Clinical and Instrumental TMJ Evaluation in Children and Adolescents with Juvenile Idiopathic Arthritis: A Case—Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
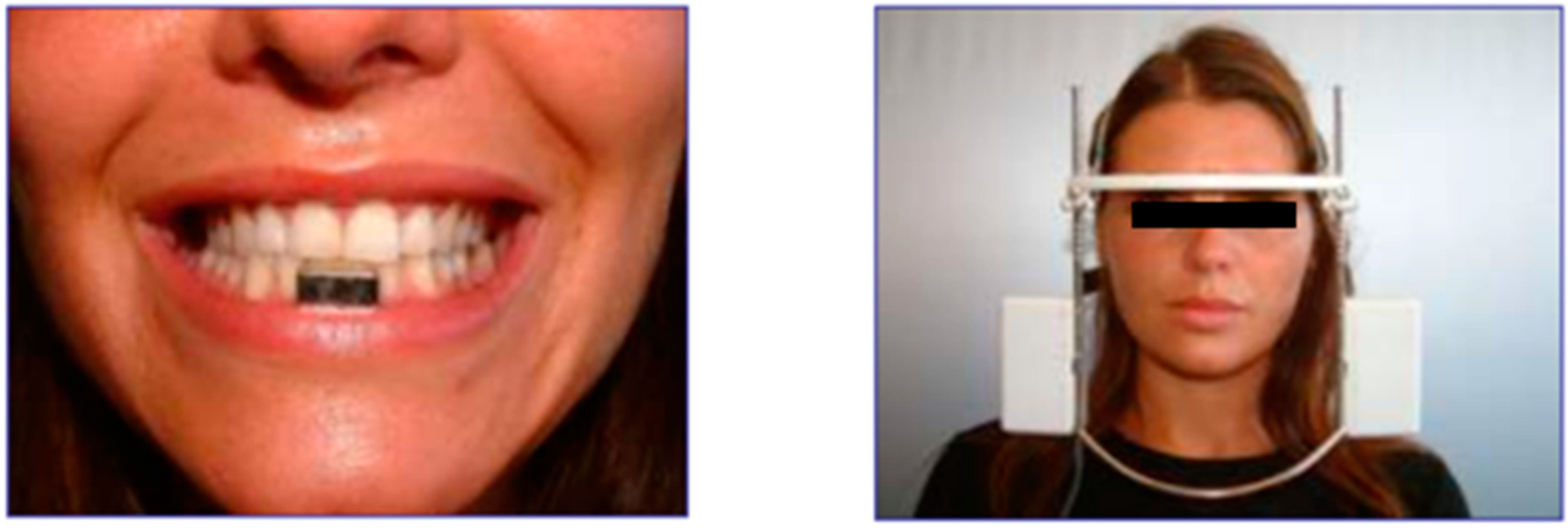
2.2. Clinical Examination Methods
2.3. Recording Equipment
- Clenching test (CLE6): the subject was asked to close their mouth and clench in normal condition without cotton rolls (true occlusal test).
- Rotation test (ROT6): The subject was asked to rotate their head, first on one side, then on the other. The operator ensured that the patient did not tilt their head or move their shoulders away from the back of the chair.
