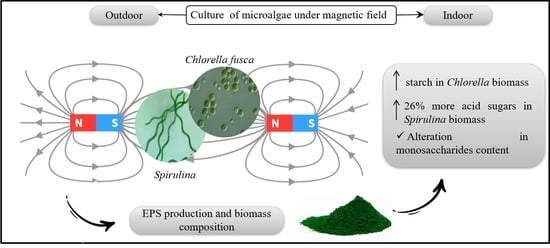
Static Magnetic Fields Effects on Polysaccharides Production by Different Microalgae Strains
Abstract
:1. Introduction
2. Materials and Methods
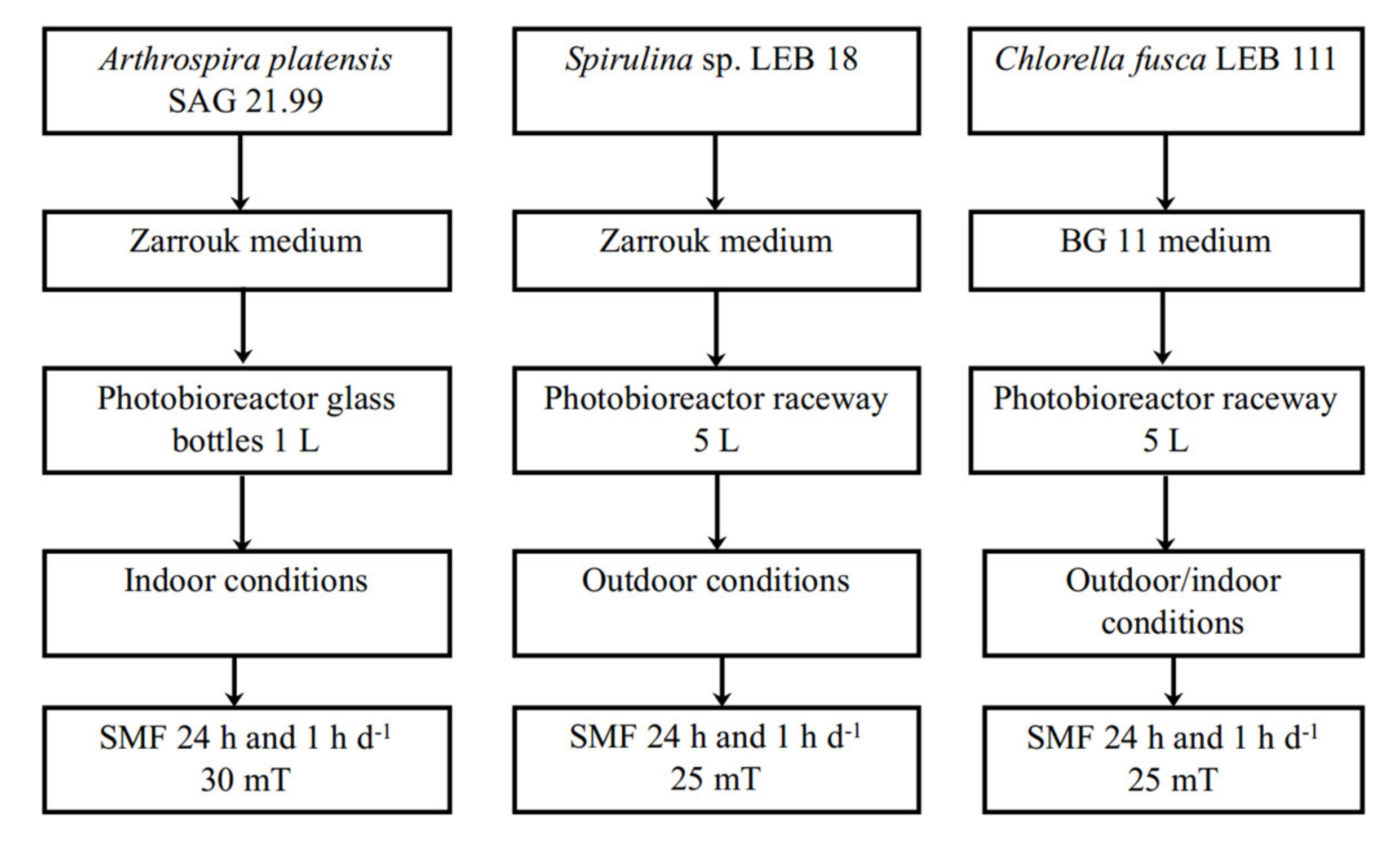
2.1. Microalgae and Culture Conditions
2.2. Static Magnetic Fields Application
2.3. Polysaccharides Extraction and Characterization
2.3.1. Starch from Chlorella fusca Biomass
2.3.2. EPS from Spirulina
2.4. Statistical Analysis
3. Results
3.1. Polysaccharides in Chlorella Microalgae Biomass
3.2. Characterization of EPS of Cyanobacteria Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Dimitriu, S. (Ed.) Polysaccharides, Structural Diversity and Functional Versatility; Dekker M: New-York, NY, USA, 2004. [Google Scholar]
- Dogra, B.; Shaheen, A.; Yong, P.; Jae, P. Biochemical properties of water soluble polysaccharides from photosynthetic marine microalgae Tetraselmis species. Macromol. Res. 2017, 25, 172–179. [Google Scholar] [CrossRef]
- Amna Kashif, S.; Hwang, Y.; Park, J. Potent biomedical applications of isolated polysaccharides from marine microalgae Tetraselmis species. Bioproc. Biosyst. Engineer. 2018, 41, 611–1620. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; el Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, R.; Liu, T.; Li, P. Partial characterization, the immune modulation and anticancer activities of sulfated polysaccharides from filamentous microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Silva, T.H.; Coimbra, M.A.; Nunes, C. Reserve, structural and extracellular polysaccharides of Chlorella vulgaris: A holistic approach. Algal Res. 2020, 45, 101757. [Google Scholar] [CrossRef]
- Sekharam, K.M.; Venkataraman, L.V.; Salimath, P.V. Structural studies of a glucan isolated from blue-green alga Spirulina platensis. Food Chem. 1989, 31, 85–91. [Google Scholar] [CrossRef]
- Nakamura, Y.J.-I.; Sakurai, A.; Inaba, Y.; Suzuki, E.; Nihei, S.; Fujiwara, S.; Tsuzuki, M.; Miyashita, H.; Ikemoto, H.; Kawachi, M.; et al. Some cyanobacteria synthesize semiamylopectin type α-Polyglucans instead of glycogen. Plant Cell Physiol. 2005, 46, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; de Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.L.; Cardoso, J.S.; Mastrantonio, D.J.S.; Bierhals, C.K.; Moreira, J.B.; Costa, J.A.V.; Morais, M.G. Microalgae starch: A promising raw material for the bioethanol production. Int. J. Biol. Macromol. 2020, 165, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; de Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.O.; Deamici, K.M.; Menestrino, B.C.; Garda, B.J.; Costa, J.A.V. Magnetic treatment of microalgae for enhanced product formation. World J. Microbiol. Biotechnol. 2017, 33, 169–173. [Google Scholar] [CrossRef]
- Deamici, K.M.; Costa, J.A.V.; Santos, L.O. Magnetic field action on outdoor and indoor cultures of Spirulina: Evaluation of growth, medium consumption and protein profile. Bioresour. Technol. 2018, 249, 168–174. [Google Scholar] [CrossRef]
