Surface Plasmon Resonance Sensor of CO2 for Indoors and Outdoors
Abstract
:1. Introduction
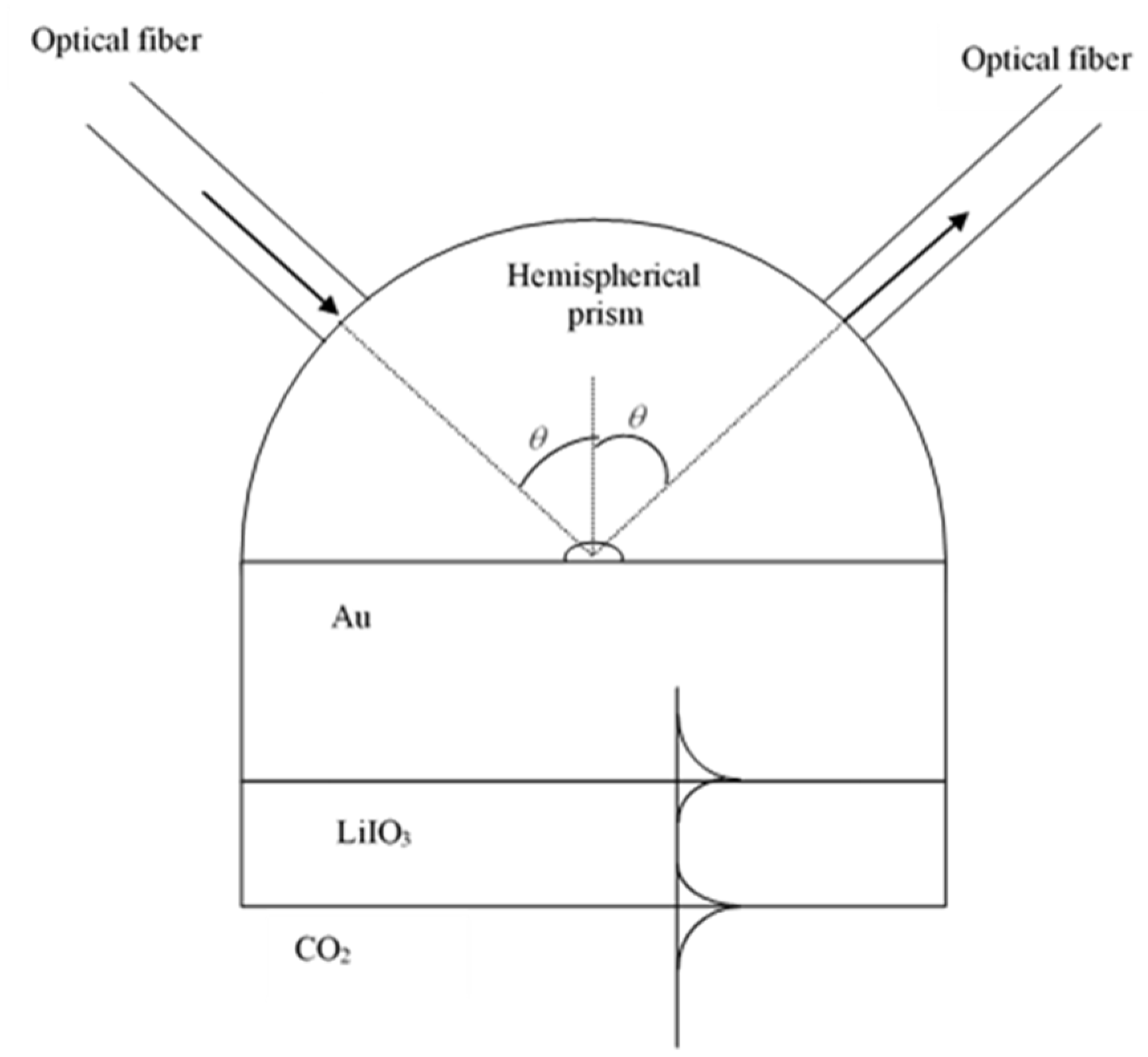
2. Design of the Plasmonic Sensor
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, J.H. CO2 at NOAA’s Mauna Loa Observatory Reaches New Milestone: Tops 400 ppm, Global Monitoring Division—ESRL-GMD. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/weekly.html (accessed on 24 June 2021).
