Recovery of Zinc and Copper from Mine Tailings by Acid Leaching Solutions Combined with Carbon-Based Materials
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Selection and Characterization
2.2. Leaching Experiments
2.3. Statistical Analysis
3. Results
3.1. Materials Characterisation
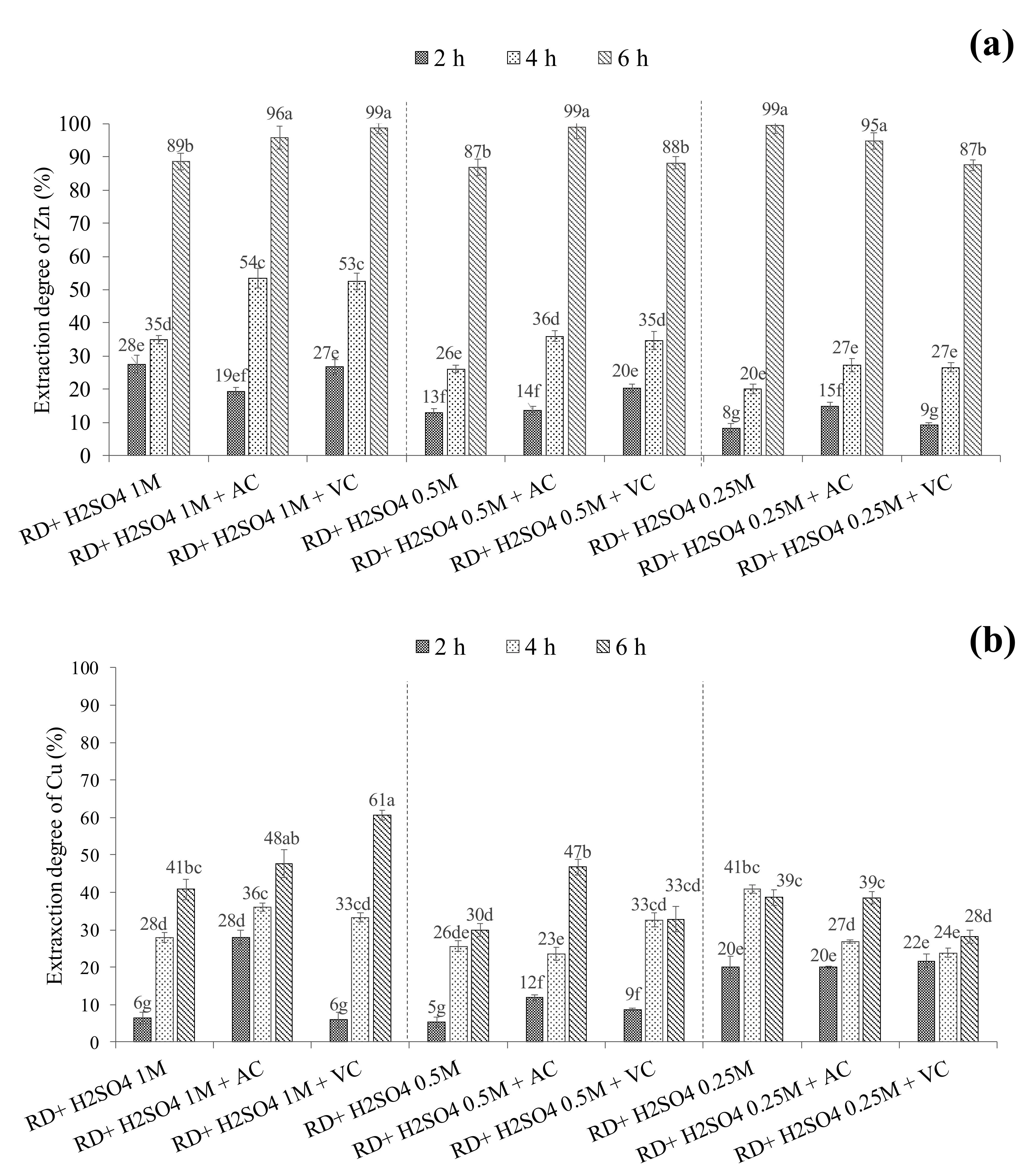
3.2. Percentage of Zn and Cu Extracted in H2SO4 Leaching Solutions
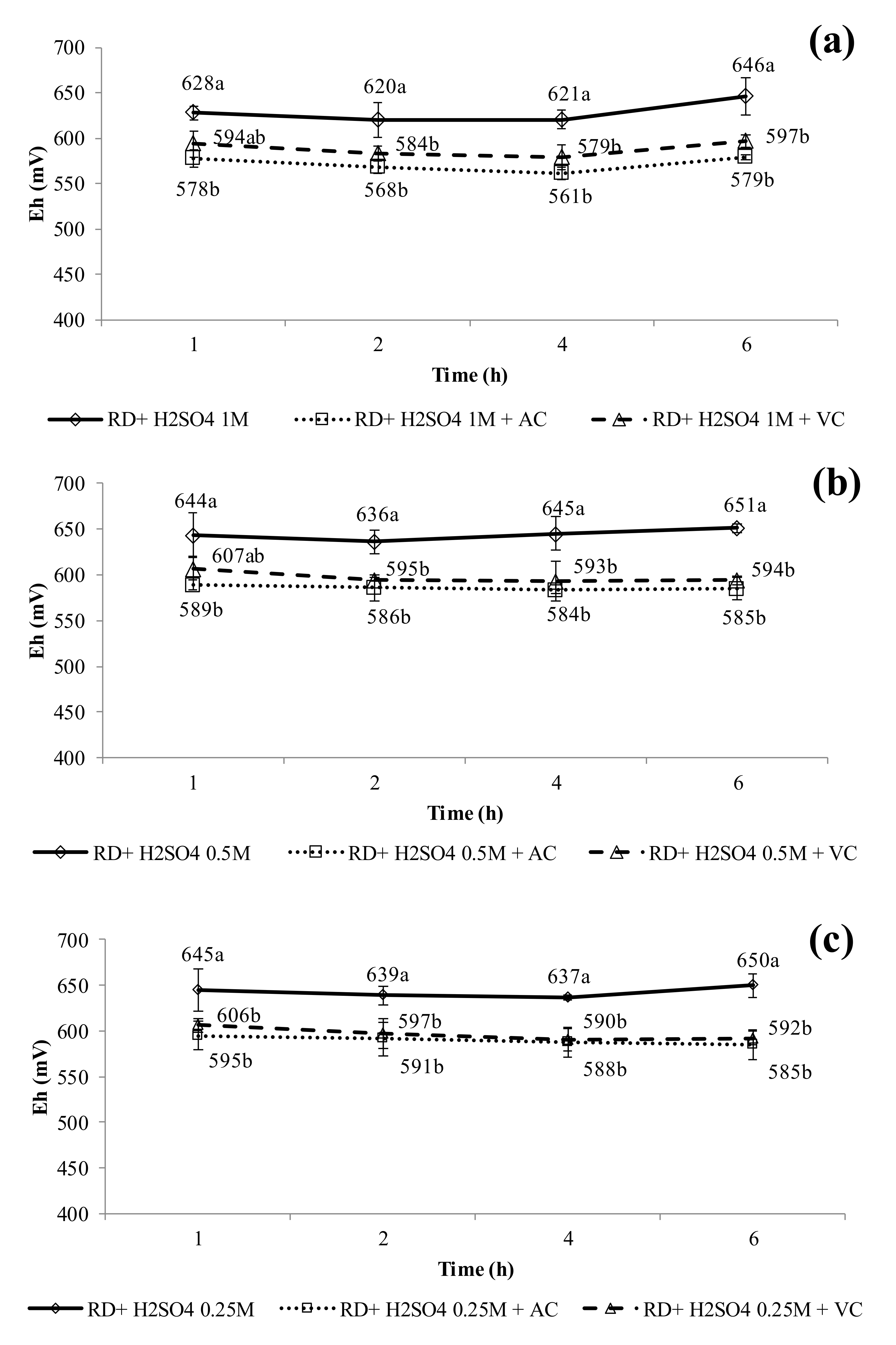
3.3. pH and Eh Evolution During Leaching Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A Framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Alcolea, A.; Ibarra, I.; Caparrós, A.; Rodríguez, R. Study of the MS response by TG-MS in an acid mine drainage efflorescence. J. Therm. Anal. Calorim. 2009, 101, 1161–1165. [Google Scholar] [CrossRef]
- Rodríguez, L.F.; Millán, R.; Álvarez, A.M.G.G.; Cardona, A.I. Estudio de una Rambla Afectada por la Actividad Minera: Rambla del Beal, Cartagena (Murcia). Inf. Técnicos Ciemat. 2010, 1195, 78. [Google Scholar]
- Dirección General del Medio Natural. Región de Murcia, Parque minero y ambiental Cabezo Rajao, una propuesta para su recuperación. Ouverture Project: Green Action II. In Consejería de Agricultura, Agua y Medio Ambiente; CARM: Murcia, Spain, 1998. [Google Scholar]
- García-García, C.; Robles-Arenas, V.M.; Peñas Castejón, J.M.; Rodriguez-Pacheco, R.L. El riesgo ambiental de los residuos minero-metalúrgicos de la Sierra Minera de Cartagena-La Unión (Murcia, España). In Proceedings of the III Congreso de la Naturaleza de la Región de Murcia, Murcia, Spain, 21–24 October 2004; pp. 1–24. [Google Scholar]
- Robles-Arenas, V.M.; Rodríguez, R.; García, C.; Manteca, J.I.; Candela, L. Sulphide-mining impacts in the physical environment: Sierra de Cartagena-La Unión (SE Spain) case study. Environ. Geol. 2006, 51, 57–64. [Google Scholar] [CrossRef]
- Alcolea, A.; Vázquez, M.; Caparrós, A.; Ibarra, I.; García, C.; Linares, R.; Rodríguez, R. Heavy metal removal of intermittent acid mine drainage with an open limestone channel. Miner. Eng. 2012, 26, 86–98. [Google Scholar] [CrossRef]