2.4. Statistical Analysis
3. Results
3.1. Muscle Palpation Values
3.2. sEMG Values
3.3. Kinesiographic Values
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crayne, C.B.; Beukelman, T. Juvenile Idiopathic Arthritis: Oligoarthritis and Polyarthritis. Pediatr. Clin. N. Am. 2018, 65, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Cimaz, R. Systemic-onset juvenile idiopathic arthritis. Autoimmun. Rev. 2016, 15, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Niibo, P.; Pruunsild, C.; Voog-Oras, Ü.; Nikopensius, T.; Jagomägi, T.; Saag, M. Contemporary management of TMJ involvement in JIA patients and its orofacial consequences. EPMA J. 2016, 7, 12. [Google Scholar] [CrossRef]
- Thatayatikom, A.; De Leucio, A. Juvenile Idiopathic Arthritis (JIA); StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Horton, D.B.; Shenoi, S. Review of environmental factors and juvenile idiopathic arthritis. Open Access Rheumatol. 2019, 11, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Giancane, G.; Alongi, A.; Ravelli, A. Update on the pathogenesis and treatment of juvenile idiopathic arthritis. Curr. Opin. Rheumatol. 2017, 29, 523–529. [Google Scholar] [CrossRef]
- Mahmud, S.A.; Binstadt, B.A. Autoantibodies in the Pathogenesis, Diagnosis, and Prognosis of Juvenile Idiopathic Arthritis. Front. Immunol. 2019, 9, 3168. [Google Scholar] [CrossRef] [PubMed]
- Barut, K.; Adrovic, A.; Şahin, S.; Kasapçopur, Ö. Juvenile Idiopathic Arthritis. Balkan Med. J. 2017, 34, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Southwood, T.R.; Baum, J.; Bhettay, E.; Glass, D.N.; Manners, P.; Maldonado-Cocco, J.; Suarez-Almazor, M.; Orozco-Alcala, J.; Prieur, A.M. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J. Rheumatol. 1998, 25, 1991–1994. [Google Scholar]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar]
- Martini, A.; Ravelli, A.; Avcin, T.; Beresford, M.W.; Burgos-Vargas, R.; Cuttica, R.; Ilowite, N.T.; Khubchandani, R.; Laxer, R.M.; Lovell, D.J.; et al. Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J. Rheumatol. 2019, 46, 190–197. [Google Scholar] [CrossRef]
- Malattia, C.; Rinaldi, M.; Martini, A. The role of imaging in juvenile idiopathic arthritis. Expert Rev. Clin. Immunol. 2018, 14, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.L.; Hoffmann, R.; Schmickler, J.; Rühlmann, M.; Challakh, N.; Haak, R.; Schmalz, G.; Ziebolz, D. Comprehensive Assessment of Orofacial Health and Disease Related Parameters in Adolescents with Juvenile Idiopathic Arthritis-A Cross-Sectional Study. J. Clin. Med. 2020, 9, 513. [Google Scholar] [CrossRef]
- Billiau, A.D.; Hu, Y.; Verdonck, A.; Carels, C.; Wouters, C. Temporomandibular joint arthritis in juvenile idiopathic arthritis: Prevalence, clinical and radiological signs, and relation to dentofacial morphology. J. Rheumatol. 2007, 34, 1925–1933. [Google Scholar] [PubMed]
- Steenks, M.H.; Giancane, G.; de Leeuw, R.R.; Bronkhorst, E.M.; van Es, R.J.J.; Koole, R.; van Bruggen, H.W.; Wulffraat, N.M. Temporomandibular joint involvement in Juvenile Idiopathic Arthritis: Reliability and validity of a screening protocol for the rheumatologist. Pediatr. Rheumatol. 2015, 13, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kjellberg, H. Juvenile chronic arthritis. Dentofacial morphology, growth, mandibular function and orthodontic treatment. Swed Dent. J. Suppl. 1995, 109, 1–56. [Google Scholar] [PubMed]
- Cedströmer, A.-L.; Andlin-Sobocki, A.; Abbu, N.; Hedenberg-Magnusson, B.; Dahlström, L.; Berntson, L. Condylar alterations and facial growth in children with juvenile idiopathic arthritis. J. Orofac. Orthop. 2020, 81, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.; Adell, R.; Kopp, S. Temporomandibular disorders in juvenile chronic arthritis patients. A clinical study. Swed Dent. J. 2000, 24, 83–92. [Google Scholar]