- Satish, U.; Mendell, M.J.; Shekhar, K.; Hotchi, T.; Sullivan, D.; Streufert, S.; Fisk, W.J. Is CO2 an Indoor Pollutant? Direct Effects of Low-to-Moderate CO2 Concentrations on Human Decision-Making Performance. Environ. Health Perspect. 2012, 120, 1671–1677. [Google Scholar] [CrossRef] [Green Version]
- Chemical Sampling Information|Carbon Dioxide|Occupational Safety and Health Administration. 2018. Available online: https://www.osha.gov/dts/chemicalsampling/data/ (accessed on 20 June 2021).
- Chen, S.J.; Hovde, D.C.; Peterson, K.A.; Marshall, A.W. Fire Detection using Smoke and Gas Sensors. Fire Safety. J. 2007, 42, 507–515. [Google Scholar] [CrossRef]
- Ge, X.D.; Kostov, Y.; Rao, G. High-stability Non-invasive Autoclavable Naked Optical CO2 Sensor. Biosens. Bioelectron. 2003, 18, 857–865. [Google Scholar] [CrossRef]
- Gorman, T.; Haxha, S. Design and Optimization of Integrated Hybrid Surface Plasmon Biosensor. Opt. Commun. 2014, 325, 175–178. [Google Scholar] [CrossRef]
- Salim, M.R.; Yaacob, M.; Ibrahim, M.H.; Azmi, A.I.; Ngajikin, N.H.; Dooly, G.; Lewis, E. An Optical Spectroscopic Based Reflective Sensor for CO2 Measurement with Signal to Noise Ratio Improvement. J. Optoelectron. Adv. Mater. 2015, 17, 519–525. [Google Scholar]
- Mi, J.G.C.; Horvath, C.; Aktary, M.; Van, V. Silicon Microring Refractometric Sensor for Atmospheric CO2 gas Monitoring. Opt. Express 2016, 25, 1773–1780. [Google Scholar] [CrossRef]
- Nuryadi, R.; Aprilia, L.; Gustiono, D. CO Gas Response of ZnO Nanostructures using Microcantilever in Dynamic Mode Operation. In Proceedings of the 7th IEEE International Nanoelectronics Conference, Chengdu, China, 9–11 May 2016; Available online: https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=7589337 (accessed on 20 June 2021).
- Vijayan, A.; Fuke, M.V.; Karekar, R.N.; Aiyer, R.C. Planar Optical Waveguide Evanescent Wave CO2 Sensor based on a Clad of Alstonia Scholaris Leaf Extract. IEEE Sens. J. 2009, 9, 13–19. [Google Scholar] [CrossRef]
- Yi, S. Temperature Compensation Methods of Nondispersive Infrared CO2 gas Sensor with Dual Ellipsoidal Optical Waveguide. Sensors Mater. 2017, 29, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Bao, B.; Melo, L.; Davies, B.; Fadaei, H.; Sinton, D.; Wild, P. Detecting Supercritical CO2 in Brine at Sequestration Pressure with an Optical Fiber Sensor. Environ. Sci. Technol. 2013, 47, 306–313. [Google Scholar] [CrossRef]
- Starecki, F.; Charpentier, F.; Doualan, J.L.; Quetel, L.; Michel, K.; Chahal, R.; Troles, J.; Bureau, B.; Braud, A.; Camy, P.; et al. Mid-IR Optical Sensor for CO2 Detection based on Fluorescence Absorbance of Dy3+: Ga5Ge20Sb10S65 fibers. Sens. Actuators B Chem. 2015, 207, 518–525. [Google Scholar] [CrossRef]
- Wysokinski, K.; Napierała, M.; Stanczyk, T.; Lipinski, S.; Nasiłowski, T. Study on The Sensing Coating of The Optical Fibre CO2 Sensor. Sensors 2015, 15, 31888–31903. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.W.; Xing, J.X.; Wang, R.H.; Rong, Q.Z.; Zhang, W.L.; Li, Y.C.; Zhang, J.Y.; Qiao, X.G. Optical Fiber Fabry–Perot Interferometric CO2 gas Sensor using Guanidine Derivative Polymer Functionalized Layer. IEEE Sens. J. 2018, 18, 1924–1929. [Google Scholar] [CrossRef]