- García Fernández, G.; Hernández Bastida, J.; Conesa Alcaraz, H.; Faz Cano, Á. Scientific Excursion n° 3: Polluted soils by mining and industrial activities in the “Campo de Cartagena” County (Murcia). In Proceedings of the Fourth International Conference on Land Degradation, Murcia, Spain, 12 September 2004; Quaderna Editorial: Murcia, Spain; pp. 1–85, ISBN 84-95781-41-7. [Google Scholar]
- Martínez, M. Las ramblas del Campo de Cartagena. Problemática ambiental de la Laguna del Mar Menor. Rev. Murc. Antropol. 2007, 14, 63–76. [Google Scholar]
- Martínez-López, S.; Martínez-Sánchez, M.J.; Gómez-Martínez, M.d.C.; Pérez-Sirvent, C. Assessment of the risk associated with mining-derived arsenic inputs in a lagoon system. Environ. Geochem. Health 2019, 42, 2439–2450. [Google Scholar] [CrossRef]
- Jiménez-Cárceles, F.J.; Álvarez-Rogel, J. Phosphorus fractionation and distribution in salt marsh soils affected by mine wastes and eutrophicated water: A case study in SE Spain. Geoderma 2008, 144, 299–309. [Google Scholar] [CrossRef]
- Alcolea, A.; Contreras, S.; Hunink, J.E.; García-Aróstegui, J.L.; Jiménez-Martínez, J. Hydrogeological modelling for the watershed management of the Mar Menor coastal lagoon (Spain). Sci. Total Environ. 2019, 663, 901–914. [Google Scholar] [CrossRef]
- Blondet, I.; Schreck, E.; Viers, J.; Casas, S.; Jubany, I.; Bahí, N.; Darrozes, J. Atmospheric dust characterisation in the mining district of Cartagena-La Unión, Spain: Air quality and health risks assessment. Sci. Total Environ. 2019, 693, 133496. [Google Scholar] [CrossRef]
- Kinnunen, P.H.M.; Kaksonen, A.H. Towards circular economy in mining: Opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 2019, 228, 153–160. [Google Scholar] [CrossRef]
- Chen, T.; Lei, C.; Yan, B.; Xiao, X. Metal recovery from the copper sulfide tailing with leaching and fractional precipitation technology. Hydrometallurgy 2014, 147–148, 178–182. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Wang, X.; Xiao, X. Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching. J. Clean. Prod. 2018, 181, 408–415. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Zhao, G. Production of zinc and lead concentrates from lean oxidized zinc ores by alkaline leaching followed by two-step precipitation using sulfides. Hydrometallurgy 2011, 110, 79–84. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Miller, J.D.; Kuhn, M.C. Chemistry of Flotation. Society of Mining Engineers; AIME: New York, NY, USA, 1985. [Google Scholar]
