In Vitro Cytotoxic Effects of Secondary Metabolites Present in Sarcopoterium Spinosum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation of Bioactive Compounds
2.3. Tumor Cell Lines
2.4. Cell Proliferation Assay
2.5. Synthesis of Stachydrine Hydroiodide
2.6. Nuclear Magnetic Resonance (NMR) Analysis
2.7. Ultra High-Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS) Analysis
2.8. Chemical Materials
2.9. Statistical Analysis
3. Results
3.1. Isolation of Cytostatic Compounds and Cell Proliferation Analysis
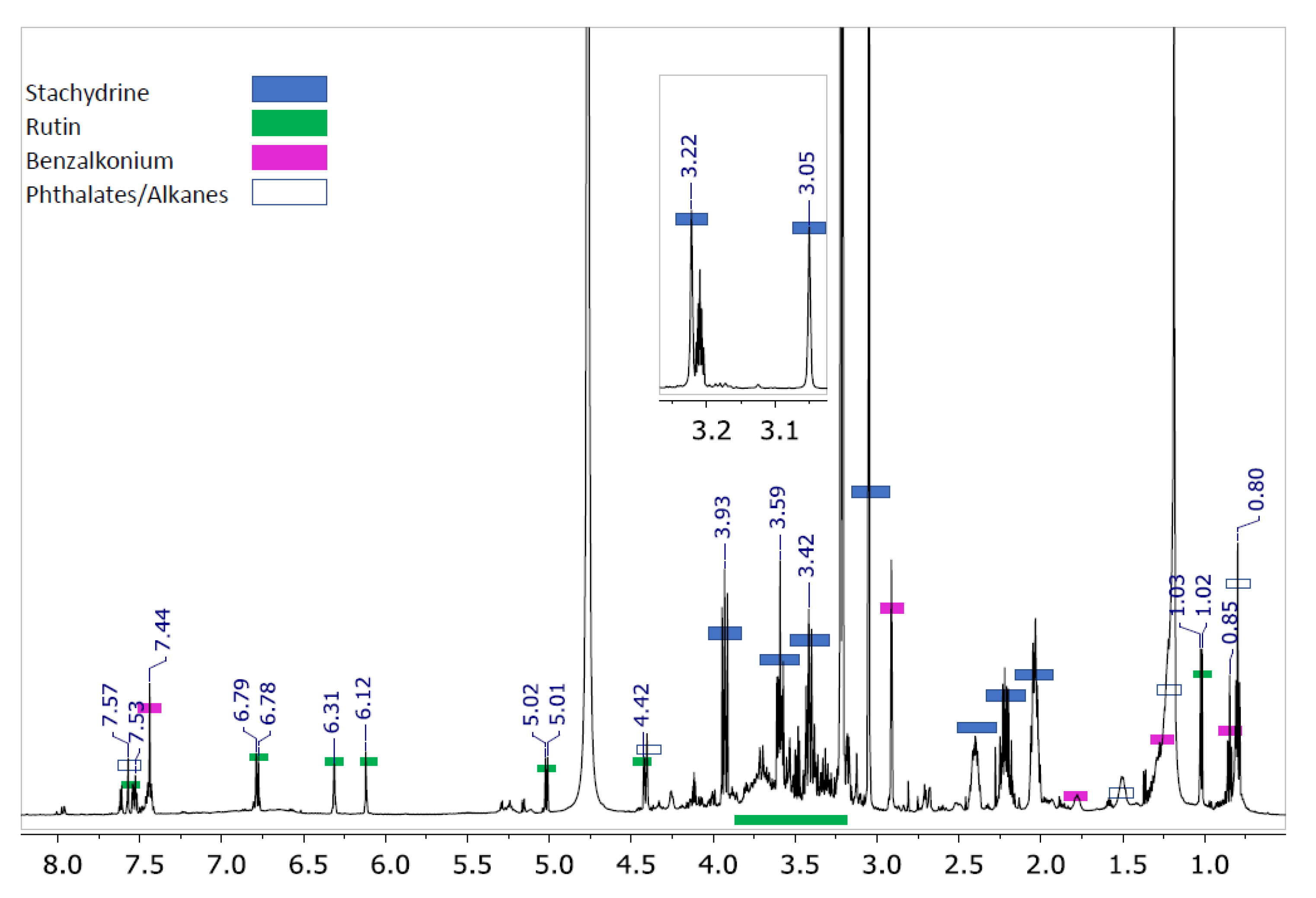
3.2. Compounds Present in the Isolated Fraction
3.3. Synthesis of Stachydrine and Its Identification
3.4. Cytostatic Effect of the Substances Present in the Most Active Sub-Fraction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today (accessed on 15 January 2021).
