Woodhead Publishing: Cambridg, UK, 2018; pp. 19–57. ISBN 978-0-08-101975-7. [Google Scholar]
- Aegerter, M.A.; Puetz, J.; Gasparro, G.; Al-Dahoudi, N. Versatile wet deposition techniques for functional oxide coatings. Opt. Mater. (Amst). 2004, 26, 155–162. [Google Scholar] [CrossRef]
- Jilani, A.; Abdel-wahab, M.S.; Hammad, A.H. Advance Deposition Techniques for Thin Film and Coating. In Modern Technologies for Creating the Thin-Film Systems and Coatings; InTech: London, UK, 2017. [Google Scholar]
- Pawar, S.M.; Pawar, B.S.; Kim, J.H.; Joo, O.-S.; Lokhande, C.D. Recent status of chemical bath deposited metal chalcogenide and metal oxide thin films. Curr. Appl. Phys. 2011, 11, 117–161. [Google Scholar] [CrossRef]
- Gorup, L.F.; Amorin, L.H.; Camargo, E.R.; Sequinel, T.; Cincotto, F.H.; Biasotto, G.; Ramesar, N.; de La Porta, F.A. Chapter 2—Methods for design and fabrication of nanosensors: The case of ZnO-based nanosensor. In Micro and Nano Technologies; Han, B., Tomer, V.K., Nguyen, T.A., Farmani, A., Kumar Singh, P.B.T.-N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 9–30. ISBN 978-0-12-819870-4. [Google Scholar]
- Cho, J.; Hwang, S.; Ko, D.-H.; Chung, S. Transparent ZnO Thin-Film Deposition by Spray Pyrolysis for High-Performance Metal-Oxide Field-Effect Transistors. Materials 2019, 12, 3423. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Pan, C.T.; Chang, S.J.; Lin, S.C. Wet Deposition Process for Thin-Film Transistors. Mater. Manuf. Process. 2014, 29, 498–503. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Walsh, F.C.; Ponce de Leon, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Tseluikin, V.N. On the Structure and Properties of Composite Electrochemical Coatings. A Review. Prot. Met. Phys. Chem. Surf. 2016, 52, 254–266. [Google Scholar] [CrossRef]
- Seshan, K. Handbook of Thin Film Deposition Processes and Techniques, 2nd ed.; Seshan, K., Ed.; William Andrew Publishing: Norwich, NY, USA, 2001; ISBN 978-0-8155-1442-8. [Google Scholar]
- Jameel, D.A. Thin Film Deposition Processes. Int. J. Mod. Phys. Appl. 2015, 1, 193–199. [Google Scholar]
- Vaz, J.F.V. Preparação e Caracterização de Filmes Finos de Ti(1-x)Si(x)N(y), Crescidos por Pulverização Catódica Reactiva em Magnetrão; Universidade do Minho: Braga, Portugal, 2000. [Google Scholar]
- de Carvalho, P.M.C.B.F. Development of New Decorative Coatings Based on Zirconium Oxynitrides; Universidade do Minho: Braga, Portugal, 2008. [Google Scholar]
- Borges, J.N.P. Otimização do Processo de Deposição de Oxinitretos Metálicos por Pulverização Reativa Magnetrão; Universidade do Minho: Braga, Portugal, 2012. [Google Scholar]
- Shimpi, K.C.; Ravindranath, K.; Jani, A.K.; Kothari, D.C.; Harindranath, C.S. Decorative coatings produced using combination of reactive arc evaporation and magnetron sputtering. Surf. Coat. Technol. 1997, 90, 115–122. [Google Scholar] [CrossRef]
- Qiu, Y.; Xian, Y.; Lu, X.; Ostrikov, K. Effect of background ionization on plasma ignition dynamics. Phys. Plasmas 2017, 24, 33503. [Google Scholar] [CrossRef]
- Depla, D.; Mahieu, S. (Eds.) Reactive Sputter Deposition; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 109, ISBN 978-3-540-76662-9. [Google Scholar]
- Langmuir, I. Oscillations in Ionized Gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef]
- Bräuer, G.; Szyszka, B.; Vergöhl, M.; Bandorf, R. Magnetron sputtering—Milestones of 30 years. Vacuum 2010, 84, 1354–1359. [Google Scholar] [CrossRef]
- Rodrigues, M.P.S.M. Filmes Finos de Nanopartículas de Ouro Dispersas Numa Matriz Dielétrica de TiO2 Com Efeito de Ressonância de Plasmão de Superfície Localizado; Universidade do Minho: Braga, Portugal, 2016. [Google Scholar]
- Iseki, T. Completely flat erosion magnetron sputtering using a rotating asymmetrical yoke magnet. Vacuum 2010, 84, 1372–1376. [Google Scholar] [CrossRef]
- Nadel, S.J.; Greene, P.; Rietzel, J.; Perata, M.; Malaszewski, L.; Hill, R. Advanced generation of rotatable magnetron technology for high performance reactive sputtering. Thin Solid Film. 2006, 502, 15–21. [Google Scholar] [CrossRef]
- Borges, J.; Vaz, F.; Marques, L. AlNxOy thin films deposited by DC reactive magnetron sputtering. Appl. Surf. Sci. 2010, 257, 1478–1483. [Google Scholar] [CrossRef]