- Costa, S.S.; Peres, B.P.; Machado, B.R.; Costa, J.A.V.; Santos, L.O. Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour. Technol. 2020, 315, 123880. [Google Scholar] [CrossRef]
- Veiga, M.C.; Fontoura, M.M.; Oliveira, M.G.; Costa, J.A.V.; Santos, L.O. Magnetic fields: Biomass potential of Spirulina sp. for food supplement. Bioproc. Biosyst. Engineer. 2020, 43, 1231–1240. [Google Scholar] [CrossRef]
- Menestrino, B.C.; Pintos, T.H.C.; Sala, L.; Costa, J.A.V.; Santos, L.O. Application of static magnetic fields on the mixotrophic culture of Chlorella minutissima for carbohydrate production. Appl. Biochem. Biotechnol. 2020, 192, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Jin, W.; Xi, T.; Yang, Q.; Han, S.-F.; Abomohra, A.E.-F. Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivates in municipal wastewater. Water Res. 2015, 86, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Schenck, J. The role of magnetic susceptibility in magnetic reso- nance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996, 23, 815–850. [Google Scholar] [CrossRef]
- Rusanowska, P.; Zieliński, M.; Dębowski, M. Impact of static magnetic field on efficiency of fine-bubble aeration of liquid. J. Ecol. Eng. 2017, 2, 130–135. (In Polish) [Google Scholar]
- Zieliński, M.; Cydzik-Kwiatkowska, A.; Zielińska, M.; Dębowski, M.; Rusanowska, P.; Kopańska, J. Nitrification in activated sludge exposed to static magnetic field. Water Air Soil Pollut. 2017, 22, 126. [Google Scholar] [CrossRef] [Green Version]
- Dini, L.; Abbro, L. Bioeffects of moderate intensity static magnetic fields on cell cultures. Micron 2005, 36, 195–217. [Google Scholar] [CrossRef]
- Yadollahpour, A.; Rashidi, S.; Ghotbeddin, Z.; Jalilifar, M.; Rezaee, Z. Electromagnetic fields for treatments of wastewater: A review of applications and future opportunities. J. Pure Appl. Microbiol. 2014, 8, 3711–3719. [Google Scholar]
- Repacholi, M.H.; Greenebaum, B. Interaction of static and extremely low frequency electric and magnetic fields with living systems: Health effects and research needs. Bioelectromagnetics 1999, 20, 133–160. [Google Scholar] [CrossRef]
- Sahebjamei, H.; Abdolmaleki, P.; Ghanati, F. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics 2007, 28, 42–47. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zeng, X.B.; Guo, S.Y. Growth of Chlorella vulgaris under different magnetic treatments. Prog. Mod. Biomed. 2006, 6, 106–108. [Google Scholar]
- Zhi-Yong, L.; Si-Yuan, G.; Lin, L.; Miao-Yan, C. Effects of electromagnetic field on the batch cultivation and nutritional composition of Spirulina platensis in an air-lift photobioreactor. Bioresour. Technol. 2007, 98, 700–705. [Google Scholar]
- Miyakoshi, J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005, 87, 213–223. [Google Scholar] [CrossRef]