- Ganesan, A.; Nolan, L.; Crabb, S.J.; Packham, G. Epigenetic therapy: Histone acetylation, DNA methylation and anti-cancer drug discovery. Curr. Cancer Drug Targets 2009, 9, 963–981. [Google Scholar] [CrossRef] [PubMed]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; Van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef]
- Litav, M.; Orshan, G. Biological flora of Israel 1. Sarcopoterium spinosum (L.) Spach. Isr. J. Bot. 1971, 20, 48–64. [Google Scholar]
- Reher, G.; Buděšínský, M. Triterpenoids from plants of the sanguisorbeae. Phytochemistry 1992, 31, 3909–3914. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D.A.; Dobhal, M.; Sharma, M.; Gupta, R.S. Antidiabetic and antioxidant potential of β-sitosterol in Strep-tozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
- Smirin, P.; Taler, D.; Abitbol, G.; Brutman-Barazani, T.; Kerem, Z.; Sampson, S.R.; Rosenzweig, T. Sarcopoterium spinosum extract as an antidiabetic agent: In vitro and in vivo study. J. Ethnopharmacol. 2010, 129, 10–17. [Google Scholar] [CrossRef]
- Yaniv, Z.; Dudai, N. Medicinal and Aromatic Plants of the Middle-East, 1st ed.; Springer Sciences and Business Media: Dordrecht, The Netherlands, 2014; p. 314. [Google Scholar]
- Aldal’In, H.K. Chemical composition of the methanolic extract from seeds, thorns and leaves of Sarcopoterium spinosum (L.) (Rosaceae) grown in Al-Tafila, Jordan. Res. Crop. 2018, 19, 315. [Google Scholar] [CrossRef]
- Henkin, Z.; Rosenzweig, T.; Yaniv, Z. Sarcopoterium spinosum. In Medicinal and Aromatic Plants of the World; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 2, pp. 151–161. [Google Scholar]
- Loizzo, M.R.; Bonesi, M.; Passalacqua, N.G.; Saab, A.; Menichini, F.; Tundis, R. Antiproliferative activities on renal, prostate and melanoma cancer cell lines of Sarcopoterium spinosum aerial parts and its major constituent tormentic acid. Anti-Cancer Agents Med. Chem. 2013, 13, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, K.; Rosenzweig, T. Sarcopoterium spinosum extract improved insulin sensitivity in mice models of glucose intolerance and diabetes. PLoS ONE 2018, 13, e0196736. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shachar, M.; Rozenberg, K.; Skalka, N.; Wollman, A.; Michlin, M.; Rosenzweig, T. Activation of Insulin Signaling in Adipocytes and Myotubes by Sarcopoterium Spinosum Extract. Nutrients 2019, 11, 1396. [Google Scholar] [CrossRef] [Green Version]
- Al-Qura’N, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef]
- Bachrach, Z.Y. Ethnobotanical studies of Sarcopoterium spinosum in Israel. Isr. J. Plant Sci. 2007, 55, 111–114. [Google Scholar] [CrossRef]
- Friedman, J.; Yaniv, Z.; Dafni, A.; Palewitch, D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J. Ethnopharmacol. 1986, 16, 275–287. [Google Scholar] [CrossRef]
- Rozenberg, K.; Wollman, A.; Ben-Shachar, M.; Argaev-Frenkel, L.; Rosenzweig, T. Anti-inflammatory effects of Sarcopo-terium spinosum extract. J. Ethnopharmacol. 2020, 249, 112391. [Google Scholar] [CrossRef] [PubMed]