- Domingues, R.P.; Rodrigues, M.S.; Lopes, C.; Pedrosa, P.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin films composed of metal nanoparticles (Au, Ag, Cu) dispersed in AlN: The influence of composition and thermal annealing on the structure and plasmonic response. Thin Solid Film. 2019, 676, 12–25. [Google Scholar] [CrossRef]
- Borges, J.; Pereira, R.M.S.; Rodrigues, M.S.; Kubart, T.; Kumar, S.; Leifer, K.; Cavaleiro, A.; Polcar, T.; Vasilevskiy, M.I.; Vaz, F. Broadband Optical Absorption Caused by the Plasmonic Response of Coalesced Au Nanoparticles Embedded in a TiO2 Matrix. J. Phys. Chem. C 2016, 120, 16931–16945. [Google Scholar] [CrossRef]
- Heilmann, A. Polymer Films with Embedded Metal Nanoparticles, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-642-07729-6. [Google Scholar]
- Koneti, S.; Borges, J.; Roiban, L.; Rodrigues, M.S.; Martin, N.; Epicier, T.; Vaz, F.; Steyer, P. Electron Tomography of Plasmonic Au Nanoparticles Dispersed in a TiO2 Dielectric Matrix. Acs Appl. Mater. Interfaces 2018, 10, 42882–42890. [Google Scholar] [CrossRef] [PubMed]
- Torrell, M.; Machado, P.; Cunha, L.; Figueiredo, N.M.; Oliveira, J.C.; Louro, C.; Vaz, F. Development of new decorative coatings based on gold nanoparticles dispersed in an amorphous TiO2 dielectric matrix. Surf. Coat. Technol. 2010, 204, 1569–1575. [Google Scholar] [CrossRef]
- Borges, J.; Kubart, T.; Kumar, S.; Leifer, K.; Rodrigues, M.S.; Duarte, N.; Martins, B.; Dias, J.P.; Cavaleiro, A.; Vaz, F. Microstructural evolution of Au/TiO2 nanocomposite films: The influence of Au concentration and thermal annealing. Thin Solid Film. 2015, 580, 77–88. [Google Scholar] [CrossRef]
- Borges, J.; Ferreira, C.G.; Fernandes, J.P.C.; Rodrigues, M.S.; Proença, M.; Apreutesei, M.; Alves, E.; Barradas, N.P.; Moura, C.; Vaz, F. Thin films of Ag–Au nanoparticles dispersed in TiO2: Influence of composition and microstructure on the LSPR and SERS responses. J. Phys. D Appl. Phys. 2018, 51, 205102. [Google Scholar] [CrossRef]
- Borges, J.; Rodrigues, M.S.; Lopes, C.; Costa, D.; Couto, F.M.; Kubart, T.; Martins, B.; Duarte, N.; Dias, J.P.; Cavaleiro, A.; et al. Thin films composed of Ag nanoclusters dispersed in TiO2: Influence of composition and thermal annealing on the microstructure and physical responses. Appl. Surf. Sci. 2015, 358, 595–604. [Google Scholar] [CrossRef]
- Meira, D.I.; Domingues, R.P.; Rodrigues, M.S.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin films of Au-Al2O3 for plasmonic sensing. Appl. Surf. Sci. 2020, 500, 144035. [Google Scholar] [CrossRef]
- Proença, M.; Borges, J.; Rodrigues, M.S.; Domingues, R.P.; Dias, J.P.; Trigueiro, J.; Bundaleski, N.; Teodoro, O.M.N.D.; Vaz, F. Development of Au/CuO nanoplasmonic thin films for sensing applications. Surf. Coat. Technol. 2018, 343, 178–185. [Google Scholar] [CrossRef]
- Proença, M.; Borges, J.; Rodrigues, M.S.; Meira, D.I.; Sampaio, P.; Dias, J.P.; Pedrosa, P.; Martin, N.; Bundaleski, N.; Teodoro, O.M.N.D.; et al. Nanocomposite thin films based on Au-Ag nanoparticles embedded in a CuO matrix for localized surface plasmon resonance sensing. Appl. Surf. Sci. 2019, 484, 152–168. [Google Scholar] [CrossRef]
- Pereira-Silva, P.; Borges, J.; Rodrigues, M.S.; Oliveira, J.C.; Alves, E.; Barradas, N.P.; Dias, J.P.; Cavaleiro, A.; Vaz, F. Nanocomposite Au-ZnO thin films: Influence of gold concentration and thermal annealing on the microstructure and plasmonic response. Surf. Coat. Technol. 2020, 385, 125379. [Google Scholar] [CrossRef]
- Domingues, R.P.; Rodrigues, M.S.; Proença, M.; Costa, D.; Alves, E.; Barradas, N.P.; Oliveira, F.J.; Silva, R.F.; Borges, J.; Vaz, F. Thin films composed of Au nanoparticles embedded in AlN: Influence of metal concentration and thermal annealing on the LSPR band. Vacuum 2018, 157, 414–421. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Borges, J.; Meira, D.I.; Costa, D.; Rodrigues, M.S.; Rebelo, R.; Correlo, V.M.; Vaz, F.; Reis, R.L. Development of label-free plasmonic Au-TiO2 thin film immunosensor devices. Mater. Sci. Eng. C 2019, 100, 424–432. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Costa, D.; Domingues, R.P.; Apreutesei, M.; Pedrosa, P.; Martin, N.; Correlo, V.M.; Reis, R.L.; Alves, E.; Barradas, N.P.; et al. Optimization of nanocomposite Au/TiO2 thin films towards LSPR optical-sensing. Appl. Surf. Sci. 2018, 438, 74–83. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Vaz, F. Enhancing the Sensitivity of Nanoplasmonic Thin Films for Ethanol Vapor Detection. Materials 2020, 13, 870. [Google Scholar] [CrossRef]