- Wu, J.S.; Yin, M.J.; Seefeldt, K.; Dani, A.; Guterman, R.; Yuan, J.Y.; Zhang, A.P.; Tam, H.Y. In Situ Mu-printed Optical Fiber-tip CO2 Sensor using a Photocross Linkable Poly (ionic liquid). Sens. Actuators B Chem. 2018, 259, 833–839. [Google Scholar] [CrossRef]
- Herminjard, S.; Sirigu, L.; Herzig, H.P.; Studemann, E.; Crottini, A.; Pellaux, J.P.; Gresch, T.; Fischer, M.; Faist, J. Surface Plasmon Resonance Sensor showing Enhanced Sensitivity for CO2 Detection in the Mid-infrared Range. Opt. Express 2009, 5, 293–303. [Google Scholar] [CrossRef]
- Lang, T.; Hirsch, T.; Fenzl, C.; Brandl, F.; Wolfbeis, O.S. Surface Plasmon Resonance Sensor for Dissolved and Gaseous Carbon Dioxide. Anal. Chem. 2012, 84, 9085–9088. [Google Scholar] [CrossRef]
- Nuryadi, R.; Mayasari, R.D.; Aprilia, L.; Yuliarto, B. Fabrication of ZnO/Au/prism-based Surface Plasmon Resonance Device for Gas Detection. Presented at International Conference on Quality in Research (QiR), Lombok, Indonesia, 10–13 August 2015. [Google Scholar] [CrossRef]
- González-Vila, A.; Ioannou, A.; Loyez, M.; Debliquy, M.; Lahem, D.; Caucheteur, C. Surface Plasmon Resonance Sensing in Gaseous Media with Optical Fiber Gratings. Opt. Letters 2018, 43, 2308–2311. [Google Scholar] [CrossRef]
- Yao, Q.F.; Ren, G.H.; Xu, K.; Zhu, L.; Khan, H.; Mohiuddin, M.; Khan, M.W.; Zhang, B.Y.; Jannat, A.; Haque, F.; et al. 2D Plasmonic Tungsten Oxide Enabled Ultrasensitive Fiber Optics Gas Sensor. Adv. Opt. Mater. 2019, 7. [Google Scholar] [CrossRef]
- Pozo, A.M.; Pérez-Ocón, F.; Rabaza, O. A Continuous Liquid-level Sensor for Fuel Tanks based on Surface Plasmon Resonance. Sensors 2016, 16, 724. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ocón, F.; Pozo, A.M.; Serrano, J.M.; Rabaza, O. Continuous Measurement with Three-in-one Plasmon Sensor in Sucrose Solutions. IEEE Sens. J. 2020. (In Press) [Google Scholar] [CrossRef]
- Sang, B.H.; Jeon, T.I. Pressure-dependent Refractive Indices of Gases by THz Time-domain Spectroscopy. Opt. Express 2016, 24, 29040–29047. [Google Scholar] [CrossRef]
- Birch, K.P.; Downs, M.J. The results of a Comparison between Calculated and Measured Values of The Refractive Index of Air. J. Phys. E Sci. Instrum. 1988, 21, 694–695. [Google Scholar] [CrossRef]
- Optical Glass|Sumita Optical Glass. Available online: http://www.sumita-opt.co.jp/en/products/optical (accessed on 20 June 2021).
- Choy, M.M.; Byer, R.L. Accurate Second-order Susceptibility Measurements of Visible and Infrared Nonlinear Crystals. Phys. Rev. B 1976, 14, 693–706. [Google Scholar] [CrossRef]
- Babar, S.; Weaver, J.H. Optical Constants of Cu, Ag, and Au Revisited. Appl. Opt. 2015, 54, 477–481. [Google Scholar] [CrossRef]
- Heavens, OS Thin Films Optics. Optical Properties of Thin Solid Films; Dover Books on Physics: New York, NY, USA, 2011; pp. 45–96. [Google Scholar]
- Ohta, K.; Ishida, H. Matrix Formalism for Calculation of Electric Field Intensity of Light in Stratified Multilayered Films. Appl. Opt. 1990, 29, 1952–1959. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Ronot-Trioli, C.; Trouillet, A.; Veillas, C.; Gagnaire, H. Monochromatic Excitation of Surface Plasmon Resonance in an Optical Fibre Refractive-index Sensor. Sens. Actuators A Phys. 1996, 54, 589–593. [Google Scholar] [CrossRef]
- Derbel, F. Performance Improvement of Fire Detectors by Means of Gas Sensors and Neural Networks. Fire Safety, J. 2004, 39, 383–398. [Google Scholar] [CrossRef]