- Wollman, A.; Daniel, T.; Rosenzweig, T. Sarcopoterium spinosum Inhibited the Development of Non-Alcoholic Steatosis and Steatohepatitis in Mice. Nutrients 2019, 11, 3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durodola, J. Tumour inhibitory effects of crude extracts from poterium spinosum. Planta Med. 1975, 27, 231–234. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Woster, P.M.; Murray, W.J. ChemInform Abstract: Synthesis and Biological Evaluation of Cyclic Analogues of 1-Carnitine as Potential Agents in the Treatment of Myocardial Ischemia. Chem. Inf. 1986, 17, 865–868. [Google Scholar] [CrossRef]
- Rathee, P.; Rathee, D.; Rathee, D.; Rathee, S. In vitro anticancer activity of stachydrine isolated from Capparis decidua on prostate cancer cell lines. Nat. Prod. Res. 2012, 26, 1737–1740. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Z.-W.; Yu, W.-J.; Liao, J.-Y.; Luo, X.-G.; Shen, Y.-J. Stachydrine, a Major Constituent of the Chinese Herb Leonurus Heterophyllus Sweet, Ameliorates Human Umbilical Vein Endothelial Cells Injury Induced by Anoxia-Reoxygenation. Am. J. Chin. Med. 2010, 38, 157–171. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Zhou, Y.-J.; Tong, Q.-Q.; Qu, C.; Kang, T.-J. The Effect of Stachydrine on the Expression of Caspase-12 in Rats with Unilateral Ureteral Obstruction. J. Urol. 2014, 192, 1549–1554. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Liao, Y.-L.; Lv, R.; Wei, H.-C. Effect of Leonurus stachydrine on myocardial cell hypertrophy. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2012, 35, 940–943. [Google Scholar]
- Servillo, L.; D’Onofrio, N.; Longobardi, L.; Sirangelo, I.; Giovane, A.; Cautela, D.; Castaldo, D.; Giordano, A.; Balestrieri, M.L. Stachydrine ameliorates high-glucose induced endothelial cell senescence and SIRT1 downregulation. J. Cell. Biochem. 2013, 114, 2522–2530. [Google Scholar] [CrossRef]
- Heinzmann, S.S.; Brown, I.J.; Chan, Q.; Bictash, M.; Dumas, E.M.; Kochhar, S.; Stamler, J.; Holmes, E.; Elliott, P.; Nicholson, J.K. Metabolic profiling strategy for discovery of nutritional biomarkers: Proline betaine as a marker of citrus consump-tion. Am. J. Clin. Nutr. 2010, 92, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Zhou, Y.; Wang, M.; Guo, C.; Cao, Z.; Zhang, R.; Peng, C. A review of pharmacological and pharmacokinetic properties of stachydrine. Pharmacol. Res. 2020, 155, 104755. [Google Scholar] [CrossRef] [PubMed]
- Marple, B.; Roland, P.; Benninger, M. Safety Review of Benzalkonium Chloride Used as a Preservative in Intranasal Solutions: An Overview of Conflicting Data and Opinions. Otolaryngol. Neck Surg. 2004, 130, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Dart, R.; Caravati, E.M.; McGuigan, M.A. Medical Toxicology, 3rd ed.; Lippincott Williams & Wilkons: Philadelphia, PA, USA, 2014; p. 1883. [Google Scholar]