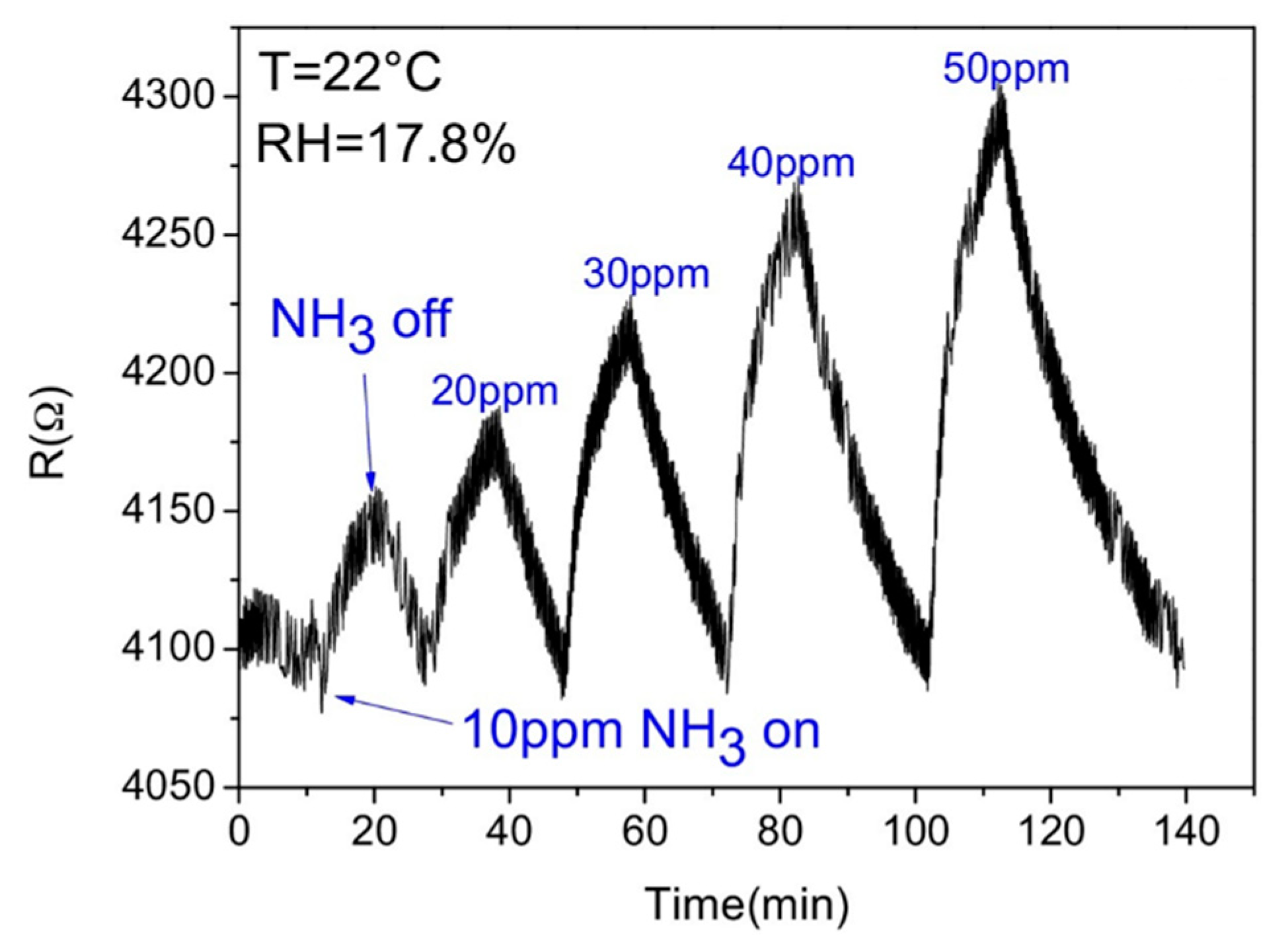
- Proença, M.; Rodrigues, M.S.; Vaz, F.; Borges, J. Carbon Monoxide (CO) Sensor Based on Au Nanoparticles Embedded in a CuO Matrix by HR-LSPR Spectroscopy at Room Temperature. IEEE Sens. Lett. 2021, 5, 1–3. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Proença, M.; Pedrosa, P.; Martin, N.; Romanyuk, K.; Kholkin, A.L.; Vaz, F. Nanoplasmonic response of porous Au-TiO2 thin films prepared by oblique angle deposition. Nanotechnology 2019, 30, 225701. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Abbasian, S.; Moshaii, A.; Vayghan, N.S.; Nikkhah, M. Ag Nanostructures Produced by Glancing Angle Deposition with Remarkable Refractive Index Sensitivity. Plasmonics 2017, 12, 631–640. [Google Scholar] [CrossRef]
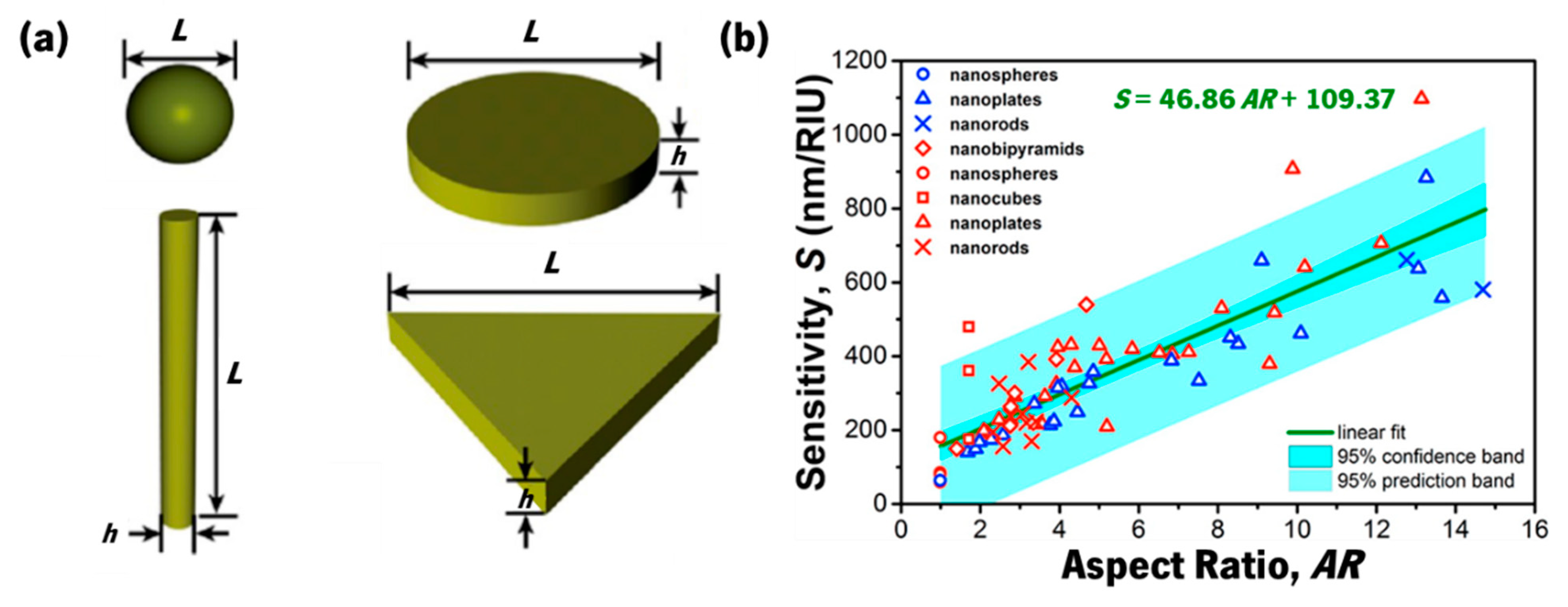
- Khan, A.U.; Zhao, S.; Liu, G. Key Parameter Controlling the Sensitivity of Plasmonic Metal Nanoparticles: Aspect Ratio. J. Phys. Chem. C 2016, 120, 19353–19364. [Google Scholar] [CrossRef]
- Edward, N.W.M.; Goswami, P. 9—Plasma-based treatments of textiles for water repellency. In The Textile Institute Book Series; Sanjay, M.R., Madhu, P., Parameswaranpillai, J., Siengchin, S., Gorbatyuk, S.M., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 215–232. ISBN 978-0-08-101212-3. [Google Scholar]
- Kechkar, S. Experimental Investigation of a Low Pressure Capacitively-Coupled Discharge. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2015. [Google Scholar]
- Ebnesajjad, S. Chapter 9—Plasma Treatment of Polymeric Materials; William Andrew Publishing: Oxford, UK, 2014; pp. 227–269. ISBN 978-0-323-26435-8. [Google Scholar]
- Shishoo, R. Introduction—The potential of plasma technology in the textile industry. In Woodhead Publishing Series in Textiles; Woodhead Publishing: Oxford, UK, 2007; pp. xv–xxx. ISBN 978-1-84569-073-1. [Google Scholar]
- Roy Choudhury, A.K. 14—Various ecofriendly finishes. In Woodhead Publishing Series in Textiles; Woodhead Publishing: Oxford, UK, 2017; pp. 467–525. ISBN 978-0-08-100646-7. [Google Scholar]
- Johansson, K.S. 20—Surface Modification of Plastics. In Plastics Design Library; Second, E., Ed.; William Andrew Publishing: New York, NY, USA, 2017; pp. 443–487. ISBN 978-0-323-39040-8. [Google Scholar]
- Alves, M.J.; Grenho, L.; Lopes, C.; Borges, J.; Vaz, F.; Vaz, I.P.; Fernandes, M.H. Antibacterial effect and biocompatibility of a novel nanostructured ZnO-coated gutta-percha cone for improved endodontic treatment. Mater. Sci. Eng. C 2018, 92, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Abelmann, L.; Lodder, C. Oblique evaporation and surface diffusion. Thin Solid Film. 1997, 305, 1–21. [Google Scholar] [CrossRef]
- Pedrosa, P.; Ferreira, A.; Cote, J.-M.; Martin, N.; Yazdi, M.A.P.; Billard, A.; Lanceros-Mendez, S.; Vaz, F. Influence of the sputtering pressure on the morphological features and electrical resistivity anisotropy of nanostructured titanium films. Appl. Surf. Sci. 2017, 420, 681–690. [Google Scholar] [CrossRef]
- Taschuk, M.T.; Hawkeye, M.M.; Brett, M.J. Glancing Angle Deposition. In Handbook of Deposition Technologies for Films and Coatings; William Andrew Publishing: Manchester, UK, 2010; ISBN 9780815520313. [Google Scholar]
- Martin, N.; Robbie, K.; Carpentier, L. Architecture of Thin Solid Films by the GLAD Technique. Nanomater. Surf. Eng. 2013, 1–30. [Google Scholar]
- Robbie, K.; Brett, M.J. Sculptured thin films and glancing angle deposition: Growth mechanics and applications. J. Vac. Sci. Technol. A 1997, 15, 1460–1465. [Google Scholar] [CrossRef]
- Knorr, T.G.; Hoffman, R.W. Dependence of Geometric Magnetic Anisotropy in Thin Iron Films. Phys. Rev. 1959, 113, 1039–1046. [Google Scholar] [CrossRef]