- Fraser, R.H.; Li, Z.; Cihlar, J. Hotspot and NDVI Differencing Synergy (HANDS): A new Technique for Burned Area Mapping Over Boreal Forest. Remote Sens. Environ. 2000, 74, 362–376. [Google Scholar] [CrossRef]
- Mu, M.; Randerson, J.T.; Van der Werf, G.R.; Giglio, L.; Kasibhatla, P.; Morton, D.; Collatz, G.J.; De Fries, R.S.; Hyer, E.J.; Prins, E.M.; et al. Daily and 3-hourly Variability in Global Fire Emissions and Consequences for Atmospheric Model Predictions of Carbon Monoxide. J. Geophys. Res. 2011, 116, 1–19. [Google Scholar] [CrossRef]

| Indoor | Outdoor | Multigas | Only CO2 | Wide Dynamic Range | Short Dynamic Range |
| [4] | [12,19] | [20,21] | [5,6,7,8,9,10,11,12,13] | [7,11,16] | [5,6,8,13,15,18] |
| Waveguide/Optical fiber sensor | Plasmonic sensor | Others | Instant response time | Fast response time | Slow response time |
| [10,11,12,13,14,15,16,20,21] | [6,17,18,19] | [5,7,8,9] | [6,17,18,19] | [5,10] | [9,14,16] |
| High resolution | Low resolution | High sensitivity | Low sensitivity | ||
| [17] | [13] | [8,17,20] | [12] |
| Altitude (m) | Sensitivity (RIU−1) and Resolution (RIU) | 400–450 ppm | 450–500 ppm | 500–550 ppm | 550–600 ppm |
|---|---|---|---|---|---|
| 0 | Sensitivity | 17.18 | 22.07 | 19.53 | 14.71 |
| Resolution × 10−5 | 5.80 | 4.55 | 5.10 | 6.80 | |
| 500 | Sensitivity | 13.39 | 20.54 | 20.76 | 17.28 |
| Resolution × 10−5 | 7.45 | 4.85 | 4.80 | 5.80 | |
| 1000 | Sensitivity | 19.37 | 24.89 | 22.03 | 16.59 |
| Resolution × 10−5 | 5.15 | 4.02 | 4.54 | 6.05 | |
| 1500 | Sensitivity | 7.51 | 16.54 | 20.65 | 20.16 |
| Resolution × 10−4 | 1.33 | 0.61 | 0.49 | 0.50 | |
| 2000 | Sensitivity | 6.01 | 14.75 | 19.70 | 20.45 |
| Resolution × 10−4 | 1.67 | 0.68 | 0.51 | 0.49 | |
| 2500 | Sensitivity | 6.68 | 14.37 | 18.98 | 20.12 |
| Resolution × 10−4 | 1.50 | 0.70 | 0.53 | 0.50 | |
| 3000 | Sensitivity | 5.09 | 12.53 | 17.68 | 19.82 |
| Resolution × 10−4 | 1.97 | 0.80 | 0.57 | 0.51 | |
| 3500 | Sensitivity | 3.88 | 10.91 | 16.28 | 19.16 |
| Resolution × 10−4 | 2.58 | 0.92 | 0.62 | 0.52 | |
| 4000 | Sensitivity | 3.02 | 9.56 | 14.92 | 18.28 |
| Resolution × 10−4 | 3.31 | 1.05 | 0.67 | 0.55 | |
| 4500 | Sensitivity | 2.59 | 8.62 | 13.79 | 17.37 |
| Resolution × 10−4 | 3.87 | 1.16 | 0.73 | 0.58 | |
| 5000 | Sensitivity | 2.36 | 7.89 | 12.79 | 16.43 |
| Resolution × 10−4 | 4.24 | 1.27 | 0.78 | 0.61 | |
| 5500 | Sensitivity | 2.41 | 7.47 | 12.04 | 15.62 |
| Resolution × 10−4 | 4.16 | 1.34 | 0.83 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Ocón, F.; Pozo, A.M.; Cortina, J.; Rabaza, O. Surface Plasmon Resonance Sensor of CO2 for Indoors and Outdoors. Appl. Sci. 2021, 11, 6869. https://doi.org/10.3390/app11156869
Pérez-Ocón F, Pozo AM, Cortina J, Rabaza O. Surface Plasmon Resonance Sensor of CO2 for Indoors and Outdoors. Applied Sciences. 2021; 11(15):6869. https://doi.org/10.3390/app11156869
Chicago/Turabian StylePérez-Ocón, Francisco, Antonio M. Pozo, Jorge Cortina, and Ovidio Rabaza. 2021. "Surface Plasmon Resonance Sensor of CO2 for Indoors and Outdoors" Applied Sciences 11, no. 15: 6869. https://doi.org/10.3390/app11156869
APA StylePérez-Ocón, F., Pozo, A. M., Cortina, J., & Rabaza, O. (2021). Surface Plasmon Resonance Sensor of CO2 for Indoors and Outdoors. Applied Sciences, 11(15), 6869. https://doi.org/10.3390/app11156869