- Bernini, J.M.; Kellenberger, C.J.; Eichenberger, M.; Eliades, T.; Papageorgiou, S.N.; Patcas, R. Quantitative analysis of facial asymmetry based on three-dimensional photography: A valuable indicator for asymmetrical temporomandibular joint affection in juvenile idiopathic arthritis patients? Pediatr. Rheumatol. Online J. 2020, 18, 10. [Google Scholar] [CrossRef]
- Zwir, L.F.; Terreri, M.T.; Castro, A.D.A.; Rodrigues, W.D.R.; Fernandes, A.R.C. Is power Doppler ultrasound useful to evaluate temporomandibular joint inflammatory activity in juvenile idiopathic arthritis? Clin. Rheumatol. 2020, 39, 1237–1240. [Google Scholar] [CrossRef]
- Hsieh, Y.-J.; Darvann, T.A.; Hermann, N.V.; Larsen, P.; Liao, Y.-F.; Kreiborg, S. Three-dimensional assessment of facial morphology in children and adolescents with juvenile idiopathic arthritis and moderate to severe TMJ involvement using 3D surface scans. Clin. Oral Investig. 2020, 24, 799–807. [Google Scholar] [CrossRef]
- Urtane, I.; Jankovska, I.; Al-Shwaikh, H.; Krisjane, Z. Correlation of temporomandibular joint clinical signs with cone beam computed tomography radiologic features in juvenile idiopathic arthritis patients. Stomatologija 2018, 20, 82–89. [Google Scholar]
- Klenke, D.; Quast, A.; Prelog, M.; Holl-Wieden, A.; Riekert, M.; Stellzig-Eisenhauer, A.; Meyer-Marcotty, P. TMJ pathomorphology in patients with JIA-radiographic parameters for early diagnosis. Head Face Med. 2018, 14, 15. [Google Scholar] [CrossRef]
- Stoustrup, P.; Twilt, M.; Spiegel, L.; Kristensen, K.D.; Koos, B.; Pedersen, T.K.; Küseler, A.; Cron, R.Q.; Abramowicz, S.; Verna, C.; et al. Clinical Orofacial Examination in Juvenile Idiopathic Arthritis: International Consensus-based Recommendations for Monitoring Patients in Clinical Practice and Research Studies. J. Rheumatol. 2017, 44, 326–333. [Google Scholar] [CrossRef]
- Stoll, M.L.; Kau, C.H.; Waite, P.D.; Cron, R.Q. Temporomandibular joint arthritis in juvenile idiopathic arthritis, now what? Pediatr. Rheumatol. Online J. 2018, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.D.; Stoustrup, P.; Küseler, A.; Pedersen, T.K.; Twilt, M.; Herlin, T. Clinical predictors of temporomandibular joint arthritis in juvenile idiopathic arthritis: A systematic literature review. Semin. Arthritis Rheum. 2016, 45, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-T.; Wu, J.-S. The Visual Analogue Scale for Rating, Ranking and Paired-Comparison (VAS-RRP): A new technique for psychological measurement. Behav. Res. Methods 2018, 50, 1694–1715. [Google Scholar] [CrossRef] [PubMed]
- Goddard, G.; Karibe, H.; McNeill, C. Reproducibility of visual analog scale (VAS) pain scores to mechanical pressure. Cranio 2004, 22, 250–256. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Tartaglia, G.M.; Luraghi, F.E.; Sforza, C. The use of surface electromyography as a tool in differentiating temporomandibular disorders from neck disorders. Man Ther. 2007, 12, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.F.; Sforza, C.; Serrao, G. The influence of crossbite on the coordinated electromyographic activity of human masticatory muscles during mastication. J. Oral Rehabil. 1999, 26, 575–581. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Tartaglia, G.M.; Galletta, A.; Grassi, G.P.; Sforza, C. The influence of occlusion on jaw and neck muscle activity: A surface EMG study in healthy young adults. J. Oral Rehabil. 2006, 33, 341–348. [Google Scholar] [CrossRef]
- De Felìcio, C.M.; Sidequersky, F.V.; Tartaglia, G.M.; Sforza, C. Electromyographic standardized indices in healthy Brazilian young adults and data reproducibility. J. Oral Rehabil. 2009, 36, 577–583. [Google Scholar] [CrossRef]
- Teethan User Manual. Document Number: ERTHN-01376-00 Published: February 2016 Copyright © 2014-2015-2016 Teethan S.p.A. All Rights Reserved. Available online: https://teethan.com/immagini/TEETHAN_User_Manual_02-07-2019_LQ_ENG.pdf (accessed on 10 February 2021).