- Bouchemi, M.; Roubeix, C.; Kessal, K.; Riancho, L.; Raveu, A.-L.; Soualmia, H.; Baudouin, C.; Brignole-Baudouin, F. Effect of benzalkonium chloride on trabecular meshwork cells in a new in vitro 3D trabecular meshwork model for glaucoma. Toxicol. In Vitro 2017, 41, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ammar, D.A.; Noecker, R.J.; Kahook, M.Y. Effects of benzalkonium chloride- and polyquad-preserved combination glau-coma medications on cultured human ocular surface cells. Adv. Ther. 2011, 28, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of Flavonoids and Hy-droxycinnamic Acids in Pak Choi Varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC–ESI-MS n and NMR and Their Quantification by HPLC–DAD. J. Agric. Food. Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Marcarini, J.C.; Tsuboy, M.S.F.; Luiz, R.C.; Ribeiro, L.R.; Hoffmann-Campo, C.B.; Mantovani, M.S. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Raghav, S.; Gupta, B.; Agrawal, C.; Goswami, K.; Das, H.; Gupta, C. (Agrawal) Anti-inflammatory effect of Ruta graveolens L. in murine macrophage cells. J. Ethnopharmacol. 2006, 104, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, J.Y.; Cho, H.C.; Kim, J.C. Antiasthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin. Arch. Pharmacal Res. 2007, 30, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-R.; Hsiao, G.; Chou, P.-H.; Shen, A.M.-Y.; Chou, D.-S. Mechanisms Involved in the Antiplatelet Activity of Rutin, a Glycoside of the Flavonol Quercetin, in Human Platelets. J. Agric. Food Chem. 2004, 52, 4414–4418. [Google Scholar] [CrossRef] [PubMed]


| Sample | IC50 Value a (μg/mL) | ||||
|---|---|---|---|---|---|
| HeLa b | MCF-7 c | Caco-2 d | MDA e | 3T3 f | |
| M-AF | >100 | 22.0 ± 2.7 | >100 | Nt | nt |
| M8 | 20.7 ± 2.5 * | 5.9 ± 1.1 * | 56.0 ± 4.2 * | Nt | nt |
| M10 | >100 | 21.2 ± 2.4 | >100 | Nt | nt |
| M8R | 5.8 ± 1.1 * | 2.2 ± 0.8 * | 9.5 ± 1.4 * | 62.5 ± 3.8 | 270.0 |
| M8N | >100 | >100 | >100 | >100 | >500 |
| L b | Stachydrine | n-Alkane | Rutin | L b | Rutin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13C NMR | 1H NMR | 13C NMR | 1H NMR | 13C NMR | 1H NMR | 13C NMR | 1H NMR | ||||
| 1 | 13.1 | 0.90 | 1 | 101.0 | 5.10 | ||||||
| 2 | 76.2 | 4.01 | 21.7 | 1.30 | 157.9 | nt | 2″ | 74.3 | 3.46 | ||
| 3 | 25.2 | 2.30 | 2.49 | 30.9 | 1.26 | 134.1 | nt | 3″ | 76.7 | 3.40 | |
| 4 | 18.4 | 2.12 | 28.4 | 1.25–1.28 | 178.0 | nt | 4″ | 70.0 | 3.25 | ||
| 5 | 66.6 | 3.68 | 3.49 | 28.8 | 1.25–1.29 | 157.0 | nt | 5″ | 75.8 | 3.31 | |
| 6 | 43.7 | 3.13 | 28.8 | 1.25–1.30 | 93.4 | 6.40 | 6″ | 67.1 | 3.37 | ||
| 7 | 51.3 | 3.30 | 28.8 | 1.25–1.31 | 164.6 | nt | 1‴ | 103.3 | 4.51 | ||
| 8 | 169.4 | - | 98.5 | 6.20 | 2‴ | 70.7 | 3.62 | ||||
| 9 | 161.5 | nt | 3‴ | 70.8 | 3.52 | ||||||
| 10 | 104.2 | nt | 4‴ | 72.5 | 3.26 | ||||||
| 1′ | 121.7 | nt | 5‴ | 68.3 | 3.43 | ||||||
| 2′ | 116.2 | 7.66 | 6‴ | 16.5 | 1.10 | ||||||
| 3′ | 144.4 | nt | |||||||||
| 4′ | 148.3 | nt | |||||||||
| 5′ | 114.6 | 6.87 | |||||||||
| 6′ | 122.1 | 7.16 | |||||||||
| Compound | Concentration (μM) | Jurkat | HeLa | MCF-7 | MDA | 3T3 |