- Niu, C.; Geng, J.; Ren, H.; Ding, L.; Xu, K.; Liang, W. The strengthening effect of a static magnetic field on activated sludge activity at low temperature. Bioresour. Technol. 2013, 150, 156–162. [Google Scholar] [CrossRef]
- Filipič, J.; Kraigher, B.; Tepuš, B.; Kokol, V.; Mandic-Mulec, I. Effects of low-density static magnetic fields on the growth and activities of wastewater bacteria Escherichia coli and Pseudomonas putida. Bioresour. Technol. 2012, 120, 225–232. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; Rosa, A.P.C.; Santos, L.O. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Shao, W.; Ebaid, R.; Abomohra, A.E.F.; Shahen, M. Enhancement of Spirulina biomass production and cadmium biosorption using combined static magnetic field. Bioresour. Technol. 2018, 265, 163–169. [Google Scholar] [CrossRef]
- Deamici, K.M.; Costa, J.A.V.; Santos, L.O. Magnetic fields as triggers of microalga growth: Evaluation of its effect on Spirulina sp. Bioresour. Technol. 2016, 220, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Use of static magnetic fields to increase CO2 biofixation by the microalga Chlorella fusca. Bioresour. Technol. 2019, 276, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, H.; Zhang, J. Effects of static magnetic field on Chlorella vulgaris: Growth and extracellular polysaccharide (EPS) production. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Morais, M.G.; Reichert, C.C.; Dalcanton, F.; Durante, A.J.; Marins, L.F.; Costa, J.A.V. Isolation and characterization of a new Arthrospira strain. Z. Naturforsch. C 2008, 63, 144–150. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Colla, L.M.; Duarte, F.P. Improving Spirulina platensis biomass yield using a fed-batch process. Bioresour. Technol. 2004, 92, 237–241. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst. Eng. 2021. [Google Scholar] [CrossRef]
- Phélippé, M.; Gonçalves, M.; Thouand, G.; Cogne, G.; Laroche, C. Characterization of the polysaccharides chemical diversity of the cyanobacteria Arthrospira platensis. Algal Res. 2019, 33, 101426. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filali-Mouhim, R.; Cornet, J.-F.; Fontane, T.; Fournet, B.; Dubertret, G. Production, isolation and preliminary characterization of the exopolysaccharide of the cyanobacterium Spirulina platensis. Biotechnol. Lett. 1993, 15, 567–572. [Google Scholar] [CrossRef]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridiumcruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.C. Colorimetric determination of neutral sugars by a resorcinol sulphuric acid micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Montreuil, J.; Spick, G.; Chosson, A.; Segard, E.; Scheppler, N. Methods of study of the structure of glycoproteins. J. Pharm. Belg. 1963, 18, 529–546. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Villay, A.; Laroche, C.; Roriz, D.; el Alaoui, H.; Delbac, F.; Michaud, P. Optimisation of culture parameters for exopolysaccharides production by the microalga Rhodella violacea. Biores. Technol. 2013, 146, 732–735. [Google Scholar] [CrossRef]