- Tahereh Asadi, T.; Azizi, A.; Lee, J.; Jahani, M. Leaching of zinc from a lead-zinc flotation tailing sample using ferric sulphate and sulfuric acid media. J. Environ. Chem. Eng. 2017, 5, 4769–4775. [Google Scholar] [CrossRef]
- Watling, H.R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- Fleming, C.A.; Brown, J.A.; Botha, M. An economic and environmental case for re-processing gold tailings in South Africa. In Proceedings of the 42nd Annual Meeting of the Canadian Mineral Processors, Otawa, ON, Canada, 19 January 2010; pp. 365–388. [Google Scholar]
- Cortés, S.; Soto, E.E.; Ordóñez, J.I. Recovery of Copper from Leached Tailing Solutions by Biosorption. Minerals 2020, 10, 158. [Google Scholar] [CrossRef]
- Jorjani, E.; Ghahreman, A. Challenges with elemental sulfur removal during the leaching of copper and zinc sulfides and from the residues: A review. Hydrometallurgy 2017, 171, 333–343. [Google Scholar] [CrossRef]
- Nakazawa, H.; Nakamura, S.; Odashima, S.; Hareyama, W. Effect of carbon black to facilitate galvanic leaching of copper from chalcopyrite in the presence of manganese (IV) oxide. Hydrometallurgy 2016, 163, 69–76. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Fidalgo, J.M.; Gascó, G.; Méndez, A. Hydrometallurgical recovery of Cu and Zn from a complex sulfide mineral by Fe3+/H2SO4 leaching in the presence of carbon-based materials. Metals 2021, 11, 286. [Google Scholar] [CrossRef]
- Nakazawa, H. Effect of carbon black on chalcopyrite leaching in sulfuric acid media at 50 °C. Hydrometallurgy 2018, 177, 100–108. [Google Scholar] [CrossRef]
- Olvera, O.G.; Dixon, D.G.; Asselin, E. Electrochemical study of the dissolution of enargite (Cu3AsS4) in contact with activated carbon. Electrochim. Acta 2013, 107, 525–536. [Google Scholar] [CrossRef]
- Medina, D.; Anderson, K.G. A review of the cyanidation treatment of copper-gold ores and concentrates. Metals 2020, 10, 897. [Google Scholar] [CrossRef]