- Berni, K.C.; Dibai-Filho, A.V.; Pires, P.F.; Rodrigues-Bigaton, D. Accuracy of the surface electromyography RMS processing for the diagnosis of myogenous temporomandibular disorder. J. Electromyogr. Kinesiol. 2015, 25, 596–602. [Google Scholar] [CrossRef]
- Augusti, D.; Augusti, G.; Re, D.; Dellavia, C.; Giannì, A.B. Effect of different dental articulating papers on SEMG activity during maximum clenching. J. Electromyogr. Kinesiol. 2015, 25, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Basmajian John, V.; De Luca, C.J. Muscles Alive. Their Functions Relieved by Electromyography, 5th ed.; William/Wilkin: Baltimore, MD, USA, 1985. [Google Scholar]
- Jankelson, B. Neuromuscular aspects of occlusion. Effects of occlusal position on the physiology and dysfunction of the mandibular musculature. Dent. Clin. N. Am. 1979, 23, 157–168. [Google Scholar]
- Cesaretti, G.; Gobbi, G. Kinesiografia e sEMG su Pazienti Affetti da Bruxismo; Bioket: San Benedetto del Tronto, Italy, 2013. [Google Scholar]
- Papini, A.; Cesaretti, G.; Defabianis, P. Kinesiographic analysis of lateral excursive movement on the horizontal plane: The retrusive component. J. Oral Sci. Rehabil. 2017, 3, 60–67. [Google Scholar]
- Müller, L.; Kellenberger, C.J.; Cannizzaro, E.; Ettlin, D.; Schraner, T.; Bolt, I.B.; Peltomäki, T.; Saurenmann, R.K. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: A pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology 2009, 48, 680–685. [Google Scholar] [CrossRef]
- Pedersen, T.K.; Küseler, A.; Gelineck, J.; Herlin, T. A prospective study of magnetic resonance and radiographic imaging in relation to symptoms and clinical findings of the temporomandibular joint in children with juvenile idiopathic arthritis. J. Rheumatol. 2008, 35, 1668–1675. [Google Scholar]
- Lucchese, A.; Carinci, F.; Brunelli, G.; Monguzzi, R. Everstick® and Ribbond® fiber reinforced composites: Scanning Electron Microscope (SEM) comparative analysis. Eur. J. Inflamm. 2011, 3, 73–79. [Google Scholar]
- Roncati, M.; Polizzi, E.; Cingano, L.; Gherlone, E.F.; Lucchese, A. An oral health aid for disabled patients. Dent. Cadmos 2013, 81, 447–452. [Google Scholar] [CrossRef]
- Stoustrup, P.; Kristensen, K.D.; Verna, C.; Küseler, A.; Herlin, T.; Pedersen, T.K. Orofacial symptoms related to temporomandibular joint arthritis in juvenile idiopathic arthritis: Smallest detectable difference in self-reported pain intensity. J. Rheumatol. 2012, 39, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, K.; Lipski, M.; Lichota, D.; Szyszka-Sommerfeld, L. Muscle Fatigue in the Temporal and Masseter Muscles in Patients with Temporomandibular Dysfunction. BioMed Res. Int. 2015, 2015, e269734. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, O. Management of Temporomandibular Disorders and Occlusion, 7th ed.; Mosby: St. Louis, MO, USA, 2013. [Google Scholar]
- Bakke, M.; Zak, M.; Jensen, B.L.; Pedersen, F.K.; Kreiborg, S. Orofacial pain, jaw function, and temporomandibular disorders in women with a history of juvenile chronic arthritis or persistent juvenile chronic arthritis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Baena, R.R.; Pastorino, R.; Gherlone, E.F.; Perillo, L.; Lupi, S.M.; Lucchese, A. Histomorphometric Evaluation of two Different Bone Substitutes In Sinus Augumentation Procedures: A Randomized Controlled Trial in Humans. Int. J. Oral Maxillofac. Implants. 2017, 32, 188–194. [Google Scholar] [CrossRef]
- Matarese, G.; Isola, G.; Ramaglia, L.; Dalessandri, D.; Lucchese, A.; Alibrandi, A.; Fabiano, F.; Cordasco, G. Periodontal biotype: Characteristic, prevalence and dimensions related to dental malocclusion. Minerva Stomatol. 2016, 65, 231–238. [Google Scholar]
- Koos, B.; Twilt, M.; Kyank, U.; Fischer-Brandies, H.; Gassling, V.; Tzaribachev, N. Reliability of Clinical Symptoms in Diagnosing Temporomandibular Joint Arthritis in Juvenile Idiopathic Arthritis. J. Rheumatol. 2014, 41, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Rongo, R.; Alstergren, P.; Ammendola, L.; Bucci, R.; Alessio, M.; D’Antò, V.; Michelotti, A. Temporomandibular joint damage in juvenile idiopathic arthritis: Diagnostic validity of diagnostic criteria for temporomandibular disorders. J. Oral Rehabil. 2019, 46, 450–459. [Google Scholar] [CrossRef]