- Smith, D.O. Anisotropy in Permalloy Films. J. Appl. Phys. 1959, 30, S264–S265. [Google Scholar] [CrossRef]
- YOUNG, N.O.; KOWAL, J. Optically Active Fluorite Films. Nature 1959, 183, 104–105. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Brett, M.J. Glancing angle deposition: Fabrication, properties, and applications of micro- and nanostructured thin films. J. Vac. Sci. Technol. A 2007, 25. [Google Scholar] [CrossRef]
- Lintymer, J.; Gavoille, J.; Martin, N.; Takadoum, J. Glancing angle deposition to modify microstructure and properties of sputter deposited chromium thin films. Surf. Coat. Technol. 2003, 174–175, 316–323. [Google Scholar] [CrossRef]
- Besnard, A.; Martin, N.; Sthal, F.; Carpentier, L.U.C.; Rauch, J.-Y. Metal-to-Dielectric transition induced by annealing of oriented titanium thin FILMS. Funct. Mater. Lett. 2012, 6, 1250051. [Google Scholar] [CrossRef]
- Pedrosa, P.; Lopes, C.; Martin, N.; Fonseca, C.; Vaz, F. Electrical characterization of Ag:TiN thin films produced by glancing angle deposition. Mater. Lett. 2014, 115, 136–139. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Nylander, C.; Liedberg, B.; Lind, T. Gas detection by means of surface plasmon resonance. Sens. Actuators 1982, 3, 79–88. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M.; Tudos, A.J. Handbook of Surface Plasmon Resonance; Royal Society of Chemistry: Cambridge, UK, 2008; ISBN 0854042679. [Google Scholar]
- Rodrigues, M.S.; Borges, J.; Gabor, C.; Munteanu, D.; Apreutesei, M.; Steyer, P.; Lopes, C.; Pedrosa, P.; Alves, E.; Barradas, N.P.; et al. Functional behaviour of TiO2 films doped with noble metals. Surf. Eng. 2015, 32, 1743294415Y.000. [Google Scholar]
- Borges, J.; Costa, D.; Antunes, E.; Lopes, C.; Rodrigues, M.S.; Apreutesei, M.; Alves, E.; Barradas, N.P.; Pedrosa, P.; Moura, C.; et al. Biological behaviour of thin films consisting of Au nanoparticles dispersed in a TiO2 dielectric matrix. Vacuum 2015, 122, 360–368. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, N.; Liu, H.; Zhou, X.; Knoll, W. Integrating plasmonic diagnostics and microfluidics. Biomicrofluidics 2015, 9, 052611. [Google Scholar] [CrossRef] [PubMed]
- SadAbadi, H.; Badilescu, S.; Packirisamy, M.; Wüthrich, R. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron. 2013, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, S.; Xiao, X.; Li, S.; Gao, F.; Zhang, Z.; Du, J. Fabricating metal nanoparticle arrays at specified and localized regions of microfluidic chip for LSPR sensing. Microelectron. Eng. 2016, 151, 7–11. [Google Scholar] [CrossRef]
- Chen, P.; Liedberg, B. Curvature of the Localized Surface Plasmon Resonance Peak. Anal. Chem. 2014, 86, 7399–7405. [Google Scholar] [CrossRef] [PubMed]
- Henkel, A.; Ye, W.; Khalavka, Y.; Neiser, A.; Lambertz, C.; Schmachtel, S.; Ahijado-Guzmán, R.; Sönnichsen, C. Narrowing the Plasmonic Sensitivity Distribution by Considering the Individual Size of Gold Nanorods. J. Phys. Chem. C 2018, 122, 10133–10137. [Google Scholar] [CrossRef]
- Søndergaard, T. Modeling of plasmonic nanostructures: Green’s function integral equation methods. Phys. Status Solidi 2007, 244, 3448–3462. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. T-matrix method in plasmonics: An overview. J. Quant. Spectrosc. Radiat. Transf. 2013, 123, 184–217. [Google Scholar] [CrossRef]
- Siad, A.; Besnard, A.; Nouveau, C.; Jacquet, P. Critical angles in DC magnetron glad thin films. Vacuum 2016, 131, 305–311. [Google Scholar] [CrossRef]
- Sun, F.; Du, C.; Fu, T.; Chen, Y.; Sun, L.; Zhang, R.; Shi, D. Optimal aspect ratio and excitation spectral region of individual AuxAg1−x alloy nanobars for plasmonic sensing. Phys. Lett. A 2020, 384, 126785. [Google Scholar] [CrossRef]
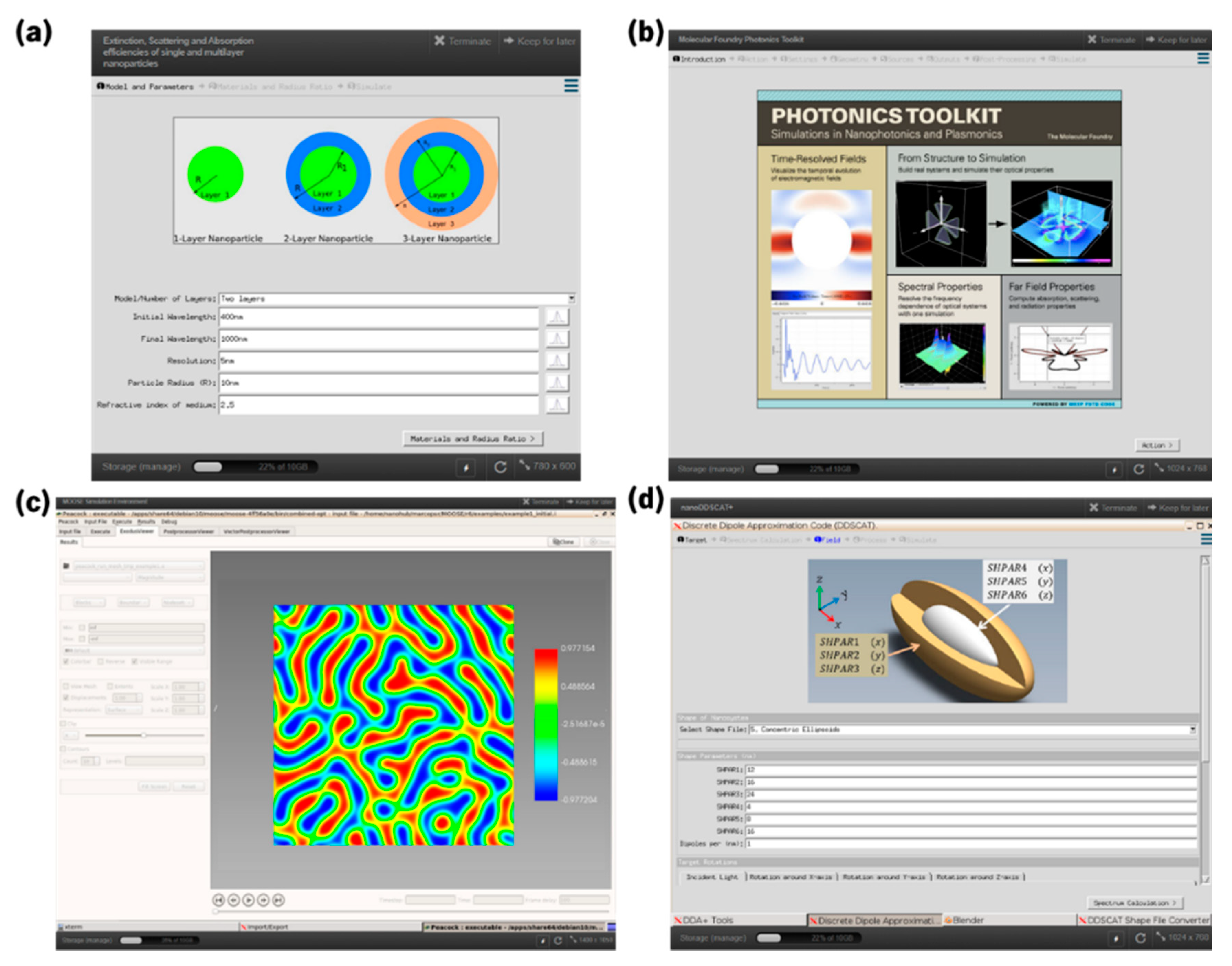
- Madhavan, K.; Zentner, L.; Farnsworth, V.; Shivarajapura, S.; Zentner, M.; Denny, N.; Klimeck, G. nanoHUB.org: Cloud-based services for nanoscale modeling, simulation, and education. Nanotechnol. Rev. 2013, 2, 107–117. [Google Scholar] [CrossRef]
- Camden, J.; Schatz, G.C. Nanosphere Optics Lab. 2016. Available online: https://nanohub.org/resources/nsoptics (accessed on 1 May 2021).