- Soanen, N.; da Silva, E.; Gardarin, C.; Michaud, P.; Laroche, C. Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour. Technol. 2016, 213, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, C.; Macao, V.; Gardarin, C.; Rihouey, C.; Picton, L.; Michaud, P.; Laroche, C. The red microalga Flintiella sanguinaria as a new exopolysaccharide producer. J. Appl. Phycol. 2018, 30, 2803–2814. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Hu, Q. Environmental effects on cell composition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2004. [Google Scholar]
- Baudelet, P.-H.; Ricochon, G.; Linder, M.; Muniglia, L. New insight into cell walls of Chlorophyta. Algal Res. 2017, 25, 333–371. [Google Scholar] [CrossRef]
- Barberousse, H.; Ruiz, G.; Gloaguen, V.; Lombardo, R.J.; Djediat, C.; Mascarell, G.; Castaing, J.C. Capsular polysaccharides secreted by building façade colonisers: Characterisation and adsorption to surfaces. Biofouling 2006, 22, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, W.; Xu, J.; Wang, Z.; Xu, J.; Yuan, Z. Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Biores. Technol. 2014, 152, 292–298. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-D.; Chang, C.-Y.; Lai, Y.-Y.; Chen, C.-Y.; Kondo, A.; Ren, N.-Q.; Chang, J.-S. Feasibility of CO2 mitigation and carbohydrate production by microalga Scenedesmus obliquus CNW-N used for bioethanol fermentation under outdoor conditions: Effects of seasonal changes. Biotechnol. Biofuels. 2017, 10, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, J.A.; Geider, R.J. Temperature and algal growth. New Phytol. 2006, 110, 441–461. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, T.; Hayashi, K.; Sankawa, U.; Maeda, M.; Nemoto, T.; Nakanishi, H. Further purification and structural analysis of calcium spirulan from Spirulina platensis. J. Nat. Prod. 1998, 61, 1101–1104. [Google Scholar] [CrossRef]
- Richert, L.; Golubic, S.; Guédès, R.L.; Ratiskol, J.; Payri, C.; Guezennec, J. Characterization of exopolysaccharides produced by cyanobacteria isolated from Polynesian microbial mats. Curr. Microbiol. 2005, 51, 379–384. [Google Scholar] [CrossRef]
- Rossi, F.; Micheletti, E.; Bruno, L.; Adhikary, S.P.; Albertano, P.; Philippis, R.D. Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling 2012, 28, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Bishop, P.L. Biodegradability of biofilm extracellular polymeric substances. Chemosphere 2003, 50, 63–69. [Google Scholar] [CrossRef]

| Assays | Starch (g 100 g−1) | Carbohydrates (g 100 g−1) | Xmax (g L−1) | |
|---|---|---|---|---|
| Indoor | Control | 6.42 ± 0.26 a | 24.84 ± 0.11 d | 0.80 ± 0.01 d |
| SMF 1 h d−1 | 7.28 ± 0.61 a,b | 24.98 ± 1.00 d | 1.36 ± 011 c | |
| SMF 24 h d−1 | 7.91 ± 0.29 b | 27.53 ± 0.52 c,d | 1.48 ± 0.11 c | |
| Outdoor | Control 1 h d−1 | 9.67 ± 0.82 c | 31.38 ± 0.28 a,b | 2.18 ± 0.02 a |