- Martínez-Sánchez, M.J.; Pérez-Sirvent, C. Niveles de Fondo y Niveles Genéricos de Referencia de Metales Pesados en Suelos de la Región de Murcia; Polytechnic University of Cartagena: Murcia, Spain, 2009. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- USEPA. Office of the Federal Registration (OFR). Appendix A to Part 423—126 Priorty pollutants. Fed Reg. 1982, 47, 52309. [Google Scholar]
- Kursunoglu, S.; Kursunoglu, N.; Hussaini, S.; Kaya, M. Selection of an appropriate acid type for the recovery of zinc from flotation tailing by the analytic hierarchy process. J. Clean. Prod. 2021, 283, 124659. [Google Scholar] [CrossRef]
- Hussaini, S.; Kursunoglu, S.; Top, S.; Thalega Ichlas, Z.; Kaya, M. Testing of 17-different leaching agents for the recovery of zinc from a carbonate-type Pb-Zn ore flotation tailing. Miner. Eng. 2021, 168, 106935. [Google Scholar] [CrossRef]
- Lorenzo-Tallafigo, J.; Romero-García, A.; Iglesias-Gonzalez, N.; Mazuelos, A.; Romero, R.; Carranza, F. A novel hydrometallurgical treatment for the recovery of copper, zinc, lead and silver from bulk concentrates. Hydrometallurgy 2020, 200, 105548. [Google Scholar] [CrossRef]

| Mineral Specie | Content (%) |
|---|---|
| Muscovite | 49.6 ± 0.1 |
| Quartz | 38.9 ± 0.9 |
| Corkite | 6.9 ± 0.5 |
| Calcite magnesium | 3.5 ± 0.1 |
| Litharge | 0.5 |
| Stannite | 0.4 |
| Calcite | 0.2 |
| Element | Content (wt%) |
|---|---|
| O | 39.37 ± 0.12 |
| Si | 20.62 ± 0.12 |
| Al | 8.32 ± 0.10 |
| Fe | 9.07 ± 0.12 |
| S | 1.94 ± 0.04 |
| K | 2.10 ± 0.07 |
| Pb | 2.18 ± 0.07 |
| Zn | 1.38 ± 0.05 |
| Ca | 0.344 ± 0.17 |
| Mg | 0.283 ± 0.014 |
| Ti | 0.252 ± 0.013 |
| Na | 0.202 ± 0.06 |
| As | 0.151 ± 0.0075 |
| P | 0.0339 ± 0.0017 |
| Ba | 0.0518 ± 0.0048 |
| Cu | 0.0435 ± 0.0022 |
| Sn | 0.0424 ± 0.0021 |
| Mn | 0.0293 ± 0.0015 |
| Zr | 0.0258 ± 0.0013 |
| Element | Background Levels 1 (mg kg−1) | Maximum Allowable Limits 2 (mg kg−1) | Mine Waste Levels (mg kg−1) |
|---|---|---|---|
| Cr | 24–45 | 38–71 | 25–80 |
| Co | 5–9 | 10–13 | ≤52 |
| Ni | 17–25 | 30–34 | ≤37 |
| Zn | 16–55 | 43–92 | 993–14,720 |
| Cu | 12–23 | 23–32 | 18–268 |
| As | 5–8 | 8–12 | ≤1930 |