- Krishna, J.B.; Jun, H.; Lasse, J. Extinction, Scattering and Absorption Efficiencies of Single and Multilayer Nanoparticles; Pennsylvania State University: Centre County, PA, USA, 2016. [Google Scholar]
- McLeod, A.S.; Schuck, P.J.; Neaton, J.B. Molecular Foundry Photonics Toolkit; University of California: Berkeley, CA, USA, 2020. [Google Scholar]
- Permann, C.J.; Gaston, D.R.; Andrš, D.; Carlsen, R.W.; Kong, F.; Lindsay, A.D.; Miller, J.M.; Peterson, J.W.; Slaughter, A.E.; Stogner, R.H.; et al. MOOSE: Enabling massively parallel multiphysics simulation. SoftwareX 2020, 11, 100430. [Google Scholar] [CrossRef]
- Clark, S.; Aagesen, L.; Schwen, D. MOOSE (Multi-physics Object Oriented Simulation Environment). 2020. Available online: https://nanohub.org/resources/moose (accessed on 1 May 2021).
- Sobh, A.N.; White, S.; Smith, J.; Sobh, N.; Jain, P.K. nanoDDSCAT+. 2019. Available online: https://nanohub.org/resources/ddaplus (accessed on 1 May 2021).
- Parsons, J.; Burrows, C.P.; Sambles, J.R.; Barnes, W.L. A comparison of techniques used to simulate the scattering of electromagnetic radiation by metallic nanostructures. J. Mod. Opt. 2010, 57, 356–365. [Google Scholar] [CrossRef]
- Loke, V.L.Y.; Huda, G.M.; Donev, E.U.; Schmidt, V.; Hastings, J.T.; Mengüç, M.P.; Wriedt, T. Comparison between discrete dipole approximation and other modelling methods for the plasmonic response of gold nanospheres. Appl. Phys. B 2014, 115, 237–246. [Google Scholar] [CrossRef]
- Mencarelli, E.; Fanò, L.; Tarpani, L.; Latterini, L. Modelling the Optical Properties of Metal Nanoparticles: Analytical vs Finite Elements Simulation. Mater. Today Proc. 2015, 2, 161–170. [Google Scholar] [CrossRef]