| SMF 1 h d−1 | 10.94 ± 0.55 d | 34.18 ± 0.68 a | 2.23 ± 0.07 a | |
| Control 24 h d−1 | 10.12 ± 1.27 c,d | 29.16 ± 1.38 b,c | 1.75 ± 0.03 b | |
| SMF 24 h d−1 | 10.08 ± 0.22 c,d | 31.45 ± 0.76 a,b | 2.30 ± 0.20 a | |
| Assays | EPS (g·100 gbiomass−1) | % NS in EPS | % UA in EPS | Xmax (g L−1) |
|---|---|---|---|---|
| Arthrospira platensis SAG 21.99 | ||||
| Control | 27.9 ± 0.01 a | 86.83 ± 0.01 b | 13.17 ± 0.01 a | 0.59 ± 0.04 a |
| SMF 24 h d−1 | 34.8 ± 0.90 a | 80.72 ± 2.34 a | 19.27 ± 2.34 b | 0.78 ± 0.05 b |
| SMF 1 h d−1 | 19.4 ± 0.06 a | 81.51 ± 0.01 a | 18.49 ± 0.01 b | 0.88 ± 0.04 c |
| Spirulina sp. LEB 18 | ||||
| Control | 49.3 ± 0.19 a | 84.47 ± 2.27 b | 15.53 ± 0.01 a | 3.40 ± 0.01 d |
| SMF 24 h d−1 | 33.8 ± 0.49 b | 85.01 ± 3.10 b | 15.00 ± 3.10 a | 3.37 ± 0.01 d |
| SMF 1 h d−1 | 33.1 ± 0.06 b | 82.98 ± 0.35 b,a | 17.02 ± 0.35 a | 3.65 ± 0.06 d |
| Monosaccharides (% Molar Ratio) | Arthrospira Platensis SAG 21.99 | Spirulina sp. LEB 18 | ||||
|---|---|---|---|---|---|---|
| Control | SMF 24 h d−1 | SMF 1 h d−1 | Control | SMF 24 h d−1 | SMF 1 h d−1 | |
| Fucose | 5.8 ± 0.01 | 10.2 ± 0.01 | 7.0 ± 0.01 | 13.34 ± 0.01 | 13.5 ± 0.02 | 15.30 ± 0.01 |
| Arabinose | 1.7 ± 0.01 | - | - | 1.77 ± 0.01 | 2.28 ± 0.01 | 1.43 ± 0.01 |
| Rhamnose | - | 4.8 ± 0.02 | 4.5 ± 0.02 | 9.77 ± 0.01 | 6.59 ± 0.01 | 4.33 ± 0.01 |
| Galactose | 7.2 ± 0.01 | 16.9 ± 0.01 | 15.5 ± 0.04 | 18.99 ± 0.01 | 21.05 ± 0.02 | 20.42 ± 0.01 |
| Glucose | 71.8 ± 0.01 | 52.4 ± 0.01 | 57.2 ± 0.04 | 19.66 ± 0.01 | 23.35 ± 0.02 | 24.51 ± 0.03 |
| Xylose | 9.9 ± 0.01 | 7.8 ± 0.02 | 6.2 ± 0.01 | 17.37 ± 0.01 | 17.62 ± 0.04 | 16.78 ± 0.02 |
| Glucuronic acid | 3.6 ± 0.01 | 8.1 ± 0.01 | 6.50 ± 0.02 | 13.45 ± 0.01 | 14.30 ± 0.01 | 15.48 ± 0.01 |
| Galacturonic acid | - | - | - | 4.18 ± 0.01 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deamici, K.M.; de Morais, M.G.; Santos, L.O.; Muylaert, K.; Gardarin, C.; Costa, J.A.V.; Laroche, C. Static Magnetic Fields Effects on Polysaccharides Production by Different Microalgae Strains. Appl. Sci. 2021, 11, 5299. https://doi.org/10.3390/app11115299
Deamici KM, de Morais MG, Santos LO, Muylaert K, Gardarin C, Costa JAV, Laroche C. Static Magnetic Fields Effects on Polysaccharides Production by Different Microalgae Strains. Applied Sciences. 2021; 11(11):5299. https://doi.org/10.3390/app11115299
Chicago/Turabian StyleDeamici, Kricelle M., Michele G. de Morais, Lucielen O. Santos, Koenraad Muylaert, Christine Gardarin, Jorge Alberto V. Costa, and Céline Laroche. 2021. "Static Magnetic Fields Effects on Polysaccharides Production by Different Microalgae Strains" Applied Sciences 11, no. 11: 5299. https://doi.org/10.3390/app11115299
APA StyleDeamici, K. M., de Morais, M. G., Santos, L. O., Muylaert, K., Gardarin, C., Costa, J. A. V., & Laroche, C. (2021). Static Magnetic Fields Effects on Polysaccharides Production by Different Microalgae Strains. Applied Sciences, 11(11), 5299. https://doi.org/10.3390/app11115299