| Se | 0.2–0.6 | 0.4–0.5 | n.d. |
| Cd | 0.1–0.4 | 0.4–0.9 | ≤65 |
| Sb | 0.5–1.6 | 2–3 | ≤220 |
| Hg | 0.1–0.4 | 0.4–1.2 | n.d. |
| Tl | 0.1–0.4 | 0.5–0.8 | n.d. |
| Pb | 3–10 | 5–34 | 75–27,780 |
| Sample | %C | %H | %N | %S | %O | H/C | O/C | Ash (wt%) | pH | Eh (mV) | SBET (m2 g−1) | Porosity (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | 85.72 | 0.88 | 0.00 | 0.00 | 13.41 | 0.12 | 0.12 | 1.00 | 8.03 | 453 | 1138.96 | 73.26 |
| VC | 80.21 | 3.12 | 0.92 | 0.00 | 15.74 | 0.47 | 0.15 | 14.94 | 8.31 | 355 | 2.18 | 63.73 |
| pH (25 °C) | Eh (mV) | |
|---|---|---|
| RD+ H2SO4 1 M | 1.02 ± 0.02 | 654 ± 2.5 |
| RD+ H2SO4 1 M + AC | 0.99 ± 0.01 | 557 ± 5.4 |
| RD+ H2SO4 1 M + VC | 0.99 ± 0.01 | 627 ± 1.3 |
| RD+ H2SO4 0.5 M | 1.08 ± 0.01 | 672 ± 3.3 |
| RD+ H2SO4 0.5 M + AC | 1.09 ± 0.01 | 558 ± 8.0 |
| RD+ H2SO4 0.5 M + VC | 1.09 ± 0.03 | 626 ± 1.7 |
| RD+ H2SO4 0.25 M | 1.21 ± 0.06 | 672 ± 2.9 |
| RD+ H2SO4 0.25 M + AC | 1.21 ± 0.03 | 530 ± 2.4 |
| RD+ H2SO4 0.25 M + VC | 1.22 ± 0.03 | 632 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, M.L.; Méndez, A.; Rodríguez-Pacheco, R.; Paz-Ferreiro, J.; Gascó, G. Recovery of Zinc and Copper from Mine Tailings by Acid Leaching Solutions Combined with Carbon-Based Materials. Appl. Sci. 2021, 11, 5166. https://doi.org/10.3390/app11115166
Álvarez ML, Méndez A, Rodríguez-Pacheco R, Paz-Ferreiro J, Gascó G. Recovery of Zinc and Copper from Mine Tailings by Acid Leaching Solutions Combined with Carbon-Based Materials. Applied Sciences. 2021; 11(11):5166. https://doi.org/10.3390/app11115166
Chicago/Turabian StyleÁlvarez, María Luisa, Ana Méndez, Roberto Rodríguez-Pacheco, Jorge Paz-Ferreiro, and Gabriel Gascó. 2021. "Recovery of Zinc and Copper from Mine Tailings by Acid Leaching Solutions Combined with Carbon-Based Materials" Applied Sciences 11, no. 11: 5166. https://doi.org/10.3390/app11115166
APA StyleÁlvarez, M. L., Méndez, A., Rodríguez-Pacheco, R., Paz-Ferreiro, J., & Gascó, G. (2021). Recovery of Zinc and Copper from Mine Tailings by Acid Leaching Solutions Combined with Carbon-Based Materials. Applied Sciences, 11(11), 5166. https://doi.org/10.3390/app11115166