- Dhawan, A.; Norton, S.J.; Gerhold, M.D.; Vo-Dinh, T. Comparison of FDTD numerical computations and analytical multipole expansion method for plasmonics-active nanosphere dimers. Opt. Express 2009, 17, 9688–9703. [Google Scholar] [CrossRef]
- Bora, T.; Dousse, A.; Sharma, K.; Sarma, K.; Baev, A.; Hornyak, G.L.; Dasgupta, G. Modeling nanomaterial physical properties: Theory and simulation. Int. J. Smart Nano Mater. 2019, 10, 116–143. [Google Scholar] [CrossRef]
- Liebsch, A.; González, P.V. Optical properties of randomly distributed particles. Phys. Rev. B 1984, 29, 6907–6920. [Google Scholar] [CrossRef]
- MacMillan, M.F.; Devaty, R.P.; Mantese, J.V. Infrared properties of Pt/Al2O3 cermet films. Phys. Rev. B 1991, 43, 13838–13845. [Google Scholar] [CrossRef]
- Vasilevskiy, M.I.; Anda, E.V. Effective dielectric response of semiconductor composites. Phys. Rev. B 1996, 54, 5844–5851. [Google Scholar] [CrossRef]
- Diaz-HR, R.; Esquivel-Sirvent, R.; Noguez, C. Plasmonic Response of Nested Nanoparticles with Arbitrary Geometry. J. Phys. Chem. C 2016, 120, 2349–2354. [Google Scholar] [CrossRef]
- Shalaev, V.M. Nonlinear Optics of Random Media; Springer Tracts in Modern Physics; Springer: Berlin/Heidelberg, Germany, 2000; Volume 158, ISBN 978-3-540-65615-9. [Google Scholar]
- DeVoe, H. Optical Properties of Molecular Aggregates. I. Classical Model of Electronic Absorption and Refraction. J. Chem. Phys. 1964, 41, 393–400. [Google Scholar] [CrossRef]
- Purcell, E.M.; Pennypacker, C.R. Scattering and Absorption of Light by Nonspherical Dielectric Grains. Astrophys. J. 1973, 186, 705. [Google Scholar] [CrossRef]
- Draine, B.T.; Flatau, P.J. Discrete-Dipole Approximation for Scattering Calculations. J. Opt. Soc. Am. A 1994, 11, 1491–1499. [Google Scholar] [CrossRef]
- Draine, B.T. The discrete-dipole approximation and its application to interstellar graphite grains. Astrophys. J. 1988, 333, 848. [Google Scholar] [CrossRef]