|---|---|---|---|---|---|---|
| Stachydrine | 100 | 100 | 28.1 ± 2.2 | 64.2 ± 3.1 | nt | 89.0 ± 3.8 |
| 10 | 100 | 81.2 ± 3.0 | 79.0 ± 4.2 | nt | 100 | |
| 1 | 100 | 89.0 ± 4.2 | 80.4 ± 4.3 | nt | 100 | |
| 0.1 | 100 | 91.2 ± 5.1 | 88.2 ± 4.1 | nt | 100 | |
| 0.01 | 100 | 95.1 ± 4.5 | 86.3 ± 4.3 | nt | 100 | |
| 0.001 | 100 | 95.0 ± 5.0 | 100 | nt | 100 | |
| Benzalkonium chloride | 100 | nt | nt | nt | nt | nt |
| 10 | nt | 12.1 ± 2.0 | 35.0 ± 2.0 | nt | 10.0 ± 1.8 | |
| 1 | 69.1 ± 3.2 | 43.0 ± 3.0 | 64.2 ± 3.1 | nt | 42.2 ± 2.9 | |
| 0.1 | 84.1 ± 4.0 | 81.1 ± 4.0 | 84.1 ± 4.2 | nt | 71.0 ± 3.4 | |
| 0.01 | 92.2 ± 5.1 | 79.3 ± 4.2 | 92.3 ± 5.0 | nt | 81.1 ± 3.9 | |
| 0.001 | 93.2 ± 5.0 | 79.1 ± 4.3 | 95.2 ± 4.5 | nt | 100 | |
| Rutin | 100 | 78.6 ± 3.1 | 100 | 100 | 94.0 ± 4.2 | 96.0 ± 4.0 |
| 10 | 85.9 ± 3.7 | 100 | 100 | 99.3 ± 5.1 | 100 | |
| 1 | 91.3 ± 4.2 | 100 | 100 | 98.0 ± 4.6 | 100 | |
| 0.1 | 100 | 100 | 100 | 98.6 ± 4.4 | 100 | |
| 0.01 | 100 | 100 | 100 | 99.3 ± 5.1 | 100 | |
| 0.001 | 100 | 100 | 100 | 100 | 100 | |
| Mixture of stachydrine, benzalkonium chloride and rutin (89% + 7% + 4%) | 0.28 ± 0.07 | 76.0 ± 3.9 | 35.5 ± 1.6 | 13.5 ± 3.1 | 52.0 ± 3.3 | |
| Mixture of stachydrine and and benzalkonium chloride a (in μM of individual compounds) | 5 + 5 | nt | nt | nt | nt | 42.0 ± 3.6 |
| 2.5 + 5 | nt | nt | nt | nt | 42.0 ± 5.9 | |
| 1 + 5 | nt | nt | nt | nt | 42.0 ± 2.1 | |
| 0.1 + 5 | nt | nt | nt | nt | 43.5 ± 3.3 | |
| 0.01 + 5 | nt | nt | nt | nt | 43.0 ± 3.4 | |
| 0.001 + 5 | nt | nt | nt | nt | 43.5 ± 5.1 | |
| Paclitaxel | 0.1 | ND | nt | 6.2 ± 1.3 | 12.1 ± 1.9 | nt |
| 0.01 | ND | 27.3 ± 2.2 | 31.0 ± 2.6 | 48.2 ± 3.1 | nt | |
| 0.001 | ND | 68.0 ± 3.2 | 60.2 ± 3.0 | 72.4 ± 3.3 | nt | |
| DMSO | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudec, J.; Mojzis, J.; Habanova, M.; Saraiva, J.A.; Hradil, P.; Liptaj, T.; Kobida, L.; Haban, M.; Holovicova, M.; Zvercova, D. In Vitro Cytotoxic Effects of Secondary Metabolites Present in Sarcopoterium Spinosum L. Appl. Sci. 2021, 11, 5300. https://doi.org/10.3390/app11115300
Hudec J, Mojzis J, Habanova M, Saraiva JA, Hradil P, Liptaj T, Kobida L, Haban M, Holovicova M, Zvercova D. In Vitro Cytotoxic Effects of Secondary Metabolites Present in Sarcopoterium Spinosum L. Applied Sciences. 2021; 11(11):5300. https://doi.org/10.3390/app11115300
Chicago/Turabian StyleHudec, Jozef, Jan Mojzis, Marta Habanova, Jorge A. Saraiva, Pavel Hradil, Tibor Liptaj, Lubomir Kobida, Miroslav Haban, Maria Holovicova, and Dominika Zvercova. 2021. "In Vitro Cytotoxic Effects of Secondary Metabolites Present in Sarcopoterium Spinosum L." Applied Sciences 11, no. 11: 5300. https://doi.org/10.3390/app11115300
APA StyleHudec, J., Mojzis, J., Habanova, M., Saraiva, J. A., Hradil, P., Liptaj, T., Kobida, L., Haban, M., Holovicova, M., & Zvercova, D. (2021). In Vitro Cytotoxic Effects of Secondary Metabolites Present in Sarcopoterium Spinosum L. Applied Sciences, 11(11), 5300. https://doi.org/10.3390/app11115300