- Manuelli, M. A peaceful man. Prog. Orthod. 2012, 13, 1. [Google Scholar] [CrossRef]
- Mohl, N.D. Reliability and validity of diagnostic modalities for temporomandibular disorders. Adv. Dent. Res. 1993, 7, 113–119. [Google Scholar] [CrossRef]
- Bertossi, D.; Giampaoli, G.; Lucchese, A.; Manuelli, M.; Albanese, M.; Nocini, R.; Nocini, P.F. The skin rejuvenation associated tratment-Fraxel laser, Microbotox, and low G prime hyaluronic acid: Preliminary results. Laser Med. Sci. 2019, 34, 1449–1455. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients with juvenile idiopathic arthritis diagnosed at least six months before by the Paediatric Department of the SS. Annunziata Hospital in Chieti, Italy | Incomplete medical history |
| Age between 5 and 20 years old | Congenital craniofacial syndrome diagnosis |
| No contemporary or previous orthodontic treatment | Presence of craniofacial bone fracture in the medical history |
| Compliance and ability to reliably indicate the presence of pain and to reliably execute instrumental tests | Previous temporomandibular joint treatments or craniofacial surgery in the medical history |
| Presence of other systemic illness |
| Muscle | Group | Mean ± SD | p-Value a |
|---|---|---|---|
| Right Sternocleidomastoid | case | 3.44 ± 3.02 | |
| control | 1.47 ± 1.39 | ||
| 0.009 * | |||
| Left Sternocleidomastoid | case | 3.50 ± 3.01 | |
| control | 1.47 ± 1.39 | ||
| 0.005 * | |||
| Right Masseter | case | 1.03 ± 1.15 | |
| control | 0.22 ± 0.42 | ||
| 0.002 * | |||
| Left Masseter | case | 1.09 ± 1.38 | |
| control | 0.41 ± 0.50 | ||
| 0.049 * | |||
| Right Temporal | case | 0.56 ± 1.34 | |
| control | 0.06 ± 0.25 | ||
| 0.018 * | |||
| Left Temporal | case | 0.50 ± 1.19 | |
| control | 0.22 ± 0.42 | ||
| 0.586 |
| Muscle | Group | Mean ± SD | p-Value a |
|---|---|---|---|
| Right MM/TA | case | 0.89 ± 0.37 | |
| control | 1.10 ± 0.60 | ||
| 0.170 | |||
| Left Sternocleidomastoid | case | 0.41 ± 0.84 | |
| control | 0.69 ± 0.97 | ||
| 0.122 |
| Group | Frequency | Percentage of Frequency | χ2 Value | p-Value a | |
|---|---|---|---|---|---|
| case | Pathological ratios | 43 | 65.2% | ||
| Physiological ratios | 23 | 34.8% | |||
| control | Pathological ratios | 35 | 53.0% | ||
| Physiological ratios | 31 | 47.0% | |||
| 2.006 | 0.157 |
| Group | Mean ± SD | p-Value a | |
|---|---|---|---|
| Mouth opening (mm) | case | 35.89 ± 6.93 | |
| control | 40.13 ± 1.58 | ||
| 0.001 * | |||
| Mouth opening deviation (mm) | case | 5.70 ± 3.06 | |
| control | 4.20 ± 1.90 | ||
| 0.024 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Attilio, M.; Di Carlo, B.; Caroccia, F.; Moscagiuri, F.; d’Angelo, D.M.; Chiarelli, F.; Festa, F.; Breda, L. Clinical and Instrumental TMJ Evaluation in Children and Adolescents with Juvenile Idiopathic Arthritis: A Case—Control Study. Appl. Sci. 2021, 11, 5380. https://doi.org/10.3390/app11125380
D’Attilio M, Di Carlo B, Caroccia F, Moscagiuri F, d’Angelo DM, Chiarelli F, Festa F, Breda L. Clinical and Instrumental TMJ Evaluation in Children and Adolescents with Juvenile Idiopathic Arthritis: A Case—Control Study. Applied Sciences. 2021; 11(12):5380. https://doi.org/10.3390/app11125380
Chicago/Turabian StyleD’Attilio, Michele, Beatrice Di Carlo, Francesco Caroccia, Francesco Moscagiuri, Debora Mariarita d’Angelo, Francesco Chiarelli, Felice Festa, and Luciana Breda. 2021. "Clinical and Instrumental TMJ Evaluation in Children and Adolescents with Juvenile Idiopathic Arthritis: A Case—Control Study" Applied Sciences 11, no. 12: 5380. https://doi.org/10.3390/app11125380
APA StyleD’Attilio, M., Di Carlo, B., Caroccia, F., Moscagiuri, F., d’Angelo, D. M., Chiarelli, F., Festa, F., & Breda, L. (2021). Clinical and Instrumental TMJ Evaluation in Children and Adolescents with Juvenile Idiopathic Arthritis: A Case—Control Study. Applied Sciences, 11(12), 5380. https://doi.org/10.3390/app11125380