- Goodman, J.J.; Draine, B.T.; Flatau, P.J. Application of fast-Fourier-transform techniques to the discrete-dipole approximation. Opt. Lett. 1991, 16, 1198–1200. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- van Dijk, M.A.; Tchebotareva, A.L.; Orrit, M.; Lippitz, M.; Berciaud, S.; Lasne, D.; Cognet, L.; Lounis, B. Absorption and scattering microscopy of single metal nanoparticles. Phys. Chem. Chem. Phys. 2006, 8, 3486–3495. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Fang, C.; Jiang, R.; Jia, H.; Lai, Y.; Wang, J.; Lin, H.-Q. Highly enhanced transverse plasmon resonance and tunable double Fano resonances in gold@titania nanorods. Nanoscale 2016, 8, 6514–6526. [Google Scholar] [CrossRef] [PubMed]
- Gisbert Quilis, N.; Lequeux, M.; Venugopalan, P.; Khan, I.; Knoll, W.; Boujday, S.; Lamy de la Chapelle, M.; Dostalek, J. Tunable laser interference lithography preparation of plasmonic nanoparticle arrays tailored for SERS. Nanoscale 2018, 10, 10268–10276. [Google Scholar] [CrossRef] [PubMed]
- Proença, M.; Rodrigues, M.S.; Borges, J.; Vaz, F. Optimization of Au:CuO Nanocomposite Thin Films for Gas Sensing with High-Resolution Localized Surface Plasmon Resonance Spectroscopy. Anal. Chem. 2020, 92, 4349–4356. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Taschuk, M.T.; Brett, M.J. Glancing Angle Deposition of Thin Films; John Wiley & Sons, Ltd.: Chichester, UK, 2014; ISBN 9781118847510. [Google Scholar]
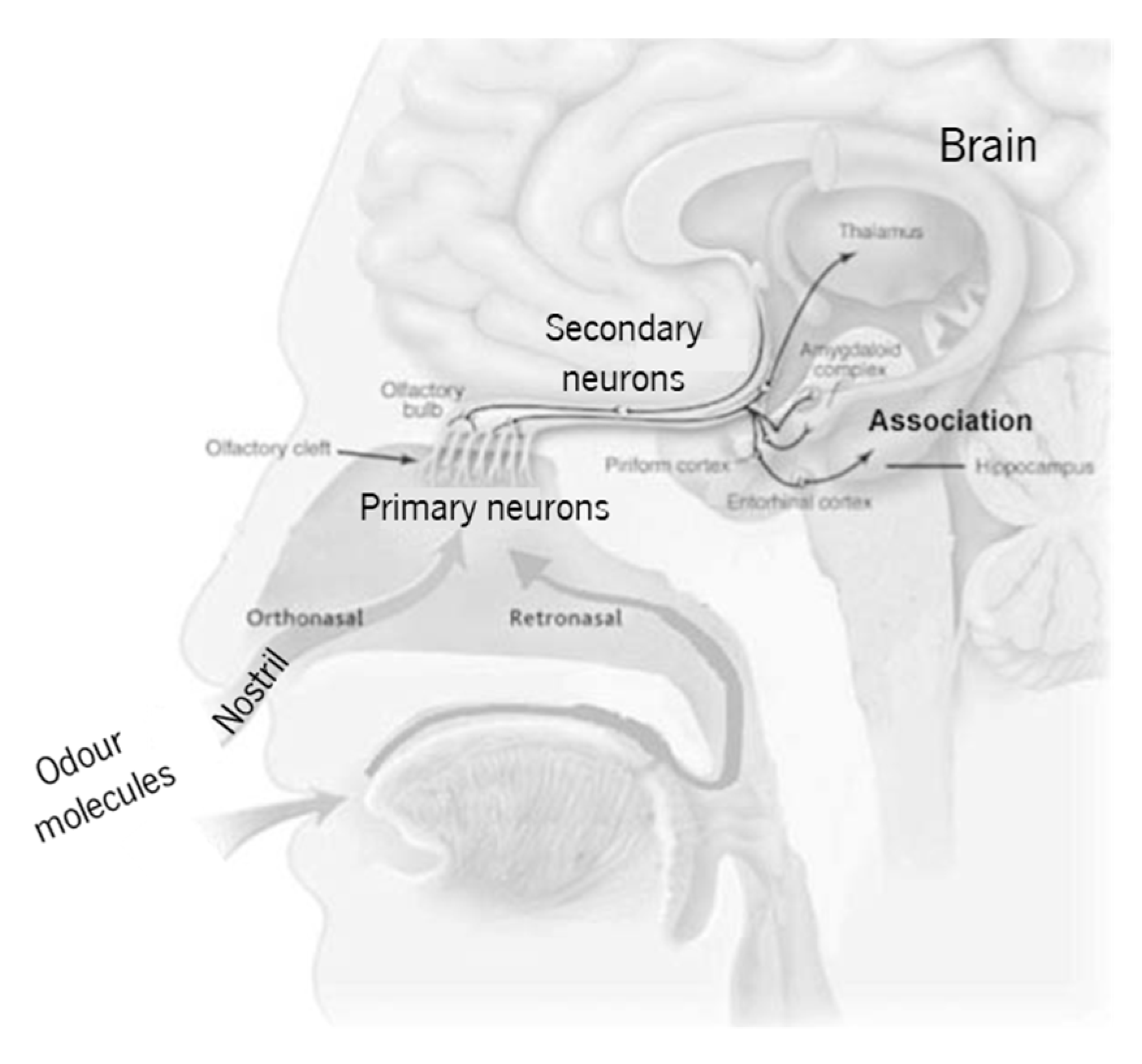
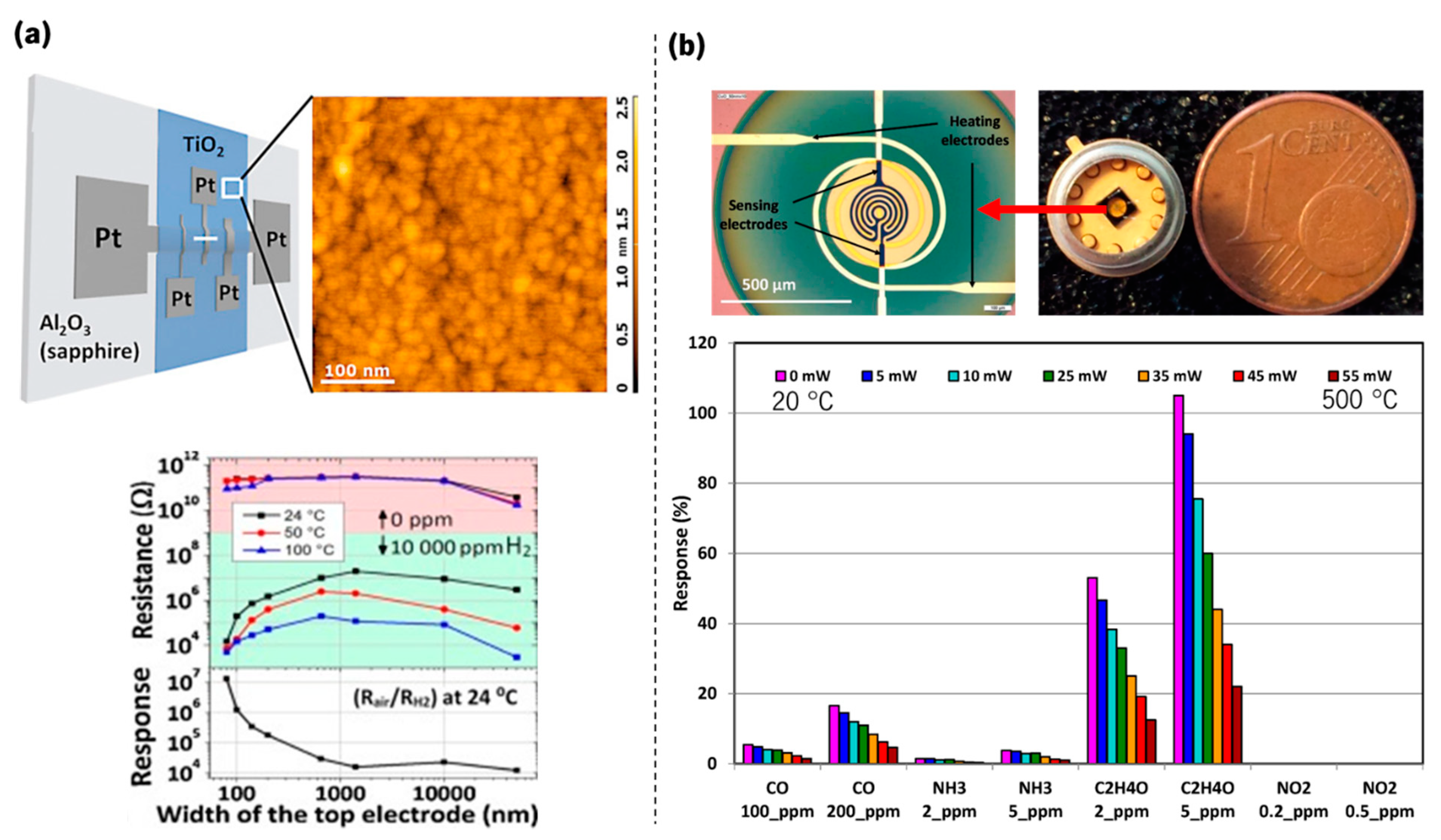

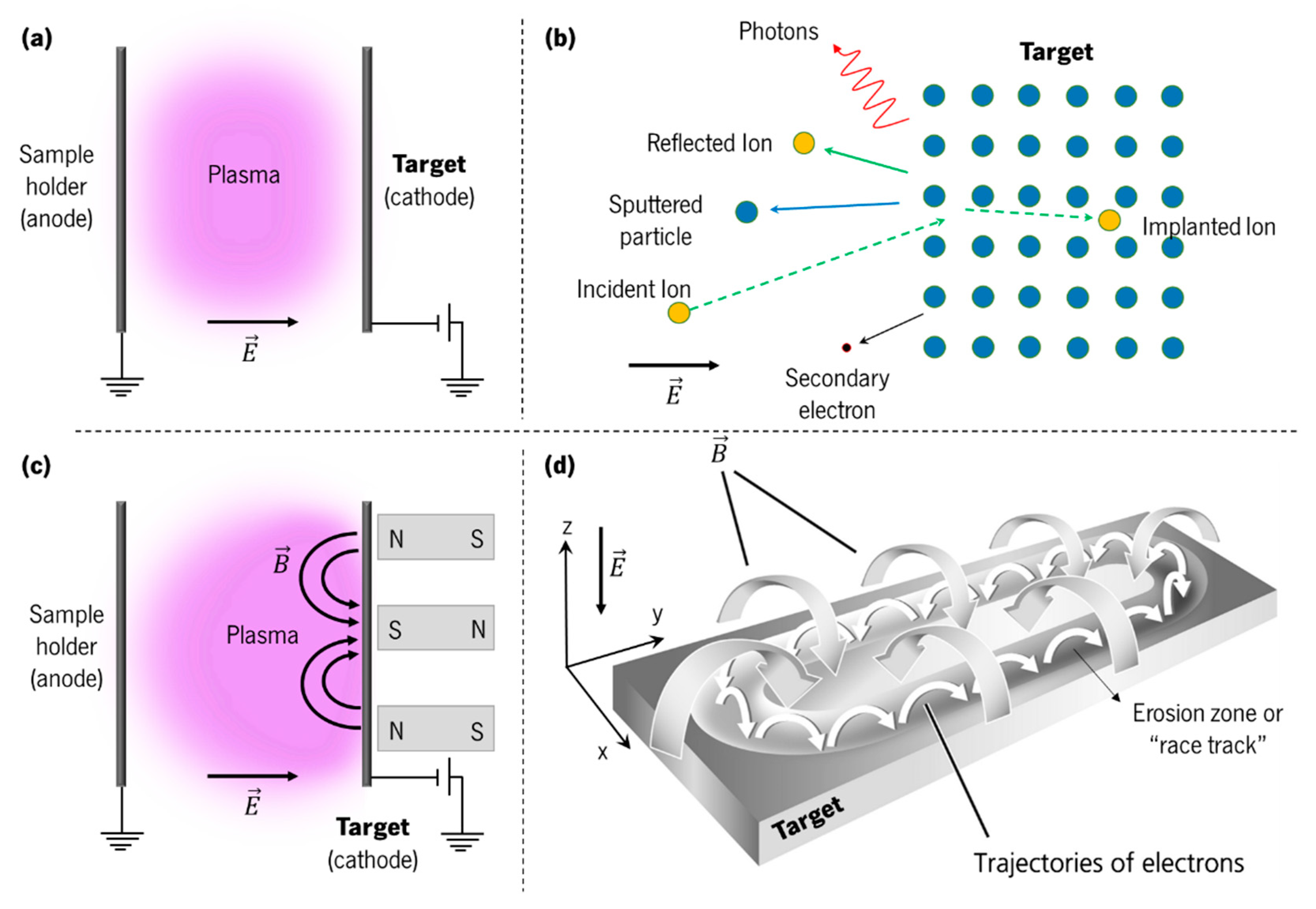


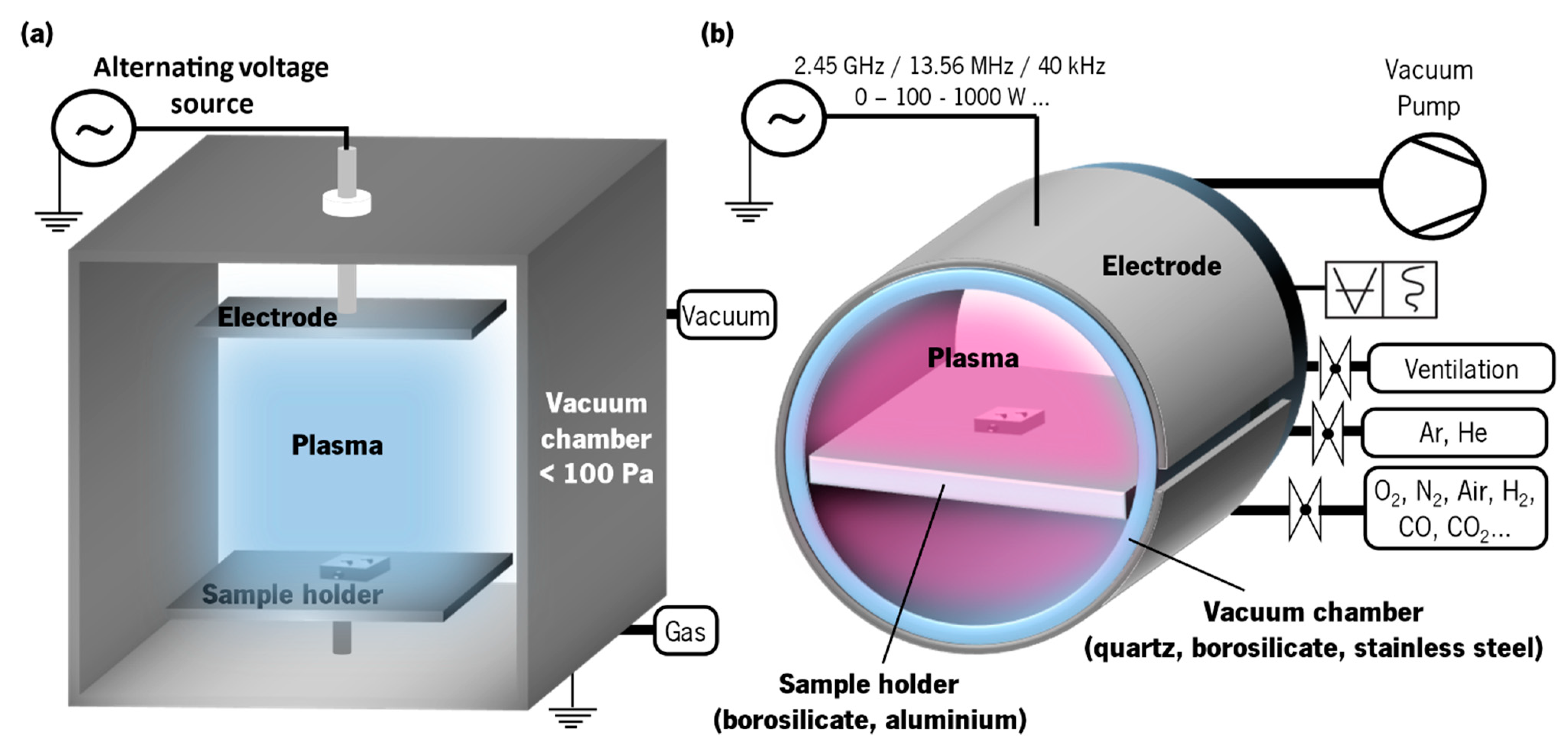
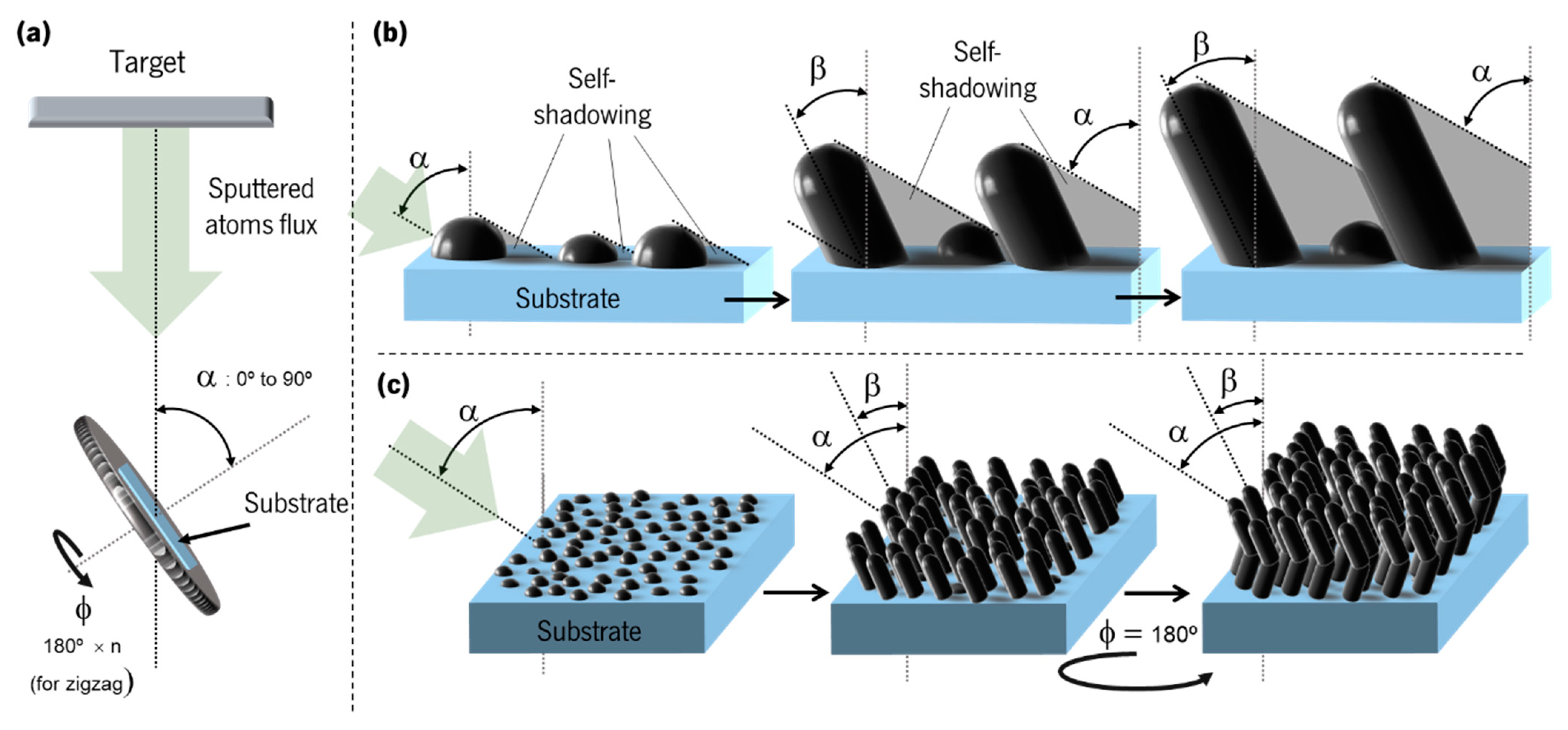
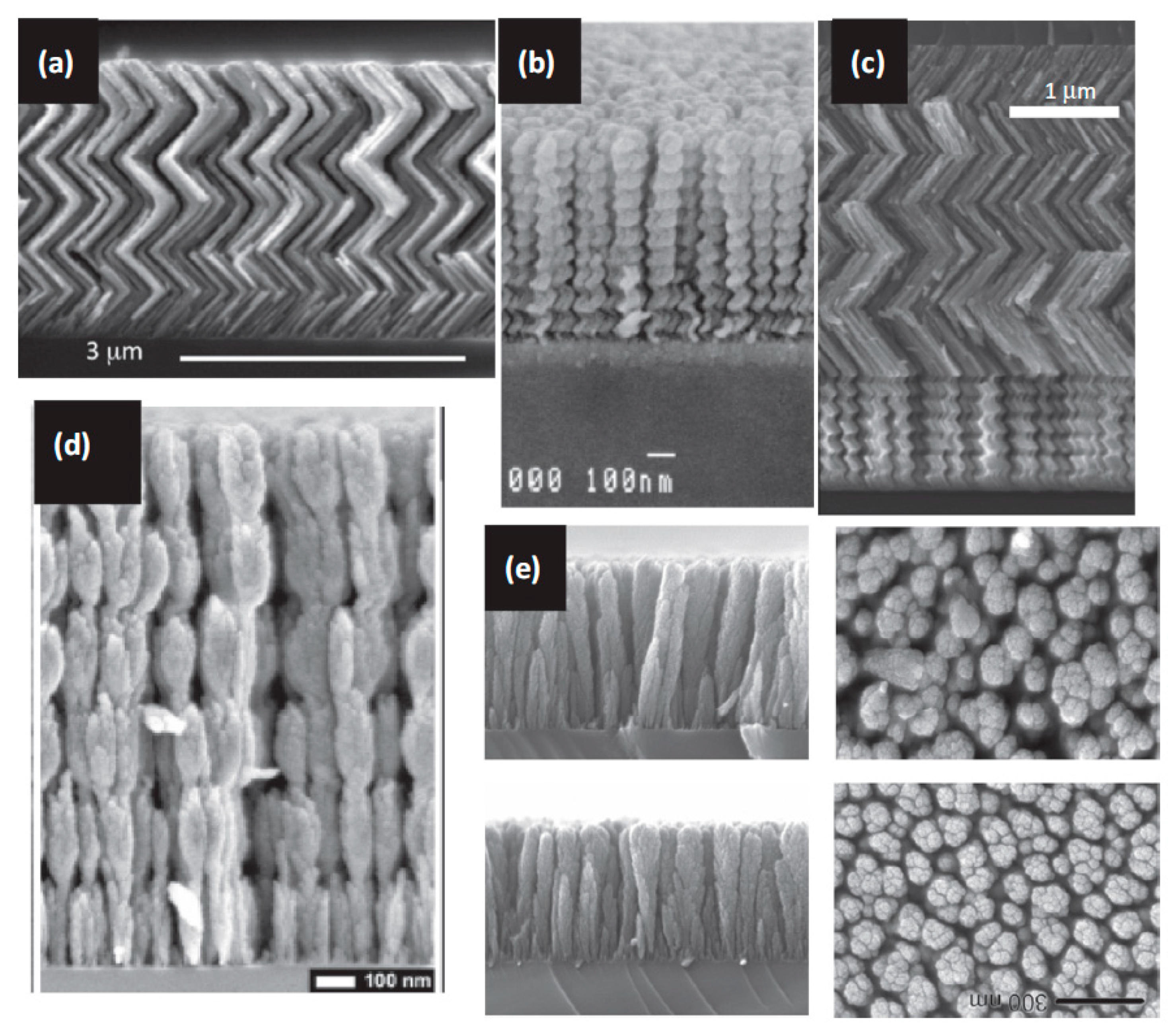
| Olfactory System | Electronic Nose System |
|---|---|
| i. Nostril | i. Sampler |
| ii. Primary neurons | ii. Sensor array |
| iii. Secondary neurons | iii. Signal conditioning and processing |
| iv. Brain | iv. Pattern recognition |
| Sensor Type | Projected Market (M EUR) |
|---|---|
| Chemical sensors—Worldwide | 36,100 [25] |
| Gas sensors—Worldwide | 1400 [26] |
| Gas sensors—Europe | 360 [26] |
| Au Nanoparticle Aspect Ratio and Shape | TiO2 Layer Thickness on Au Nanoparticle (nm) | RIS (nm.RIU−1) |
|---|---|---|
| 1 (sphere) | - | 52 |
| 1 (sphere) | 4 | 33 |
| 1 (sphere) | 8 | 21 |
| 2 (prolate) | - | 158 |
| 2 (prolate) | 4 | 74 |
| 2 (prolate) | 8 | 46 |
| 2 (oblate) | 106 | |
| 2 (oblate) | 4 | 57 |
| 2 (oblate) | 8 | 39 |
| 3 (prolate) | - | 305 |
| 4 (prolate) | - | 414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.S.; Borges, J.; Lopes, C.; Pereira, R.M.S.; Vasilevskiy, M.I.; Vaz, F. Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies. Appl. Sci. 2021, 11, 5388. https://doi.org/10.3390/app11125388
Rodrigues MS, Borges J, Lopes C, Pereira RMS, Vasilevskiy MI, Vaz F. Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies. Applied Sciences. 2021; 11(12):5388. https://doi.org/10.3390/app11125388
Chicago/Turabian StyleRodrigues, Marco S., Joel Borges, Cláudia Lopes, Rui M. S. Pereira, Mikhail I. Vasilevskiy, and Filipe Vaz. 2021. "Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies" Applied Sciences 11, no. 12: 5388. https://doi.org/10.3390/app11125388
APA StyleRodrigues, M. S., Borges, J., Lopes, C., Pereira, R. M. S., Vasilevskiy, M. I., & Vaz, F. (2021). Gas Sensors Based on Localized Surface Plasmon Resonances: Synthesis of Oxide Films with Embedded Metal Nanoparticles, Theory and Simulation, and Sensitivity Enhancement Strategies. Applied Sciences, 11(12), 5388. https://doi.org/10.3390/app11125388











